Abstract
Purpose.
Posterior capsular opacification (PCO), the most prevalent side effect of cataract surgery, occurs when residual lens epithelial cells (LECs) undergo fiber cell differentiation or epithelial-to-mesenchymal transition (EMT). Here, we used a murine cataract surgery model to investigate the role of the Zeb proteins, Smad interacting protein 1 (Sip1) and δ-crystallin enhancer-binding factor 1 (δEF1), during PCO.
Methods.
Extracapsular extraction of lens fiber cells was performed on wild-type and Sip1 knockout mice. Protein expression patterns were assessed at multiple time points after surgery using confocal immunofluorescence. βB1-Crystallin mRNA levels were measured using quantitative RT-PCR. We used Transfac searches to identify δEF1 binding sites in the βB1-crystallin promoter and transfection analysis to test the ability of δEF1 to regulate βB1-crystallin expression.
Results.
δEF1, which, in other systems, can activate fibrotic genes (e.g., α-smooth muscle actin) and repress epithelial genes, upregulates by 48 hours after fiber cell removal. In culture, δEF1 repressed βB1-crystallin promoter activity, suggesting that it may also turn off lens gene expression following surgery, contributing to “fibrotic PCO” development. Sip1 also upregulates in LECs by 48 hours, but analysis of Sip1 knockout lenses demonstrated that Sip1 does not play a major role in EMT or fiber cell differentiation after surgery. However, Sip1 knockout LECs do express the ectodermal marker keratin 8, suggesting that Sip1 may limit the reprogramming of residual LECs to an embryonic state.
Conclusions.
Zeb transcription factors likely play important, but distinct roles in PCO development after cataract surgery.
Keywords: cataract surgery, epithelial-to-mesenchymal transition, lens regeneration, mouse disease model, posterior capsular opacification, Zeb
Zeb transcription factor expression upregulates following lens fiber cell removal in an animal model. The results presented correlate δEF1 expression with lens epithelial fibrosis while Sip1 upregulation appears to prevent dedifferentiation of lens cells to a precursor fate.
Introduction
The lens is a transparent tissue composed of two distinct cell types: the lens epithelial cells (LECs), which form a single layer covering the anterior surface; and the postmitotic, elongated fiber cells, which are found in the posterior of the lens.1 The entire lens is surrounded by the lens capsule, a thick basement membrane that serves both structural and signaling roles in addition to serving as a barrier between the lens cells and the remainder of the ocular environment.2 The lens grows as epithelial cells in the equatorial region divide and differentiate into lens fiber cells. This process of differentiation continues throughout the life of the organism, leading to layers of sequentially newer lens fiber cells in the outermost layers of the lens.3
The loss of lens transparency, known as cataract, is a major disease of the lens and can result from numerous insults, including oxidative and other age-associated damage, mutations in important lens genes, and diabetes.4 All cataracts, regardless of etiology, are most commonly treated by extracapsular cataract extraction (ECCE), during which the fiber cells and the central anterior lens capsule are removed, leaving the remaining lens capsule behind to anchor an implanted artificial intraocular lens (IOL).5 However, since the equatorial lens epithelium is tightly attached to the capsule, these cells are difficult to remove, remain on the capsule, and often proliferate and migrate into the visual axis to form the common side effect, posterior capsular opacification (PCO).6,7 Posterior capsular opacification can limit final visual acuity after cataract surgery, even with laser capsulotomy treatment,8,9 and it is the major barrier to the efficient function of artificial lenses that can restore the full range of accommodative vision after cataract removal.10
There are two primary mechanisms responsible for the development of PCO: aberrant differentiation of residual LECs into lens fiber cells and epithelial-to-mesenchymal transition (EMT) of LECs.7 Epithelial-to-mesenchymal transition in the lens, like other tissues, is characterized by an upregulation of mesenchymal markers, such as α-smooth muscle actin (α-SMA), and downregulation of epithelial cell markers, such as E-cadherin,11 and appears to be driven by transforming growth factor β (TGF-β) and downstream Smad signaling.12 The residual epithelial cells that attempt to differentiate into lens fibers also cause problems as a result of their inability to properly organize, ultimately leading to the formation of Soemmering's ring and Elschnig pearls.7,13–15 The combined effects of EMT and fiber cell differentiation result in capsular wrinkling, decreased visual acuity, and inefficient function of accommodating IOLs.7,9,10
Currently, the Zeb family of transcription factors is best known for its roles in mediating EMT and fibrosis in numerous diseases,16 particularly cancer. Notably though, the Zeb family member δEF1 (also known as Zeb1, AREB6, and ZFHX1A) was first identified as a transcriptional repressor of the δ-crystallin gene during chicken lens development,17 while the other known Zeb family member, Sip1 (also known as Zeb2 or ZFHX1B), plays crucial roles in mammalian lens vesicle closure,18 lens fiber cell migration, and the downregulation of ectodermal markers during lens morphogenesis.19 Here, we investigated the expression pattern of the Zeb transcription factors in relation to other events during PCO pathogenesis in a mouse model of ECCE.
Methods
Animals and Surgical Removal of Lens Fiber Cells
All experiments using animals were approved by the University of Delaware Institutional Animal Care and Use Committee (approval No. 1039) and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice harboring a Sip1 gene with exon seven flanked by LoxP sites (Sip1flox(ex7)/Zeb2tm1.1Yhi)20 were mated to MLR10 Cre mice,21 resulting in conditional knockout mice lacking Sip1 from the lens beginning at embryonic day 10.5, as done previously.19 Lens fiber cell extraction was performed on one eye of adult wild-type (C57Bl/6<har>) and the Sip1 conditional knockout mice to model ECCE as previously described.22,23 At 12 hours, 48 hours, or 5 days after the initial surgery, mice were again anesthetized and lens fiber cell removal was performed on the previously unoperated eye to create time zero surgical controls. The mice were then immediately killed and the operated eyes were then either embedded for immunofluorescence or the lens capsular bag was isolated for RNA isolation.
Immunofluorescence
Immunofluorescence was performed as previously described.24 Briefly, postsurgical eyes were embedded in Tissue Freezing Media (TFM; Triangle Biomedical, Durham, NC, USA), and 16-μm-thick frozen sections were prepared and placed on Colorfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA). Slides were fixed in 1:1 acetone/methanol and blocked in 1% bovine serum albumin in 1× phosphate-buffered saline (PBS) for 1 hour, unless otherwise noted. Tissue was then covered with a dilution of primary antibody for 1 to 2 hours. Slides were washed three times in PBS, then incubated for 1 hour with a mixture containing a 1:2000 dilution of DRAQ5 (Biostatus Limited, Leicestershire, UK), a 1:250 dilution of an FITC-conjugated monoclonal α-SMA IgG (F3777; Sigma-Aldrich Corp., St. Louis, MO, USA), and a 1:200 dilution of the appropriate species secondary anti-IgG conjugated to Alexafluor 568 (Molecular Probes, Eugene, OR, USA). Slides were again washed three times in PBS, covered with p-phenylenediamine antifade media, and coverslipped. Epithelial whole mounts were also dissected from adult C57Bl/6<har> mouse lenses and immunostained using similar techniques as described above. For the Pax6 and E-cadherin immunostaining, a protocol similar to that for Sip119 was used. All of the primary antibodies and respective dilutions used in this study can be found in the Table. All experiments were repeated with at least three biological replicates and slides were viewed with either a Zeiss 510 LSM or Zeiss 780 LSM confocal microscope (Carl Zeiss Microscopy, Thornwood, NY, USA). All comparisons of expression pattern were done between slides generated from the same staining experiment and imaged on the same day under the same imaging settings. In some cases, brightness and/or contrast of obtained images was adjusted in Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA) for optimum viewing on diverse computer screens. However, in all cases, such adjustments were applied equally to both experimental and control images to retain the validity of the comparison.
