Abstract
Bacillus sp. strain ZYK, a member of the phylum Firmicutes, is of interest for its ability to reduce nitrate and selenite and for its resistance to arsenic under anaerobic conditions. Here we describe some key features of this organism, together with the complete genome sequence and annotation. The 3,575,797 bp long chromosome with its 3,454 protein-coding and 70 RNA genes, and the information gained from its sequence will be relevant to the elucidation of microbially-mediated transformations of nitrogen, selenium and arsenic in paddy soil.
Keywords: anaerobic, spore-forming, Gram-positive, nitrate-reduction, selenite-reduction, arsenic resistance, paddy soil, Bacillaceae
Introduction
Bacillus sp. ZYK (=DSM 26460 =CGMCC 1.5179) was isolated from a paddy soil in Dehong, Yunnan, China and is an anaerobic nitrate-reducing, Gram-positive bacterium [1]. Strain ZYK belongs to the genus Bacillus, and based on 16S rRNA phylogeny, is most closely related to Bacillus azotoformans isolated from garden soil, which is capable of reducing nitrate, nitrite, nitrous oxide, and nitric oxide under anaerobic conditions [2-4]. Strain ZYK is capable of nitrate-reduction under anaerobic conditions and, in addition, demonstrated selenite-reducing ability and arsenic resistance (unpublished data). Bacillus spp. are commonly found in paddy soil and may play important roles in elemental cycling during periodically changing redox conditions [5-8]. Therefore, strain ZYK is a suitable model for studying the properties of genes involved in denitrification, selenite-reduction and arsenic resistance pathways of paddy soil bacteria. Here we summarize the features of Bacillus sp. strain ZYK and provide a description of its sequenced genome, now available for detailed analysis.
Classification and features
Based on 16S rRNA gene phylogeny and genome information, strain ZYK was a member of the genus Bacillus, most closely related to Bacillus azotoformans (AB363732), with a sequence similarity of 96.3% based on a Blast analysis [9] of the most recent release of the Greengenes database [10]. A phylogenetic tree (Figure 1) was constructed using the Maximum likelihood method under the default settings for complete sequences of genes encoding 16S rRNA derived from sequenced genomes of Bacillus spp., along with the sequences of representative members of the genus.
Figure 1.
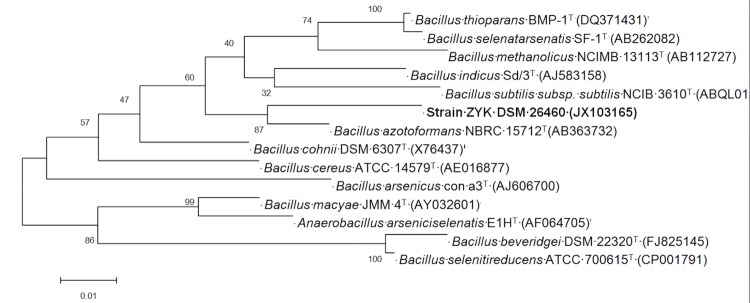
Phylogenetic tree highlighting the position of Bacillus sp. ZYK relative to selected Bacillus species. The strains and their corresponding GenBank accession numbers of 16S rRNA genes are as indicated. The tree, based on 1,545 positions, was built with MEGA 5 [11] using the Maximum likelihood method. Bar: 0.01substitutions per nucleotide position.
