Abstract
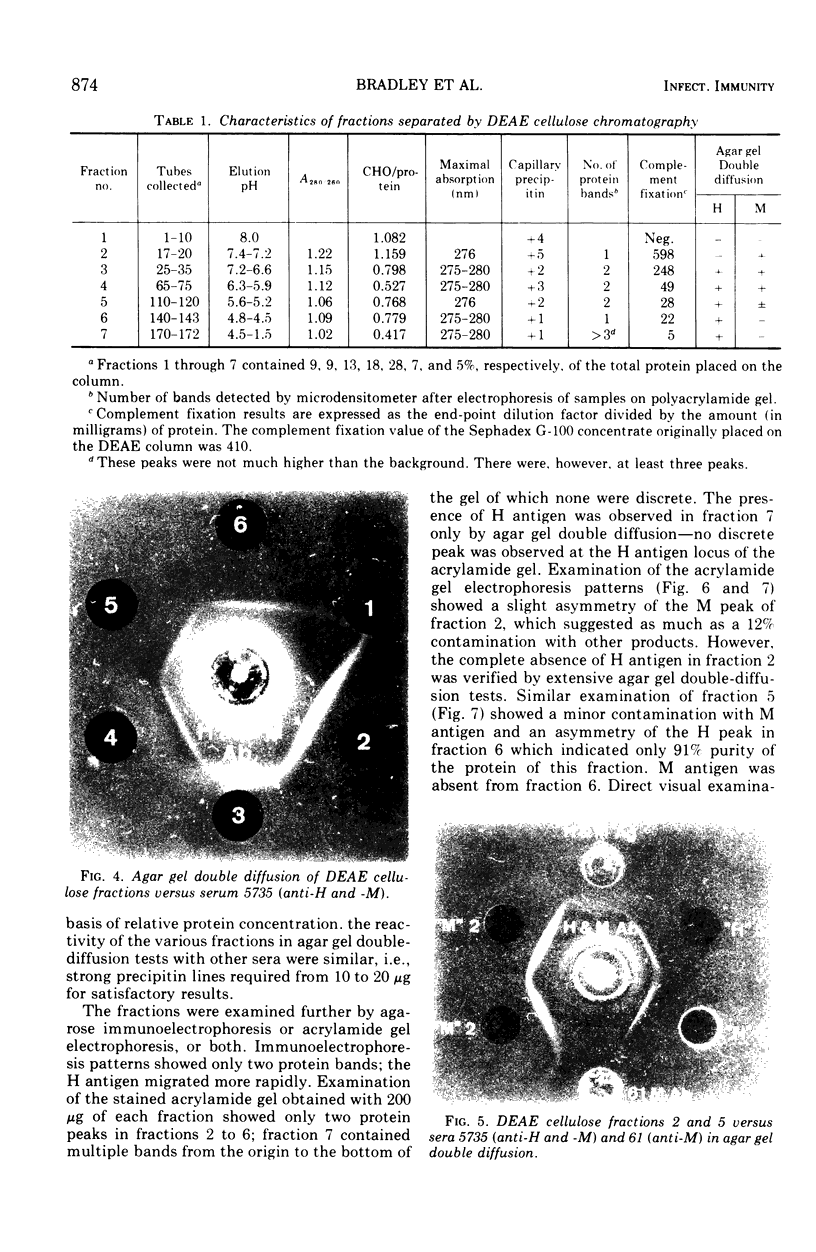
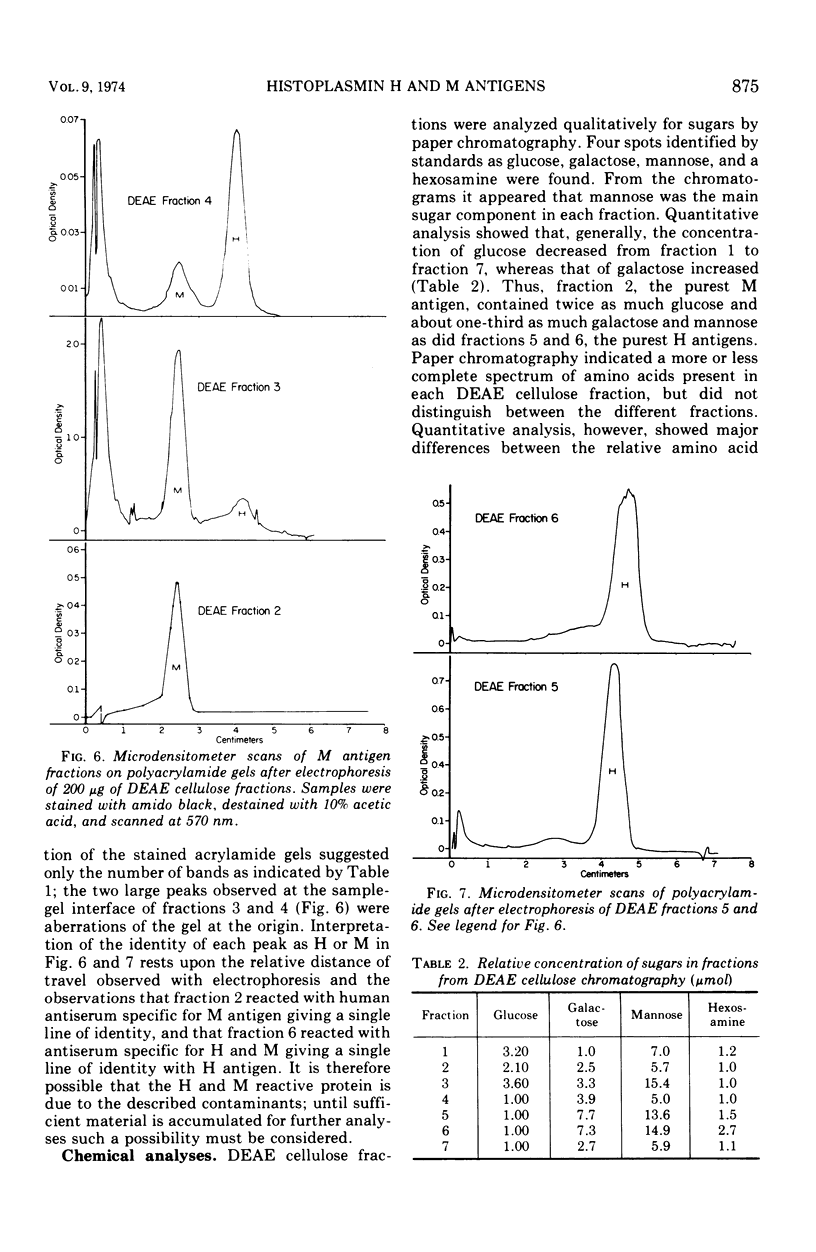
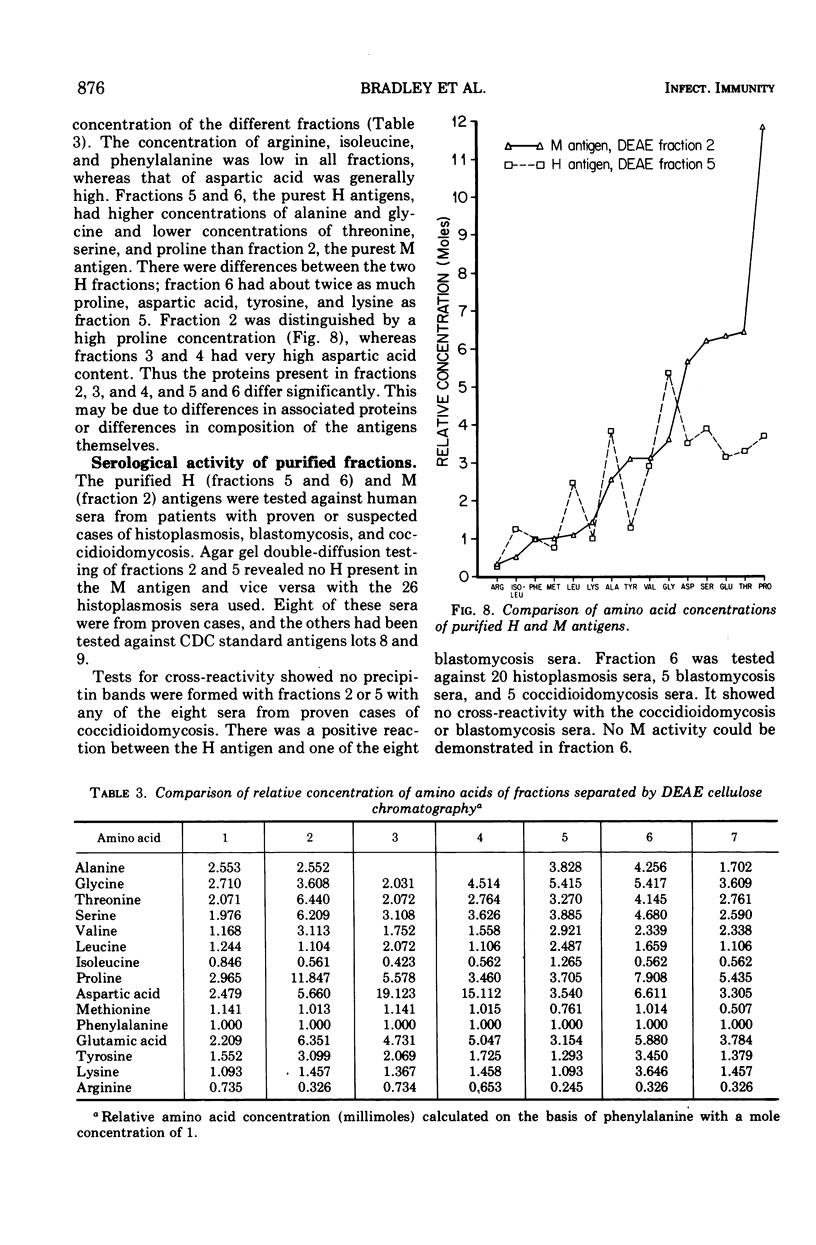
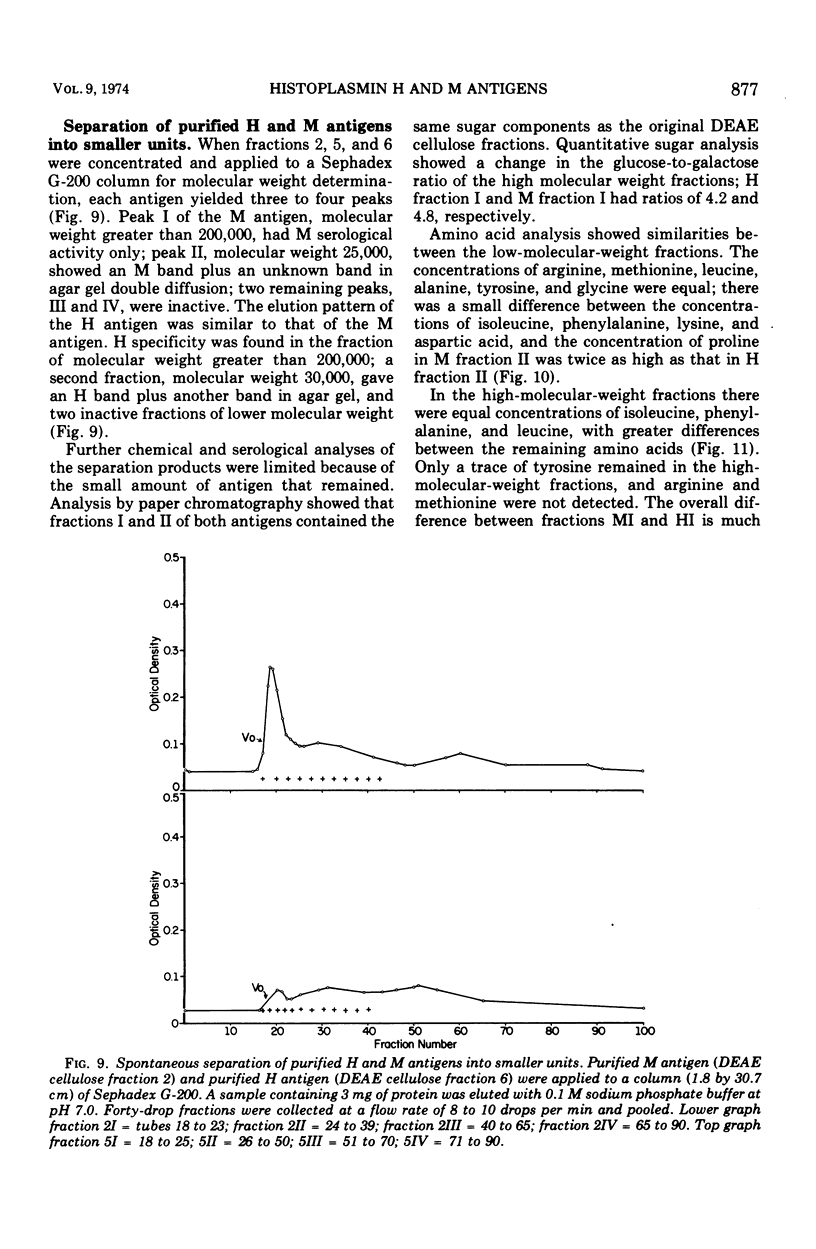
To obtain purified H and M antigens suitable as primary standards in the serological diagnosis of histoplasmosis by agar gel double-diffusion tests, H and M reactive components of histoplasmin were fractionated by column chromatography by using Sephadex G-100, Sephadex G-200, and diethylaminoethyl cellulose. Six fractions from diethylaminoethyl cellulose were reactive in agar gel double-diffusion, complement fixation, and capillary precipitin tests. When examined by electrophoresis on acrylamide gel, one M antigen (fraction 2, molecular weight greater than 200,000) and two H antigens (fractions 5 and 6, molecular weight greater than 200,000) each gave essentially a single protein band. In agar gel double-diffusion and complement fixation tests with sera from patients with proven cases of histoplasmosis, blastomycosis, or coccidioidomycosis, these two fractions of H antigen and the one of M antigen reacted only with sera from proven or suspect cases of histoplasmosis and showed reactivity with those sera known to contain only the anti-H or anti-M antibody, respectively. Fraction 2 (M antigen) and fractions 5 and 6 (H antigens) had carbohydrate-to-protein ratios of 1.60, 0.77 and 0.78, respectively. Both antigens contained galactose, glucose, mannose, and hexosamine, with mannose being the predominant sugar. Fraction 2 was characterized by a high proline and glucose content, whereas fractions 5 and 6 contained higher concentrations of galactose, mannose, glycine, and alanine. Each of these products appeared to separate into two active fractions, one of a molecular weight greater than 200,000 and one of a molecular weight less than 35,000. The M antigen component of fraction 2 was still characterized by a high proline content, whereas the H antigen components of fractions 5 and 6 had a high content of glutamic acid, serine, glycine, and proline.