Table.
Dilutions, Product Numbers, and Protocols Used for the Antibodies Used in This Study
|
Gene |
Company |
Product No. |
Fixation |
Blocking |
Secondary |
Dilution |
| DRAQ5 | Biostatus Limited | DR50200 | A:M or 4% pfa in 1× PBS | BSA or goat serum | - | 1:2000 |
| α-SMA | Sigma-Aldrich Corp. | F3777 | A:M or 4% pfa in 1× PBS | BSA or goat serum | FITC conjugated | 1:250 |
| E-cadherin | Cell Signaling | 4065 | 4% pfa in 1× PBS | Goat serum | Anti-rabbit | 1:100 |
| Aquaporin 0 | Millipore | ab3071 | A:M | 1% BSA | Anti-rabbit | 1:200 |
| Pax6 | Millipore | AB2237 | 4% pfa in 1× PBS | Goat serum | Anti-rabbit | 1:200 |
| c-Maf (M-153) | Santa Cruz Biotechnology | sc-7866 | A:M | 1% BSA | Anti-rabbit | 1:100 |
| Prox1 | Belecky-Adams et al., 1997; Duncan et al., 2002 | A:M | 1% BSA | Anti-rabbit | 1:500 | |
| Zeb1 (E-20) (δEF1) | Santa Cruz Biotechnology | sc-10572 | A:M | 1% BSA | Anti-goat | 1:200 |
| Sip1 (H-260) | Santa Cruz Biotechnology | sc-48789 | 4% pfa in 1× PBS | Goat serum | Anti-rabbit | 1:100 |
| Keratin 8 | Developmental Hybridoma Bank | Troma-1 | A:M | 1% BSA | Anti-rat | 1:100 |
A:M, 1:1 acetone:methanol solution; pfa, paraformaldehyde.
Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
RNA was extracted from postsurgical mouse lenses at 0 hours and 5 days post surgery using the SV Total RNA Isolation System (Invitrogen, Carlsbad, CA, USA). Complementary DNA was synthesized from these samples with the RT2qPCR Primer Assay (SABiosciences, Valencia, CA, USA) according to the manufacturer's instructions. Quantitative RT-PCR was performed using an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Samples were prepared in a MicroAmp Optical 96-Well Reaction Plate (Life Technologies, Grand Island, NY, USA). Each well contained 1 μL cDNA, 12.5 μL SYBR Green Master Mix (SABiosciences), 1 μL each of forward and reverse primers, and H2O to 25 μL. These experiments used mouse-specific primers for βB1-crystallin (forward: CAG GGC CTG ATG GCA AGG GAA; reverse: CCT GCT CGA AGA CGA TCA GCC)25 and the internal control β2-microglobulin (PPM03562A; SABioscience). Statistical analyses were done using log (base 10)–transformed data in a nested ANOVA. The mean and standard deviation (SD) were then calculated for the log-transformed data and subsequently back transformed, thus providing the mean fold-change, a positive standard deviation, and a negative standard deviation.
βB1Crystallin Promoter Analysis and CAT Reporter Constructs
The βB1-crystallin binding site analysis was done with Mat Inspector 2.226 and TFSEARCH 1.3,27 and the reported δEF1 sites were confirmed using Promo to search Transfac version 8.3.28 The chicken βB1-crystallin promoter (−432/+30) chloramphenicol acetyltransferase (CAT) construct has been previously described.29 The construct designed to mutate the δEF1 binding site at −422/−413 was made by amplifying the region with the following primers containing the specified restriction sites: 5′-TAA GCA TGC CTG GGT ATG CTA GTA TAC AAG GAC AGA AAC-3′ (−432/−403, A-SphI and AccI) and 5′-CCC TCT AGA CAG CTG CTG CTT CTT GTT GGA G-3′ (+30, B-XbaI). This fragment was subcloned, then ligated into the SphI and XbaI sites of the pBasic CAT vector. Constructs designed to mutate the δEF1 binding sites at −292/−283 and −216/−207 were created by ligating the regions upstream and downstream of each δEF1 element into pBasic CAT, replacing 10 bp of the element with an AccI restriction site. The upstream regions −432/−293 and −432/−217 were amplified by PCR with the following primers: 5′-TAT GCA TGC CTG GGT ATG CCC AAG GTG-3′ (−432, A-SphI) and either 5′-CGC GTC GAC GCG ATC CAG CAC-3′ (−293, B-AccI) or 5′-TAC GTA TAC ACG CCG CGC CAC-3′ (−217, B-AccI). The resulting PCR products were subcloned, then each was digested and ligated into the SphI and AccI sites of either the −282/+30/CAT or the −206/+30/CAT truncation construct, both of which have been previously described.30 The construction of all plasmids was confirmed by sequencing.
Transient Transfections
N/N1003A rabbit lens epithelial cells31 were maintained at 35°C, 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% rabbit serum. For transfections, N/N1003A cells were plated at a concentration of 5 × 105 cells per 60-mm dish and allowed to grow approximately 24 hours before transfection. Transfection was performed with Lipofectamine-Plus Reagents (Invitrogen) according to the manufacturer's suggested protocol. Plates were transfected in triplicate with 2.5 μg βB1-crystallin/CAT reporter plasmids, 0.5 μg pCMV β-galactosidase (β-gal) (BD Biosciences Clontech, Mountain View, CA, USA), and 0.5 μg pCMV δEF1.17 Cells were harvested approximately 48 hours after the transfections. The cells were washed in PBS and then resuspended in 100 μL 0.25 M Tris buffer, pH 7.8. The cells were subjected to several rounds of freeze/thaw, and then the CAT and β-gal activity of the cell extracts were measured as previously described.29 Statistical analysis was done using the Student's t-test, and the P values less than 0.05 (95% significance) are reported here.
Results
βB1-Crystallin Gene Expression Decreases and Known Regulators of This Gene Are Altered After Fiber Cell Removal in a Mouse ECCE Model
βB1-Crystallin protein expression is a commonly used marker of lens fiber cell differentiation; however, in the adult lens, its mRNA is abundant in LECs,32 whereas its expression has been reported to downregulate as LECs undergo EMT.33 To confirm these observations, we performed qRT-PCR to determine the βB1-crystallin transcript levels in LECs remaining in the capsular bag immediately after lens fiber cell removal (zero hours post surgery) and at 5 days after surgery, a time point by which remnant LECs consistently show an upregulation of mesenchymal markers, particularly α-SMA. We found that βB1-crystallin mRNA levels decreased 110 fold (+0.016/−0.006; P = 0.004) in remnant LECs by 5 days post surgery compared to time zero.