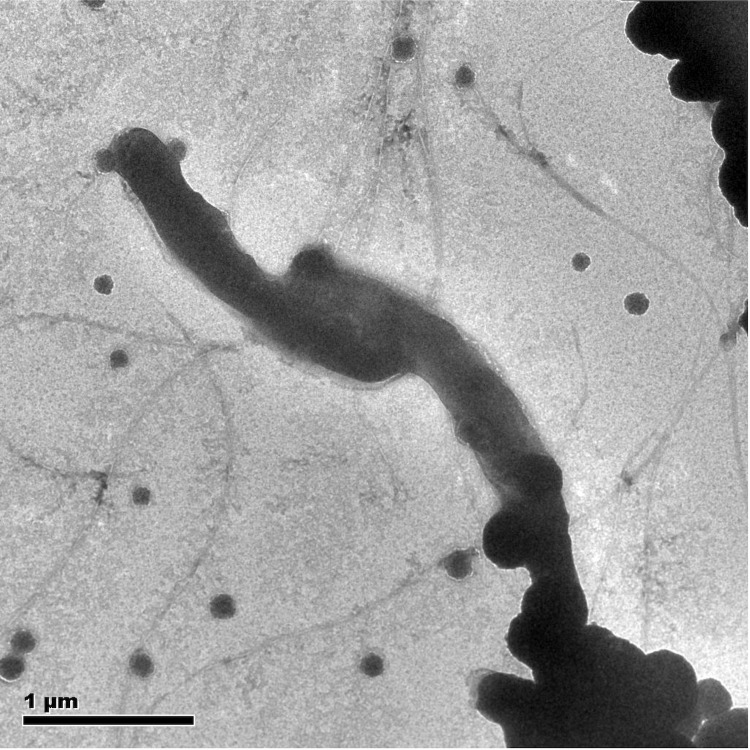
Strain ZYK is an anaerobic, Gram-positive, spore-forming, motile, rod-shaped (0.2-0.3 µm wide and 1.5-2.0 µm long) (Figure 2). The strain grew optimally at pH 7.0-7.2 (range 6.0-7.6), 30-40°C (range 21-45°C) and at low salinity (NaCl range 0-1.1%) (Table 1) in freshwater anaerobic medium [24]. On anaerobic LB agar, strain ZYK forms small, white colonies with entire edges (data not shown). Carbon substrates utilized for growth by strain ZYK included D-glucose, maltose, lactose, and sucrose. Strain ZYK reduces nitrate and selenite under anaerobic conditions in freshwater medium.
Figure 2.
Transmission electron microscopy of strain ZYK. Scale bar corresponds to 1.0 μm.
Table 1. Classification and general features of strain ZYK according to the MIGS recommendations [1].
| MIGS ID | Property | Term | Evidence codes |
|---|---|---|---|
| Classification | Domain Bacteria | TAS [12] | |
| Phylum Firmicutes | TAS [13-15] | ||
| Class Bacilli | TAS [16,17] | ||
| Order Bacillales | TAS [18,19] | ||
| Family Bacillaceae | TAS [18,20] | ||
| Genus Bacillus | TAS [18,21,22] | ||
| Strain ZYK | IDA | ||
| Gram stain | Positive | IDA | |
| MIGS-37.1 | Cell shape | Rod-shaped | NAS |
| MIGS-37.2 | Motility | Motile | NAS |
| MIGS-37.3 | Sporulation | Sporulating | NAS |
| MIGS-37.9 | Cell arrangement | Single | NAS |
| MIGS-37.12 | Optimum pH | 7.0 | NAS |
| MIGS-6 | Optimum temperature | 30°C | NAS |
| Salinity | 0-1.1% | IDA | |
| MIGS-22 | Oxygen requirement | Strict | NAS |
| Carbon source | D-Glucose, Maltose, lactose, sucrose | IDA | |
| MIGS-6 | Habitat | Paddy soil | NAS |
| MIGS-15 | Biotic relationship | Free-living | NAS |
| Pathogenicity | None-pathogen | NAS | |
| Biosafety level | 1 | NAS | |
| MIGS-4 | Geographic location | Dehong, Yunnan, China | NAS |
| MIGS-4.1 | Latitude | 24o64´70"N | NAS |
| MIGS-4.2 | Longitude | 98o53´45"E | NAS |
| MIGS-4.5 | Isolation | Paddy soil | NAS |
Evidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [23]. If the evidence code is IDA, the property was directly observed by one of the authors.
Genome project history
Bacillus sp. ZYK was selected for sequencing because of its phylogenetic affiliation with a lineage of paddy soil bacteria that may influence elemental cycling in paddy fields. The genome project is deposited in the Genomes OnLine Database (GOLD) as project Gi22906, and the complete genome sequence is in GenBank under accession number ANOK00000000 (Table 2). A summary of the main project information is shown in Table 2.