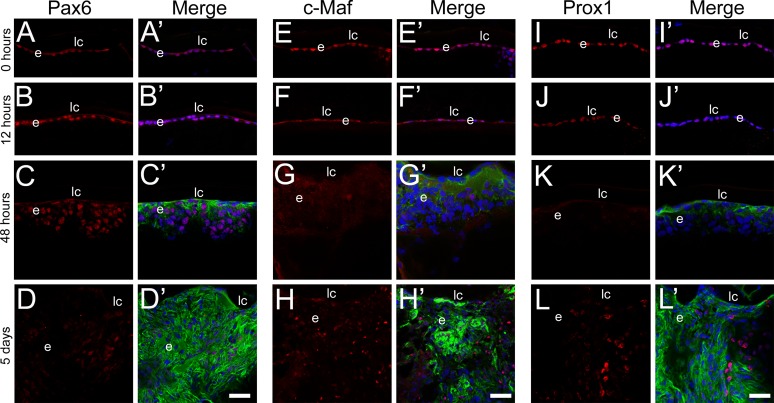
Paired box 6 (Pax6), a lens epithelial cell preferred transcription factor,34,35 has previously been shown to be a transcriptional repressor of the βB1-crystallin promoter34,36 and has been reported to downregulate as LECs undergo both EMT11 and lens fiber cell differentiation.34 After fiber cell removal, Pax6 levels remained unchanged in remnant LECs during the initial 48 hours after surgery (Figs. 1A–C), only downregulating at the 5-day time point (Fig. 1D). However, the extent of Pax6 downregulation at this time point was not consistent in all cells, implying that it may contribute to the different cell types formed during PCO. Conversely, v-maf musculoaponeurotic fibrosarcoma oncogene homolog (c-Maf) and prospero homeobox 1 (Prox1) transcription factors, known to activate βB1-crystallin transcription,30,36 appeared to be expressed at low levels in the epithelium at 0 hours after surgery (Figs. 1E, 1I), downregulated by 12 hours post surgery (Figs. 1F, 1J), and were largely undetectable in remnant cells by 48 hours after fiber cell removal (Figs. 1G, 1K). By 5 days post surgery, small pockets of robust c-Maf and Prox1 expression were detected (Figs. 1H, 1L) in regions without detectable α-SMA expression, suggesting that this recovered expression may be helping to drive the fiber cell differentiation that begins at that time.
Figure 1.
Time course of lens epithelial (Pax6, [A–D]), myofibroblast (α-SMA, [A′–L′]), and lens fiber cell (c-Maf [E–H] and Prox1 [I–L]) marker expression in lens cells remaining behind in a mouse model of cataract surgery determined by confocal immunofluorescence analysis. At the time of surgery, the residual lens epithelial cells express detectable levels of Pax6 (A), c-Maf (E), and Prox1 (I) but do not have detectable levels of α-SMA (A′, E′, I′). Similar results were obtained 12 hours after surgery (B, F, J), although c-Maf (F) and Prox1 (J) levels appear to decline. At 48 hours post surgery, α-SMA (C′, G′, K′) has increased and although Pax6 is still detectable (C), c-Maf (G) and Prox1 (K) are not. However, by 5 days post surgery, Pax6 is no longer detected in the remnant cells (D), while α-SMA expression is robust (D′, H′, L′) and the fiber cell markers c-Maf (H) and Prox1 (L) are localized to discrete cell islands that tend to not express α-SMA (H′, L′). Prime panels (e.g., [A′]) show the respective transcription factor expression (red) merged with nuclei (DRAQ5, blue) and α-SMA expression (green). Scale bars: 35 μm. e, residual lens epithelial cells/transitioning epithelial cells; lc, lens capsule.
While it is possible that the changes in Pax6, c-Maf, and Prox1 protein expression indicated here are partially responsible for the observed downregulation in βB1-crystallin mRNA 5 days after surgery, the complex function of these factors suggests that this is not the full explanation. Thus, we sought to find other transcriptional repressors that may be responsible for the downregulation of βB1-crystallin expression in LECs, following lens fiber cell removal in our cataract surgery model.
δEF1 Is a Potential Regulator of βB1-Crystallin Expression During PCO
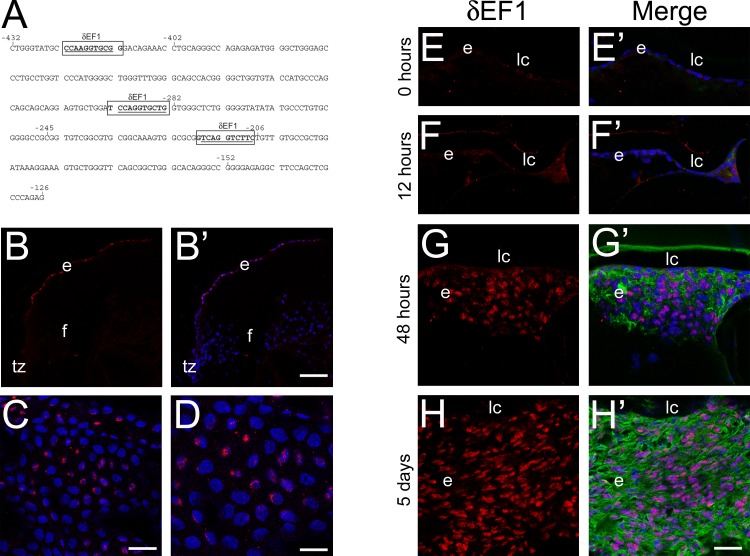
A search for cis-elements within the βB1-crystallin promoter was performed to locate possible elements contributing to the repression of βB1-crystallin in the residual LECs. Three potential δEF1 elements were identified (Fig. 2A) within the promoter (−432/+30) at positions −422/−413, −292/−283, and −216/−207.
Figure 2.
δEF1 expression is robustly upregulated in remnant cells expressing α-SMA and has binding sites present in the βB1-crystallin promoter. (A) Sequence analysis of the −432/−126 βB1-crystallin promoter region revealed three binding sites for δEF1 at −422/−413, −292/−283, and −216/−207. The bases targeted for substitution are underlined. (B–D) δEF1 protein is expressed in only select cells in the adult mouse lens epithelium, indicated in the confocal microscopy images of δEF1 (red) immunolocalization in sectioned adult mouse lenses (B, B′) (scale bar: 77 μm) and adult mouse epithelial whole mount explants at two magnifications shown in (C) (scale bar: 27 μm) and (D) (scale bar: 17 μm). (E–H) Confocal microscopy images of δEF1 protein expression 0 hours (E), 12 hours (F), 48 hours (G), and 5 days (H) after surgery indicate that δEF1 expression upregulates 2 days after lens fiber cell removal and remains high after 5 days in cells expressing high levels of α-SMA (G′, H′) (scale bar: 35 μm). Prime panels (e.g., [A′]) show δEF1 expression (red) merged with nuclei (DRAQ5, blue) and α-SMA expression (green; post surgery only). e, lens epithelial cells/transitioning epithelial cells post surgery; f, lens fiber cells.
Immunolocalization of δEF1 in the normal mouse lens was completed to determine if its expression pattern was consistent with a role in the repression of βB1-crystallin expression following lens fiber cell removal. While δEF1 protein was not detected in the embryonic mouse lens (data not shown), consistent with prior reports,37 δEF1 protein was found in a few scattered epithelial cells in the adult lens (Figs. 2B–D). Furthermore, between 12 (Fig. 2F) and 48 (Fig. 2G) hours after fiber cell removal, δEF1 protein levels dramatically increased and remained robust 5 days post surgery (Fig. 2H) in cells expressing α-SMA.
Cotransfection of a δEF1 expression vector with a −432/+30 βB1-crystallin promoter/CAT reporter construct consistently resulted in decreased reporter gene activity in the lens-derived N/N1003A cell line (Fig. 3A). The contribution of the predicted δEF1 binding sites to this repression was assessed by making substitution mutations for each of the putative δEF1 elements within the −432/+30 promoter (Fig. 3B). Notably, mutation of any of the three sites activated the expression of the promoter approximately two-fold, indicating the removal of a repressive element from the promoter (Fig. 3A). Cotransfection of these mutant promoter constructs with δEF1 revealed that a βB1-crystallin promoter construct harboring a mutation at −422/−413 was still repressed by δEF1 at an extent similar to the wild-type promoter; however, mutation of either the −292/−283 or −216/−207 δEF1-like elements reduced the δEF1-mediated repression, suggesting that both sites are required for δEF1 to repress the βB1-crystallin promoter (Fig. 3A). The upregulation of δEF1 in LECs following fiber cell removal (Fig. 2), its known roles in repressing epithelial specific genes, such as E-cadherin,16,38 and its ability to repress βB1-crystallin expression, all suggest that δEF1 may mediate, at least in part, the phenotypic change of LECs into myofibroblasts during the development of fibrotic PCO.
Figure 3.
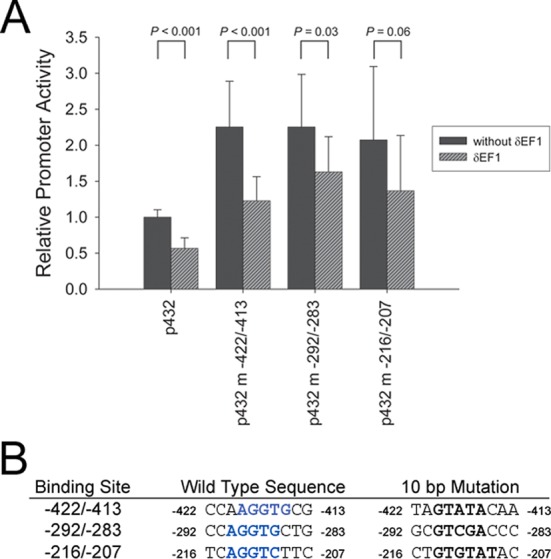
δEF1 appears to repress the βB1-crystallin promoter in cell culture. (A) δEF1 represses the wild-type βB1-crystallin promoter in cotransfection experiments, while mutation of any of the three predicted δEF1 sites leads to increased promoter activity in the N/N1003A lens epithelial cell line (−422/−413 site: P = 0.0002; −292/−283 site: P = 0.0004; −216/−207 site: P = 0.007). (B) Mutations were made in the δEF1 binding sites (blue bold) found in the βB1-crystallin promoter by inserting the AccI restriction enzyme recognition sequence (black bold) in the middle of the sites.
δEF1's Sister Protein Sip1 Also Increases in LECs After Fiber Cell Removal, but Does Not Appear to Mediate EMT During PCO
Unlike δEF1, Sip1, the other member of the Zeb transcription factor family, is expressed in the lens from the lens placode stage onward,19 and this expression is particularly prominent in the peripheral epithelium and cortical fiber cells of the embryonic and adult mouse lens.39 Early in development, Sip1 is required for the separation of the lens vesicle from the head ectoderm,18 and later, it is critical for downregulation of head ectoderm markers and lens fiber cell tip migration necessary to form the lens sutures.19 However, in other systems, Sip1 has been reported to function in the maintenance of fibrotic phenotypes, similar to δEF1, although this has not been previously explored in the lens.16,40,41
Consistent with Sip1's distribution in the intact adult lens,39 its protein levels were relatively low in remnant LECs immediately following fiber cell removal (Fig. 4A). However, Sip1 protein expression upregulated by 48 hours after surgery (Fig. 4B) and continued to be highly expressed through the 5-day time point (Fig. 4C), although the highest expression was seen in islands of cells that do not robustly express α-SMA (Fig. 4C′).
Figure 4.
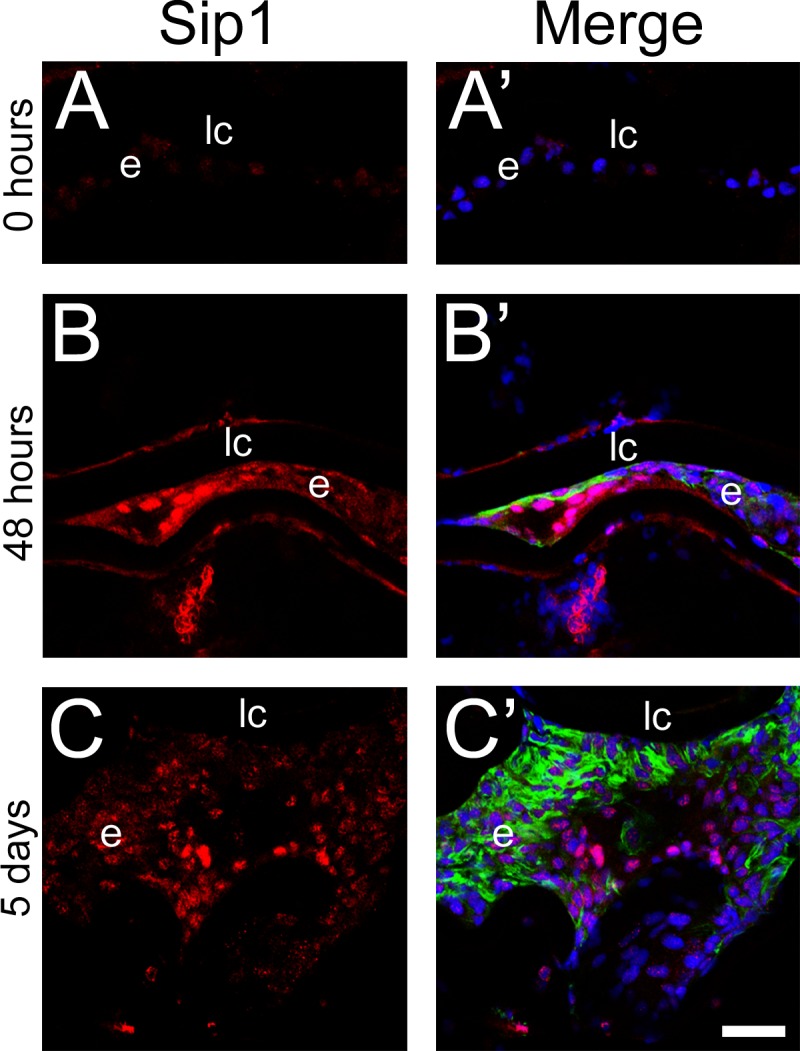
Sip1 expression upregulates in remnant lens cells 2 days after lens fiber cell removal from the mouse eye and remains high after 5 days. Immunohistochemical confocal microscopy showing a time course of Sip1 protein expression at 0 hours (A), 48 hours (B), and 5 days (C). Prime panels (e.g. [A′]) show Sip1 expression (red) merged with nuclei (DRAQ5, blue) and α-SMA expression (green). Scale bar: 35 μm. e, residual lens epithelial cells/transitioning epithelial cells.