Table 2. Genome sequencing project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Complete |
| MIGS-28 | Libraries used | Two libraries 500 bp PCR-free library, 2000 bp index library |
| MIGS-29 | Sequencing platforms | Illumina |
| MIGS-31.2 | Fold coverage | 140× |
| MIGS-30 | Assemblers | SOAPdenovo 1.05 |
| MIGS-32 | Gene calling method | Glimmer 3.0 |
| Locus TAG | D612 | |
| Genbank ID | ANOK00000000 | |
| Genbank Date of Release | January 15, 2013 | |
| GOLD ID | Gi22906 | |
| NCBI taxon ID | 1191699 | |
| MIGS-13 | Source material identifier | DSMZ 26460, CGMCC 1.5179 |
| MIGS-38.2 | Project relevance | Agricultural, Bioremediation, Environmental |
Growth conditions and DNA isolation
For the preparation of genomic DNA, one colony was picked from an anaerobic LB agar plate, and grown in anaerobic freshwater medium at 30°C [24]. A culture (1.0 ml) at 0.6 OD600nm was inoculated into 100 ml of anaerobic freshwater media. Cells were collected by centrifugation after growing to 0.6 OD600nm. Cells were suspended in TE buffer (10 mM NaCl, 20 mM Tris-HCl, 1.0 mM EDTA, pH 8.0), and treated with lysozyme to lyse the cell wall. SDS and proteinaseK were added to denature and degrade proteins. Cell lysates were extracted with phenol-chloroform and the nucleic acids were precipitated by addition of isoamylol. The nucleic acid pellet was washed with 100% ethanol, dissolved in double distilled water and then treated with RNase A [25].
Genome sequencing and assembly
The genome of ZYK was sequenced at the Beijing Genomics Institute (BGI) using Illumina paired-end sequencing. Draft assemblies were based on 4,233,334 reads totaling 380 Mb of 500 bp PCR-free library and 2,184,080 reads totaling 196 Mb of 2,000 bp index library. The SOAPdenovo software package independently developed by BGI (version 1.05 [26],) was used for sequence assembly and quality assessment. To achieve optimal assembly results, the key parameter K was set at 43 after several adjustments. Gaps between contigs were closed by KRSKGF software, version 1.2 (independently developed by BGI) and Gapcloser, version 1.10. The complete nucleotide sequence of Bacillus sp. strain ZYK and its annotation can be found online at the IMG (Integrated Microbial Genome) portal of JGI [27], as well at the genome resource site of NCBI [28].
Genome annotation
Genes were identified using Glimmer, version 3.0 [29]. The predicted CDSs were translated and used to search KEGG, COG, SwissPort, TrEMBL, NR and GO databases. These data sources were combined to assert a product description for each predicted protein. Transposons were identified using RepeatMaster (with Repbase) and RepeatProteinMasker (with its own database) software. Tandem repeat sequences were predicted by TRF (Tandem Repeat Finder) software. The rRNA, tRNA and sRNA were predicted by using rRNAmmer [30], tRNAscan [31] and Rfam [32] software, respectively.
Genome properties
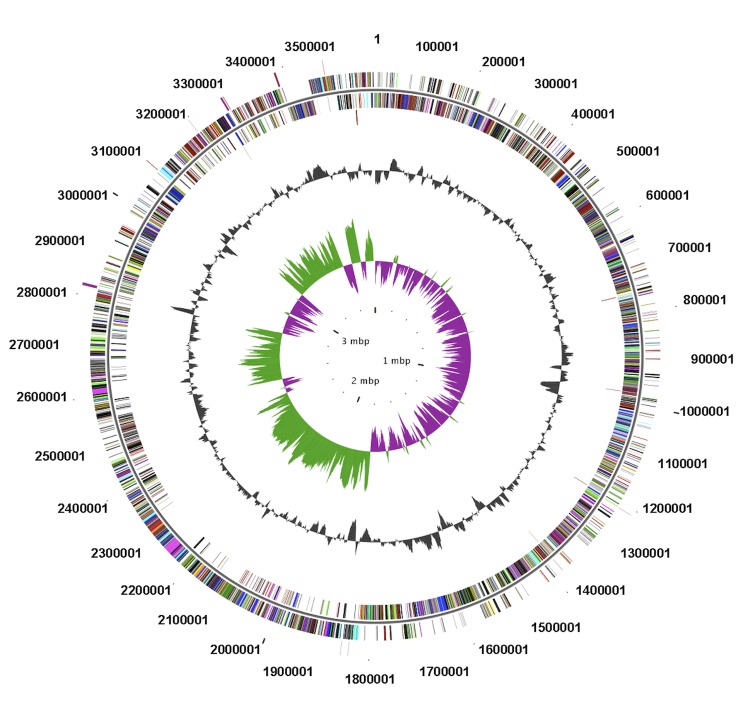
The genome consists of a circular chromosome of 3,575,797 bp in size with a GC content of 36.1% (Figure 3, Table 3). Of the 3,454 predicted genes, 70 are RNA genes, 136 are secreted protein coding genes, and 3,318 are non-secreted protein coding genes. Of the total predicted genes, 2,030 represent COG functional categories. The distribution of genes into COG functional categories is presented in Figure 3 and Table 4.