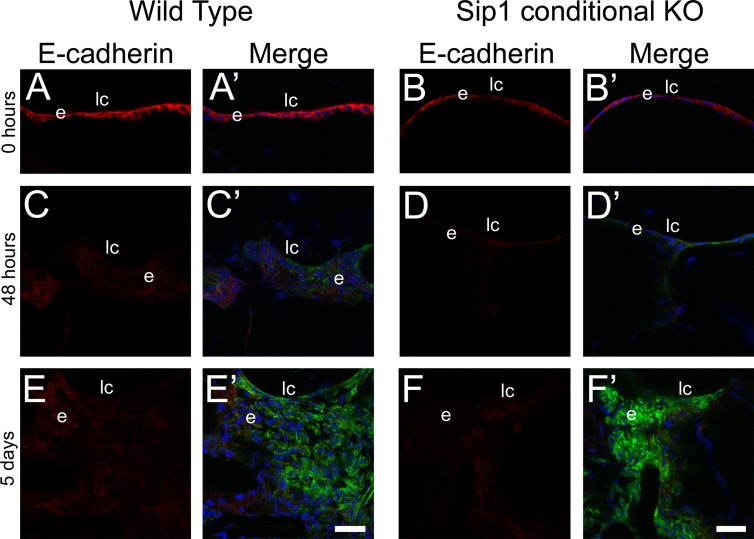
To test the function of Sip1 in PCO, we subjected mice whose lenses lack Sip1 (Sip1 protein is downregulated from the lens vesicle stage onward via MLR10 Cre-mediated deletion of a Sip1 flox allele)19 to lens fiber cell removal and compared the progression of cellular changes observed with that for wild-type mice. Wild-type and Sip1 conditional knockout lenses both expressed E-cadherin in LECs immediately following surgery (Figs. 5A, 5B), although levels appeared slightly lower in the LECs of the Sip1 knockout, consistent with prior observations in intact lenses.19 By 48 hours after surgery, both wild-type and Sip1 knockout LECs lost detectable E-cadherin expression and began to upregulate the expression of α-SMA (Figs. 5C, 5D). The number of cells and extent of α-SMA expression continued to increase in both wild-type and Sip1-deficient lenses, suggesting that Sip1 does not play a major role in EMT during the formation of fibrotic PCO. Thus, we sought to test whether Sip1 could play a role in the development of the “regenerative” or “pearl-type” PCO that arises from LEC differentiation into dysmorphic lens fiber cells42 by assessing the onset of fiber cell marker expression following surgery in Sip1 knockout lenses.
Figure 5.
Epithelial-to-mesenchymal transition still occurs after surgery when Sip1 is not present. Confocal microscopy images of E-cadherin and α-SMA protein expression in wild-type (A, A′, C, C′, E, E′) and Sip1 knockout lenses (B, B′, D, D′, F, F′) at 0 hours (A, B), 48 hours (C, D), and 5 days (E, F) showing downregulation of E-cadherin in the 48-hour and 5-day samples in both the wild-type and Sip1 conditional knockouts. Prime panels (e.g. [A′]) show E-cadherin expression (red) merged with nuclei (DRAQ5, blue) and α-SMA expression (green). Scale bars: 35 μm. e, residual lens epithelial cells/transitioning epithelial cells.
Sip1 Does Not Appear to Regulate Fiber Cell Differentiation but Plays a Distinct Role During PCO, Functioning to Prevent Ectodermal Gene Expression During LEC Reprogramming
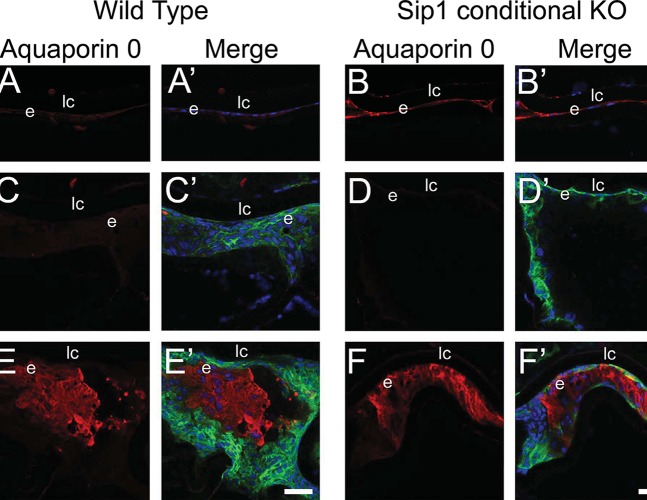
Aquaporin 0, a lens fiber cell marker,43 was not detectable in the LECs of wild-type lenses immediately following surgery (Fig. 6A), but low levels of aquaporin 0 were detected in LECs from Sip1 knockout lenses at this time point (Fig. 6B). By 48 hours after surgery, this expression was markedly decreased (Fig. 6D), being expressed at levels similar to those found in the wild-type (Fig. 6C). However, by 5 days post surgery, islands of aquaporin 0–positive cells, which do not express α-SMA, were detected in both wild-type (Fig. 6E) and Sip1-deficient (Fig. 6F) remnant LECs. Therefore, although Sip1 is expressed in remnant LECs after surgery, and it is a major player in EMT/fibrosis in other contexts,16,40,44,45 loss of this protein does not seem to greatly affect either the progression of EMT/LEC fibrosis or aberrant fiber cell differentiation during PCO.
Figure 6.
Fiber cell differentiation still occurs after surgery when Sip1 is not present. Aquaporin 0 expression is low at 0 hours (A) and 48 hours (C) after cataract surgery in the wild-type and is expressed at slightly higher levels when Sip1 is not present at 0 hours (B), although these levels downregulate by 48 hours (D). At 5 days after surgery, in both the wild-type (E) and Sip1 knockout lenses (F), aquaporin 0 is upregulated, demonstrating that aberrant fiber cell differentiation is still occurring. Prime panels (e.g., [A′]) show aquaporin 0 expression (red) merged with nuclei (DRAQ5, blue) and α-SMA expression (green). Scale bars: 35 μm. e, residual lens epithelial cells/transitioning epithelial cells.
Our prior investigations of Sip1 knockout lenses have found that Sip1 plays a major role during lens development, repressing the expression of genes abundant in the head ectoderm in lens progenitors as they are isolated from this layer during lens vesicle closure. One of these genes codes for keratin (K8), a protein abundant in simple epithelia and the corneal limbus,46 whose expression is prominent in the head ectoderm and the lens vesicle, but is lost from the normal mouse lens by embryonic day 12.5.19 This prior work has shown that expression of K8 is still detected in Sip1 knockout lenses until E16.5, although it is lost thereafter.
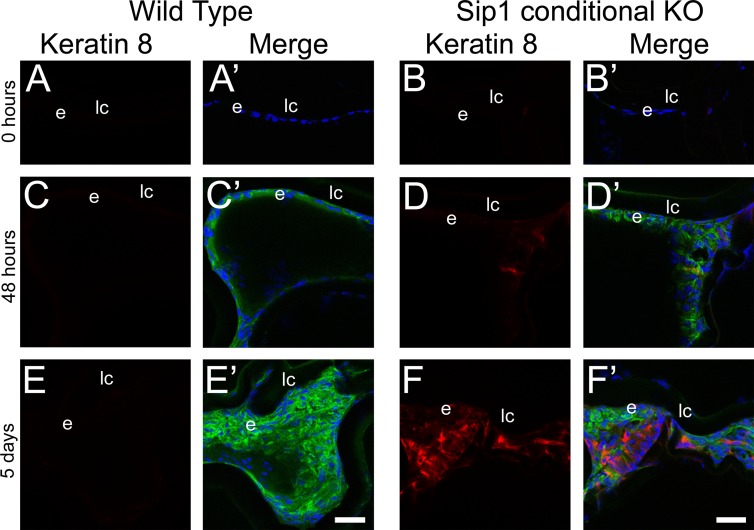
Consistent with these data, K8 was not detected in either wild-type (Fig. 7A) or Sip1 knockout (Fig. 7B) LECs immediately following lens fiber cell removal. Further, while K8 expression was not detected in the wild-type LECs at any of the postsurgical time points investigated (Figs. 7A, 7C, 7E), K8 expression was initiated in Sip1-deficient LECs by 48 hours post surgery (Fig. 7D) and this expression was more robust at 5 days post surgery in cells that also showed immunoreactivity for α-SMA (Fig. 7F′). These data may suggest that the upregulation of Sip1 following lens fiber cell removal may prevent LECs from reprogramming back to a head ectoderm fate as they respond to the injury.
Figure 7.
Keratin 8 is ectopically expressed in remnant Sip1 knockout lens cells. Keratin 8 is not expressed in the wild-type residual epithelial cells after fiber cell removal at 0 hours (A), 48 hours (C), or 5 days (E). In the Sip1 conditional knockout lens, there is little to no keratin 8 protein immediately following surgery at 0 hours (B). However, when Sip1 is not present, keratin 8 begins to upregulate at 48 hours (D) with robust expression being detected by 5 days post surgery (F). Prime panels (e.g., [A′]) show keratin 8 expression (red) merged with nuclei (DRAQ5, blue) and α-SMA expression (green). Scale bars: 35 μm. e, residual lens epithelial cells/transitioning epithelial cells.
Discussion
Posterior capsular opacification is the major complication reported after cataract surgery7,10 and leads to increased light scatter and decreased visual acuity, causing patients to perceive a recurrence of their cataract. During PCO, the residual LECs interpret the surgery as an injury to the tissue, inducing a wound-healing response that promotes the proliferation and migration of residual epithelial cells into the optical axis and their subsequent differentiation into either lens fiber cells or myofibroblasts. Although new and more advanced IOL materials and designs have decreased the rate of PCO in the year following surgery, the long-term incidence of PCO is still significant. A recent survey of postmortem eyes revealed that some degree of PCO occurs in all operated eyes 3 to 6 years after surgery, while some visual impairment due to PCO (regardless of IOL model) will occur in all patients 10 to 15 years after the initial cataract surgery.7 Although treatment of PCO, using a neodymium:yttrium aluminum garnet (Nd:YAG) laser, is a routine procedure, it can, in some cases, lead to ocular complications, including glaucoma, corneal damage, macular edema, and retinal detachment.47 Thus, it is desirable to fully understand the molecular basis of PCO in order to prevent this prevalent cataract surgery side effect.
To date, our knowledge of the mechanisms underlying PCO is limited and has largely focused on the mechanisms responsible for the generation of the myofibroblasts that drive the pathogenesis of fibrotic PCO.11,48–50 Most of this literature has led to the general idea that, following cataract surgery, the high levels of latent TGF-β present in the eye are activated, leading to increased Smad2/3 signaling in the remnant LECs, prompting them to proliferate, migrate, and undergo EMT. The progression of EMT can be followed by monitoring the loss of epithelial markers, such as E-cadherin11 and Pax6, and the upregulation of myofibroblast markers, such as α-SMA. The loss of E-cadherin and elevation of α-SMA expression were also observed in the mouse ECCE model used here by 48 hours after surgery, while the downregulation of Pax6 levels was observed by 5 days post surgery. While not recapitulated in some experimental models of PCO, the other prominent response of LECs following cataract surgery is to enter the lens fiber cell differentiation pathway, presumably in an attempt to regenerate the damaged lens.7 The molecular features of this response, including the upregulation of c-Maf and Prox1, transcription factors important for fiber cell differentiation in the intact lens,30,36,51–53 as well as aquaporin 0, a weak water channel/cell adhesion molecule abundant in lens fiber cells,43 were first consistently observed at 5 days post surgery in our ECCE model. This upregulation correlates well with a prior report suggesting that the genes expressed in the capsular bags in this mouse model often resemble the preoperative lens by 3 weeks after surgery.23
βB1-Crystallin protein is expressed at high levels in the lens fiber cells of all vertebrates54,55 and is often used as a marker of lens fiber cell differentiation.56,57 In early development, the fiber cell–preferred expression of βB1-crystallin appears to result from strict transcriptional regulation of its promoter.58 However, βB1-crystallin mRNA is reasonably abundant in the mammalian adult lens epithelium,32 although its protein is not,59 perhaps owing to translational control.25 During PCO, βB1-crystallin has been identified as a marker of fiber differentiation and is not expressed in cells undergoing TGF-β–induced EMT.48 Our results confirmed that βB1-crystallin mRNA is expressed in the adult lens epithelium and showed that its expression levels drop sharply within 5 days after fiber cell removal, consistent with the reductions in βB1-crystallin expression previously detected by microarray analysis 1 week after surgery.33
Notably, the expression patterns of known βB1-crystallin regulators—Pax6 (a repressor),34 c-Maf and Prox1 (both activators)30,36—imply that additional factors may be involved in the complex expression of this gene during PCO. δEF1, a two-handed zinc-finger transcription factor of the Zeb family, is known to play a role in normal tissue development as well as the EMT that occurs during wound healing and cancer progression.16,17,60 Heterozygous mutations in the δEF1 gene result in posterior polymorphous corneal dystrophy, which is characterized by the transition of the corneal endothelium to an epithelial phenotype.61 δEF1 is also a known repressor of δ-crystallin, a taxon-specific crystallin found in avian lenses.17 While δEF1 is expressed throughout the early embryonic chicken lens, becoming somewhat more restricted to the lens epithelium of the posthatching lens (data not shown), δEF1 expression is very low in the embryonic mouse lens (data not shown37) and is only weakly detected in scattered LECs of adult mice. However, δEF1 protein levels elevate sharply in the LECs remaining in the capsular bag after lens fiber cell removal, with timing similar to the upregulation of the expression of the EMT marker α-SMA.
In other cell types, δEF1 forms a complex with Smad3 and serum response factor during TGF-β–stimulated EMT and can directly transactivate the α-SMA promoter.62 Further, δEF1 is a direct transcriptional repressor of E-cadherin expression, and thus δEF1 can act as a critical molecular switch regulating EMT in diverse epithelial cells.38 Since the timing of δEF1 protein upregulation in LECs undergoing EMT correlates with the upregulation of α-SMA, it is possible that δEF1 is a critical player in TGF-β–induced EMT in the lens. This contention is further supported by the observation that δEF1 can transcriptionally repress βB1-crystallin expression in cotransfection experiments and this repression is dependent on the consensus δEF1 binding sites found in the βB1-crystallin promoter.
Thus, the upregulation of δEF1 protein expression observed in remnant LECs after fiber cell removal implies a dual role for this gene in PCO development, whereby it activates the expression of α-SMA associated with “fibrotic PCO” and represses the expression of the lens fiber cell marker, βB1-crystallin, which is associated with “pearl-type PCO.” Additional work is required to definitively prove this functional connection, ideally using a lens-specific knockout or postcataract surgery knockdown of δEF1, but we speculate that δEF1 likely acts as a molecular switch between EMT and fiber cell differentiation during PCO progression.
Notably, δEF1's sister protein, Sip1, is expressed in the peripheral epithelium and cortical fiber cells of the adult mouse, but it is largely absent from the central epithelial cells.39 In the mouse cataract surgery model used here, the expression of Sip1, like δEF1, upregulated between 12 and 48 hours after surgery, consistent with the hypothesis that Sip1 could also be involved in the progression of PCO, although this upregulation was not uniformly seen in all cells. Notably, the role of the ZEB proteins in cancer EMT-like processes has been well established16,44 and, in this context, decreasing Sip1 and δEF1 levels is sufficient to restore epithelial cell characteristics, with full reversal of EMT accomplished by inhibiting Rho kinase activity in combination with ZEB protein knockdown.40 However, in our ECCE mouse model, the loss of Sip1 from the lens did not affect the expression of either EMT or fiber cell markers in the residual LECs. This is not altogether surprising, as deletion of Sip1 from the lens after vesicle closure did not result in defective expression of typical lens differentiation markers (including crystallins and aquaporin 0) or genes involved in EMT.19 Thus, these results imply that Sip1 does not play a major role in either the downregulation of βB1-crystallin mRNA levels in LECs, EMT of LECs, or aberrant fiber cell differentiation during PCO after cataract surgery, although compensation by δEF1 cannot be ruled out.