Figure 3.
Graphical representation of circular map of the chromosome of strain ZYK. From outside to the center: Genes on forward strand (colored by COG categories), Genes on reverse strand (colored by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content, GC skew.
Table 3. Genome statistics.
| Attribute | Value | % of total |
|---|---|---|
| Genome size (bp) | 3,575,797 | 100.00 |
| DNA coding region (bp) | 3,002,982 | 83.98 |
| DNA G+C content (bp) | 1,290,862 | 36.10 |
| Total genes | 3454 | 100.00 |
| RNA genes | 70 | 2.03 |
| Protein-coding genes (bp) | 3,002,982 | 83.98 |
| Genes with function prediction | 3261 | 94.41 |
| Genes assigned to COGs | 2,030 | 58.77 |
| Genes assigned to Pfam domains (bp) | 617,696 | 17.27 |
| Genes with signal peptides | 169 | 4.89 |
| Genes with transmembrane helices | 132 | 3.82 |
| CRISPR repeats | 84 | 0.09 |
Table 4. Number of genes associated with the 25 general COG functional categories.
| Code | Value | %agea | Description |
|---|---|---|---|
| J | 149.0 | 6.5 | Translation |
| A | 0.0 | 0.0 | RNA processing and modification |
| K | 164.0 | 7.1 | Transcription |
| L | 119.0 | 5.2 | Replication, recombination and repair |
| B | 1.0 | 0.04 | Chromatin structure and dynamics |
| D | 27.0 | 1.2 | Cell cycle control, mitosis and meiosis |
| Y | 0.0 | 0.0 | Nuclear structure |
| V | 24.0 | 1.0 | Defense mechanisms |
| T | 162.0 | 7.0 | Signal transduction mechanisms |
| M | 95.0 | 4.1 | Cell wall/membrane biogenesis |
| N | 75.0 | 3.3 | Cell motility |
| Z | 0.0 | 0.0 | Cytoskeleton |
| W | 0.0 | 0.0 | Extracellular structures |
| U | 44.0 | 1.9 | Intracellular trafficking and secretion |
| O | 97.0 | 4.2 | Posttranslational modification, protein turnover, chaperones |
| C | 155 | 6.7 | Energy production and conversion |
| G | 79.0 | 3.4 | Carbohydrate transport and metabolism |
| E | 239.0 | 10.4 | Amino acid transport and metabolism |
| F | 61.0 | 2.7 | Nucleotide transport and metabolism |
| H | 93.0 | 4.0 | Coenzyme transport and metabolism |
| I | 97.0 | 4.2 | Lipid transport and metabolism |
| P | 127.0 | 5.5 | Inorganic ion transport and metabolism |
| Q | 38.0 | 1.7 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 261.0 | 11.3 | General function prediction only |
| S | 193.0 | 8.4 | Function unknown |
| - | 1424 | 41.2 | Not in COGs |
a) The total is based on the total number of protein coding genes in the annotated genome.
Insights into the genome sequence
Bacillus sp. ZYK can reduce nitrate and selenite under anaerobic conditions (unpublished data). The inspection of the genome of strain ZYK confirmed the presence of nitrate reductase coding genes, in support of the physiological data. Genes for a respiratory nitrate reductase corresponding to a heterotrimeric structure with four subunits, including narG, narH, narI and narJ present in the genome of strain ZYK. Genes encoding a second type of nitrate reductase, Nap (periplasmic nitrate reductase) including napA, napB, and napD were also found in the ZYK genome. We also identified in the genome a formate-dependent nitrite reductase coding gene (nrfA) and a copper-containing nitrite reductase coding gene (nirK).