However, our data do point at an important, heretofore unrecognized, role for Sip1 in limiting LEC dedifferentiation following lens injury/cataract surgery. Dedifferentiation associated with lens regeneration has been observed in adult newts, which can completely regenerate lenses via a process in which the dorsal iris pigmented epithelium is dedifferentiated and then redifferentiated into a lens through the increased expression of lens development regulators.63,64 In contrast, mammals do not have this ability, apparently owing to the inability to completely reprogram cells to an embryonic state. Even so, in some cases, mammals can reform morphologically recognizable lenses after lens fiber cell removal if an intact lens capsule with attached LECs is left behind,23,65 although the process is inefficient and optically clear lenses seldom result. During this process, mammalian residual LECs also appear to go through a limited dedifferentiation, whereby the adult epithelial cells will express lower levels of lens structural genes.33 However, it has been recently reported that newt iris pigmented epithelial cells are intrinsically more efficient in forming “normal”-appearing lenses than mouse LECs when cultured in Matrigel, suggesting that adult mouse LECs never manage to dedifferentiate sufficiently for this process to be efficient.66
In mammals, K8, an intermediate filament protein normally only found in the stem cells of the corneal limbus in the adult eye,46 is abundant in all cells of the head ectoderm, but downregulates sharply in lens precursors as the lens vesicle develops into the early lens.19 In contrast, this downregulation of K8 expression does not occur promptly in lenses lacking Sip1, and K8 protein is still detectable in lens cells until the latter stages of embryonic lens development when it is finally lost. These data suggest that, during normal lens development, Sip1 functions to turn off the expression of genes that are normally expressed in the head ectoderm so that lens development can proceed normally.19
Our observation that Sip1 conditional knockout lenses ectopically upregulated K8 expression at the same time post surgery that wild-type lenses normally upregulate Sip1 suggests that Sip1 is a transcriptional repressor of K8 expression following cataract surgery. Thus, we hypothesize that the expression of Sip1 during PCO may inhibit the dedifferentiation of LECs to an embryonic state after surgery in a manner similar to that used when turning off the expression of ectodermal markers during normal lens development.19
To date, most investigations of PCO have focused on the molecular mechanisms resulting in fibrotic PCO/EMT and pearl-type PCO/fiber cell differentiation following injury. Overall, the present study suggests that the Zeb family member δEF1 likely plays a role in the repression of pearl-type PCO and promotion of fibrotic PCO, although this idea needs to be directly tested experimentally. In contrast, Sip1, the other known Zeb family member, does not appear to affect the fiber cell differentiation or EMT pathways in the lens after surgery, but seems to play a critical role in repressing genes whose expression is typically found in the head ectoderm. This suggests that Sip1 is involved in blocking cellular dedifferentiation following lens injury and may limit the ability of mammalian LECs to efficiently regenerate the lens following cataract surgery.
Acknowledgments
We thank Hisato Kondoh, PhD, for his kind gift of the δEF1 expression plasmid and Dylan Audette for his help with the collection of chicken lenses.
Supported by the National Eye Institute Grant EY12221 (MKD), and grants from the Delaware Institutional Networks for Biomedical Research Excellence (GM103446), Fight for Sight (JRT), Howard Hughes Medical Institute (ARY), and Sigma Xi (JRT). ALM was a Chemistry-Biology Interface Fellow at the University of Delaware.
Disclosure: A.L. Manthey, None; A.M. Terrell, None; Y. Wang, None; J.R. Taube, None; A.R. Yallowitz, None; M.K. Duncan, None
References
- 1. Piatigorsky J. Lens differentiation in vertebrates: a review of cellular and molecular features. Differentiation. 1981; 19: 134–153 [DOI] [PubMed] [Google Scholar]
- 2. Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009; 88: 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassnett S, Beebe DC. Lens fiber differentiation. In: Lovicu FJ, Robinson ML. eds Development of the Ocular Lens. New York: Cambridge University Press; 2004; 214–244 [Google Scholar]
- 4. Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005; 365: 599–609 [DOI] [PubMed] [Google Scholar]
- 5. Riaz Y, Mehta JS, Wormald R, et al. Surgical interventions for age-related cataract. Cochrane Database Syst Rev. 2006; 18: CD001323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009; 88: 257–269 [DOI] [PubMed] [Google Scholar]
- 7. Apple DJ, Escobar-Gomez M, Zaugg B, Kleinmann G, Borkenstein AF. Modern cataract surgery: unfinished business and unanswered questions. Surv Ophthalmol. 2011; 56: S3–S53 [DOI] [PubMed] [Google Scholar]
- 8. van Bree MC, Zijlmans BL, van den Berg TJ. Effect of neodymium:YAG laser capsulotomy on retinal straylight values in patients with posterior capsule opacification. J Cataract Refract Surg. 2008; 34: 1681–1686 [DOI] [PubMed] [Google Scholar]
- 9. van Bree MC, van den Berg TJ, Zijlmans BL. Posterior capsule opacification severity, assessed with straylight measurement, as main indicator of early visual function deterioration. Ophthalmology. 2013; 120: 20–33 [DOI] [PubMed] [Google Scholar]
- 10. Spalton D. Preventing PCO. EyeWorld Online. http://eyeworld.org/article-preventing-pco. Published February 2011. Accessed August 22, 2014 [Google Scholar]
- 11. de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005; 179: 43–55 [DOI] [PubMed] [Google Scholar]
- 12. Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci. 2004; 45: 1946–1953 [DOI] [PubMed] [Google Scholar]
- 13. Marcantonio JM, Vrensen GF. Cell biology of posterior capsular opacification. Eye (Lond). 1999; 13 (pt 3b): 484–488 [DOI] [PubMed] [Google Scholar]
- 14. Matsushima H, Mukai K, Obara Y, Yoshida S, Clark JI. Analysis of cytoskeletal proteins in posterior capsule opacification after implantation of acrylic and hydrogel intraocular lenses. J Cataract Refract Surg. 2004; 30: 187–194 [DOI] [PubMed] [Google Scholar]
- 15. Kappelhof JP, Vrensen GF, de Jong PT, Pameyer J, Willekens BL. The ring of Soemmerring in man: an ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1987; 225: 77–83 [DOI] [PubMed] [Google Scholar]
- 16. Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009; 66: 773–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamachi Y, Kondoh H. Overlapping positive and negative regulatory elements determine lens-specific activity of the d1-crystallin enhancer. Mol Cell Biol. 1993; 13: 5206–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshimoto A, Saigou Y, Higashi Y, Kondoh H. Regulation of ocular lens development by Smad-interacting protein 1 involving Foxe3 activation. Development. 2005; 132: 4437–4448 [DOI] [PubMed] [Google Scholar]
- 19. Manthey AL, Lachke SA, FitzGerald PG, et al. Loss of Sip1 leads to migration defects and retention of ectodermal markers during lens development. Mech Dev. 2014; 131: 86–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higashi Y, Maruhashi M, Nelles L, et al. Generation of the floxed allele of the SIP1 (Smad-interacting protein 1) gene for Cre-mediated conditional knockout in the mouse. Genesis. 2002; 32: 82–84 [DOI] [PubMed] [Google Scholar]
- 21. Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci. 2004; 45: 1930–1939 [DOI] [PubMed] [Google Scholar]
- 22. Desai VD, Wang Y, Simirskii VN, Duncan MK. CD44 expression is developmentally regulated in the mouse lens and increases in the lens epithelium after injury. Differentiation. 2010; 79: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA. Lens regeneration in mice: implications in cataracts. Exp Eye Res. 2004; 78: 297–299 [DOI] [PubMed] [Google Scholar]
- 24. Reed NA, Oh DJ, Czymmek KJ, Duncan MK. An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods. 2001; 253: 243–252 [DOI] [PubMed] [Google Scholar]
- 25. Taube JR, Gao CY, Ueda Y, Zelenka PS, David LL, Duncan MK. General utility of the chicken bB1-crystallin promoter to drive protein expression in lens fiber cells of transgenic mice. Transgenic Res. 2002; 11: 397–410 [DOI] [PubMed] [Google Scholar]
- 26. Quandt K, Frech K, Karas H, Wingender E, MatInd Werner T. and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995; 23: 4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heinemeyer T, Wingender E, Reuter I, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998; 26: 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002; 18: 333–334 [DOI] [PubMed] [Google Scholar]
- 29. Duncan MK, Roth HJ, Thompson M, Kantorow M, Piatigorsky J. Chicken bB1-crystallin: gene sequence and evidence for functional conservation of promoter activity between chicken and mouse. Biochim Biophys Acta. 1995; 1261: 68–76 [DOI] [PubMed] [Google Scholar]
- 30. Chen X, Taube JR, Simirskii VI, Patel TP, Duncan MK. Dual roles for Prox1 in the regulation of the chicken betaB1-crystallin promoter. Invest Ophthalmol Vis Sci. 2008; 49: 1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddan JR, Chepelinsky AB, Dziedzic DC, Piatigorsky J, Goldenberg EM. Retention of lens specificity in long-term cultures of diploid rabbit lens epithelial cells. Differentiation. 1986; 33: 168–174 [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of a-, b-, and g-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45: 3608–3619 [DOI] [PubMed] [Google Scholar]
- 33. Medvedovic M, Tomlinson CR, Call MK, Grogg M, Tsonis PA. Gene expression and discovery during lens regeneration in mouse: regulation of epithelial to mesenchymal transition and lens differentiation. Mol Vis. 2006; 12: 422–440 [PubMed] [Google Scholar]
- 34. Duncan MK, Haynes JI II, Cvekl A, Piatigorsky J. Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific b-crystallin genes. Mol Cell Biol. 1998; 18: 5579–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang W, Cveklova K, Oppermann B, Kantorow M, Cvekl A. Quantitation of PAX6 and PAX6(5a) transcript levels in adult human lens, cornea, and monkey retina. Mol Vis. 2001; 7: 1–5 [PMC free article] [PubMed] [Google Scholar]
- 36. Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken bB1-crystallin gene expression. J Biol Chem. 2004; 279: 11088–11095 [DOI] [PubMed] [Google Scholar]
- 37. Takagi T, Moribe H, Kondoh H, Higashi Y. dEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development. 1998; 125: 21–31 [DOI] [PubMed] [Google Scholar]
- 38. Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005; 24: 2375–2385 [DOI] [PubMed] [Google Scholar]
- 39. Grabitz AL, Duncan MK. Focus on molecules: Smad Interacting Protein 1 (Sip1, ZEB2, ZFHX1B). Exp Eye Res. 2012; 101: 105–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das S, Becker BN, Hoffmann FM, Mertz JE. Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol. 2009; 10: 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandewalle C, Comijn J, De Craene B, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005; 33: 6566–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Iongh RU, Duncan MK. Growth factor signaling in lens fiber differentiation. In: Saika S, Werner L, Lovicu FJ. eds Lens Epithelium and Posterior Capsular Opacification. Tokyo, Japan: Springer Japan KK; 2014: 81–104 [Google Scholar]
- 43. FitzGerald PG, Bok D, Horwitz J. The distribution of the main intrinsic membrane polypeptide in ocular lens. Curr Eye Res. 1985; 4: 1203–1218 [DOI] [PubMed] [Google Scholar]
- 44. Xia M, Hu M, Wang J, et al. Identification of the role of Smad interacting protein 1 (SIP1) in glioma. J Neurooncol. 2009; 97: 225–232 [DOI] [PubMed] [Google Scholar]
- 45. Bindels S, Mestdagt M, Vandewalle C, et al. Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene. 2006; 25: 4975–4985 [DOI] [PubMed] [Google Scholar]
- 46. Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells. 2012; 30: 2032–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Billotte C, Berdeaux G. Adverse clinical consequences of neodymium:YAG laser treatment of posterior capsule opacification. J Cataract Refract Surg. 2004; 30: 2064–2071 [DOI] [PubMed] [Google Scholar]
- 48. Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFbeta-induced anterior subcapsular cataract. Dev Neurosci. 2004; 26: 446–455 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Li W, Zang X, et al. MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest Ophthalmol Vis Sci. 2013; 54: 323–332 [DOI] [PubMed] [Google Scholar]
- 50. Chong CC, Stump RJ, Lovicu FJ, McAvoy JW. TGFbeta promotes Wnt expression during cataract development. Exp Eye Res. 2009; 88: 307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duncan MK, Cui W, Oh DJ, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002; 112: 195–198 [DOI] [PubMed] [Google Scholar]
- 52. Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000; 127: 307–317 [DOI] [PubMed] [Google Scholar]
- 53. Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999; 21: 318–322 [DOI] [PubMed] [Google Scholar]
- 54. Ueda Y, Duncan MK, David LL. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci. 2002; 43: 205–215 [PubMed] [Google Scholar]
- 55. Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest Ophthalmol Vis Sci. 2002; 43: 216–224 [PubMed] [Google Scholar]
- 56. Duncan MK, Xie L, David LL, et al. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2004; 45: 3589–3598 [DOI] [PubMed] [Google Scholar]
- 57. Sawada K, Agata K, Yoshiki A, Eguchi G. A set of anti-crystallin monoclonal antibodies for detecting lens specificities: beta-crystallin as a specific marker for detecting lentoidogenesis in cultures of chicken lens epithelial cells. Jpn J Ophthalmol. 1993; 37: 355–368 [PubMed] [Google Scholar]
- 58. Duncan MK, Li X, Ogino H, Yasuda K, Piatigorsky J. Developmental regulation of the chicken bB1-crystallin promoter in transgenic mice. Mech Dev. 1996; 57: 79–89 [DOI] [PubMed] [Google Scholar]
- 59. Wang-Su ST, McCormack AL, Yang S, et al. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest Ophthalmol Vis Sci. 2003; 44: 4829–4836 [DOI] [PubMed] [Google Scholar]
- 60. Funahashi J, Sekido R, Murai K, Kamachi Y. Kondoh H. d-crystallin enhancer binding protein dEF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993; 119: 433–446 [DOI] [PubMed] [Google Scholar]
- 61. Krafchak CM, Pawar H, Moroi SE, et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005; 77: 694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nishimura G, Manabe I, Tsushima K, et al. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev Cell. 2006; 11: 93–104 [DOI] [PubMed] [Google Scholar]
- 63. Eguchi G, Eguchi Y, Nakamura K, Yadav MC, Millan JL, Tsonis PA. Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nat Commun. 2011; 2: 384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Henry JJ, Tsonis PA. Molecular and cellular aspects of amphibian lens regeneration. Prog Retin Eye Res. 2010; 29: 543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gwon A. Lens regeneration in mammals: a review. Surv Ophthalmol. 2006; 51: 51–62 [DOI] [PubMed] [Google Scholar]
- 66. Hoffmann A, Nakamura K, Tsonis PA. Intrinsic lens forming potential of mouse lens epithelial versus newt iris pigment epithelial cells in three-dimensional culture. Tissue Eng Part C Methods. 2014; 20: 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]