An arsenate reductase coding gene (arsC) was identified with 77% similarity to the Bacillus megaterium arsC gene (AJ515540). An arsenite efflux pump gene was also identified as arsB with 78% similarity to Bacillus sp. CDB3 arsB gene (AF178758.2). Two DMSO reductase genes have 59.2% and 60.3% similarity with Desulfosporosinus orientis DMSO reductase (Fe-S cluster containing hydrogenase coding gene) and Bacillus sp. 1NLA3E DMSO reductase (dimethylsulfoxide reductase, chain B), respectively. The discovery of an arsenate reductase coding gene (arsC) and DMSO reductase sequences suggests that the reduction capabilities of strain ZYK are broader than expected, and that other substrates be tested. Particularly, we are interested in determining whether selenite reduction activity in ZYK is mediated by a hydrogenase [33], a nitrite reductase [34] or a DMSO reductase. While the reduction of selenite to elemental selenium is a common feature of diverse microorganisms, the genes responsible for this process remain largely uncharacterized and virtually nothing is known about their regulation [33-35], or their interactions with other respiratory pathways. In addition to Bacillus sp. ZYK, the genomes of two bacteria capable of selenite reduction, Bacillus selenitireducens (NC_014219.1) [36] and Desulfirispirillum indicum S5 [37,38], have been sequenced. The investigation of the functional genes of strain ZYK will consequently enhance the understanding of the electron acceptor utilization pathways in microorganisms, and how nitrogen, selenium and arsenic cycling is mediated by microorganisms active in paddy soil. Further study of these reductase gene-coding sequences may reveal the importance of the Bacillus genus in elemental cycling in paddy soils.
Acknowledgements
We gratefully acknowledge the technically support of Beijing Genomics Institute (BGI), which worked on sequencing and annotation of this genome. This work was financially supported by the National Natural Science Foundation of China (No. 41090280) and (No. 41090282).
References
- 1.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichinoty F, Durand M, Job C, Mandel M, Garcia JL. Etude morphologique, physiologique et taxonomique de Bacillus azotoformans. Can J Microbiol 1978; 24:608-617 10.1139/m78-099 [DOI] [PubMed] [Google Scholar]
- 3.Pichinoty F, De Barjac H, Mandel M, Asselineau J. Description of Bacillus azotoformans. Int J Syst Bacteriol 1983; 33:660-662 10.1099/00207713-33-3-660 [DOI] [Google Scholar]
- 4.Suharti J, Heering HA, De Vries S. NO Reductase from Bacillus azotoformans Is a Bifunctional Enzyme Accepting Electrons from Menaquinol and a Specific Endogenous Membrane-Bound Cytochrome c551. Biochemistry 2004; 43:13487-13495 10.1021/bi0488101 [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K, Hayano K. Distribution and identification of proteolytic Bacillus spp. in paddy field soil under rice cultivation. Can J Microbiol 1993; 39:674-680 10.1139/m93-097 [DOI] [PubMed] [Google Scholar]
- 6.Wang XJ, Yang J, Chen XP, Sun GX, Zhu YG. Phylogenetic diversity of dissimilatory ferric iron reducers in paddy soil of Hunan, South China. J Soils Sediments 2009; 9:568-577 10.1007/s11368-009-0113-x [DOI] [Google Scholar]
- 7.Li H, Peng JJ, Weber KA, Zhu YG. Phylogenetic diversity of Fe(III)-reducing microorganisms in rice paddy soil: enrichment cultures with different short-chain fatty acids as electron donors. J Soils Sediments 2011; 11:1234-1242 10.1007/s11368-011-0371-2 [DOI] [Google Scholar]
- 8.Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M. Biogeochemistry of paddy soils. Geoderma 2010; 157:1-14 10.1016/j.geoderma.2010.03.009 [DOI] [Google Scholar]
- 9.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403-410 [DOI] [PubMed] [Google Scholar]
- 10.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl Environ Microbiol 2006; 72:5069-5072 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 2011; 28:2731-2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons NE, Murray RGE. Proposals Concerning the Higher Taxa of Bacteria. Int J Syst Bacteriol 1978; 28:1-6 10.1099/00207713-28-1-1 [DOI] [Google Scholar]
- 14.Garrity GM, Holt JG. The Road Map to the Manual. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 119-169. [Google Scholar]
- 15.Murray RGE. The Higher Taxa, or, a Place for Everything...? In: Holt JG (ed), Bergey's Manual of Systematic Bacteriology, First Edition, Volume 1, The Williams and Wilkins Co., Baltimore, 1984, p. 31-34. [Google Scholar]
- 16.List no. 132. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol 2010; 60:469-472 10.1099/ijs.0.022855-0 [DOI] [PubMed] [Google Scholar]
- 17.Ludwig W, Schleifer KH, Whitman WB. Class I. Bacilli class nov. In: De Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 3, Springer-Verlag, New York, 2009, p. 19-20. [Google Scholar]
- 18.Skerman VBD. McGowanV, SneathPHA. Approved Lists of Bacterial Names. Int J Syst Bacteriol 1980; 30:225-420 10.1099/00207713-30-1-225 [DOI] [Google Scholar]
- 19.Prévot AR. In: Hauderoy P, Ehringer G, Guillot G, Magrou. J., Prévot AR, Rosset D, Urbain A (eds), Dictionnaire des Bactéries Pathogènes, Second Edition, Masson et Cie, Paris, 1953, p. 1-692. [Google Scholar]
- 20.Fischer A. Untersuchungen über bakterien. Jahrbücher für Wissenschaftliche Botanik 1895; 27:1-163 [Google Scholar]
- 21.Cohn F. Untersuchungen über Bakterien. Beitr Biol Pflanz 1872; 1:127-224 [Google Scholar]
- 22.Gibson T, Gordon RE. Genus I. Bacillus Cohn 1872, 174; Nom. gen. cons. Nomencl. Comm. Intern. Soc. Microbiol. 1937, 28; Opin. A. Jud. Comm. 1955, 39. In: Buchanan RE, Gibbons NE (eds), Bergey's Manual of Determinative Bacteriology, Eighth Edition, The Williams and Wilkins Co., Baltimore, 1974, p. 529-550. [Google Scholar]
- 23.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology: tool for the unification of biology. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widdel F, Bak F. Gram-negative mesophilic sulfate reducing bacteria. In The Prokaryotes. Balows, A., Trüper, H.G., Dworkin, M., Harder, W., and Schleifer, K.H. 1992, New York: Springer, pp 3352–3378. [Google Scholar]
- 25.Marmur J. A procedure for the isolation of DNA from microorganisms. J Mol Biol 1961; 3:208-218 10.1016/S0022-2836(61)80047-8 [DOI] [Google Scholar]
- 26.The SOAPdenovo software package. http://soap.genomics.org.cn/soapdenovo.html
- 27.Integrated Microbial Genome portal of JGI. http://genomesonline.org/cgi-bin/GOLD/bin/GOLDCards.cgi?goldstamp=Gi22906
- 28.NCBI http://www.ncbi.nlm.nih.gov/genome?db=genome&cmd=Retrieve&dopt=Overview&list+uids=1609
- 29.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res 1999; 27:4636-4641 10.1093/nar/27.23.4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe TM, Eddy SR. tRNAscanSE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955-964 10.1093/nar/25.5.0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007; 35:3100-3108 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res 2005; 33:D121-D124 10.1093/nar/gki081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeMoll-Decker H, Macy JM. The periplasmic nitrite reductase of Thauera selenatis may catalyze the reduction of selenite to elemental selenium. Arch Microbiol 1993; 160:241-247 [Google Scholar]
- 34.Tomei FA, Barton LL, Lemanski CL, Zocco TG. Reduction of selenate and selenite to elemental selenium by Wolinella succinogenes. Can J Microbiol 1992; 38:1328-1333 10.1139/m92-219 [DOI] [Google Scholar]
- 35.Bao P, Huang H. HuZY, HäggblomMM, ZhuYG. Impact of temperature, CO2 fixation and nitrate reduction on selenium reduction, by a paddy soil Clostridium strain. J Appl Microbiol 2013; 114:703-712 10.1111/jam.12084 [DOI] [PubMed] [Google Scholar]
- 36.Switzer Blum J, Burns Bindi A, Buzzelli J, Stolz JF, Oremland RS. Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch Microbiol 1998; 171:19-30 10.1007/s002030050673 [DOI] [PubMed] [Google Scholar]
- 37.Rauschenbach I, Yee N, Häggblom MM, Bini E. Energy metabolism and multiple respiratory pathways revealed by genome sequencing of Desulfurispirillum indicum strain S5. Environ Microbiol 2011; 13:1611-1621 10.1111/j.1462-2920.2011.02473.x [DOI] [PubMed] [Google Scholar]
- 38.Bini E, Rauschenbach I, Narasingarao P, Starovoytov V, Land M, Hauser L, Jeffries CD, Held B, Bruce D, Detter C, et al. Complete genome sequence of Desulfurispirillum indicum strain S5T. Stand Genomic Sci 2011; 5:371-378 10.4056/sigs.2425302 [DOI] [PMC free article] [PubMed] [Google Scholar]