Full text
PDF

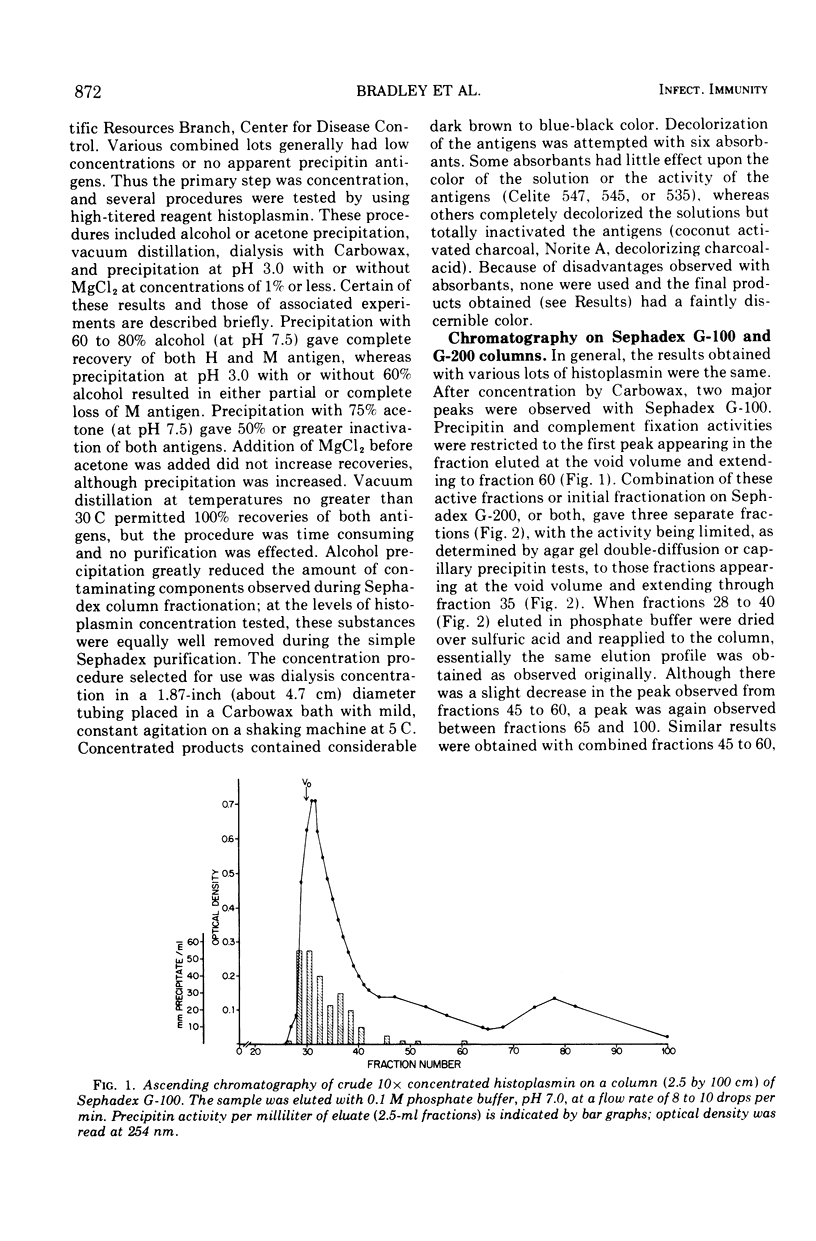
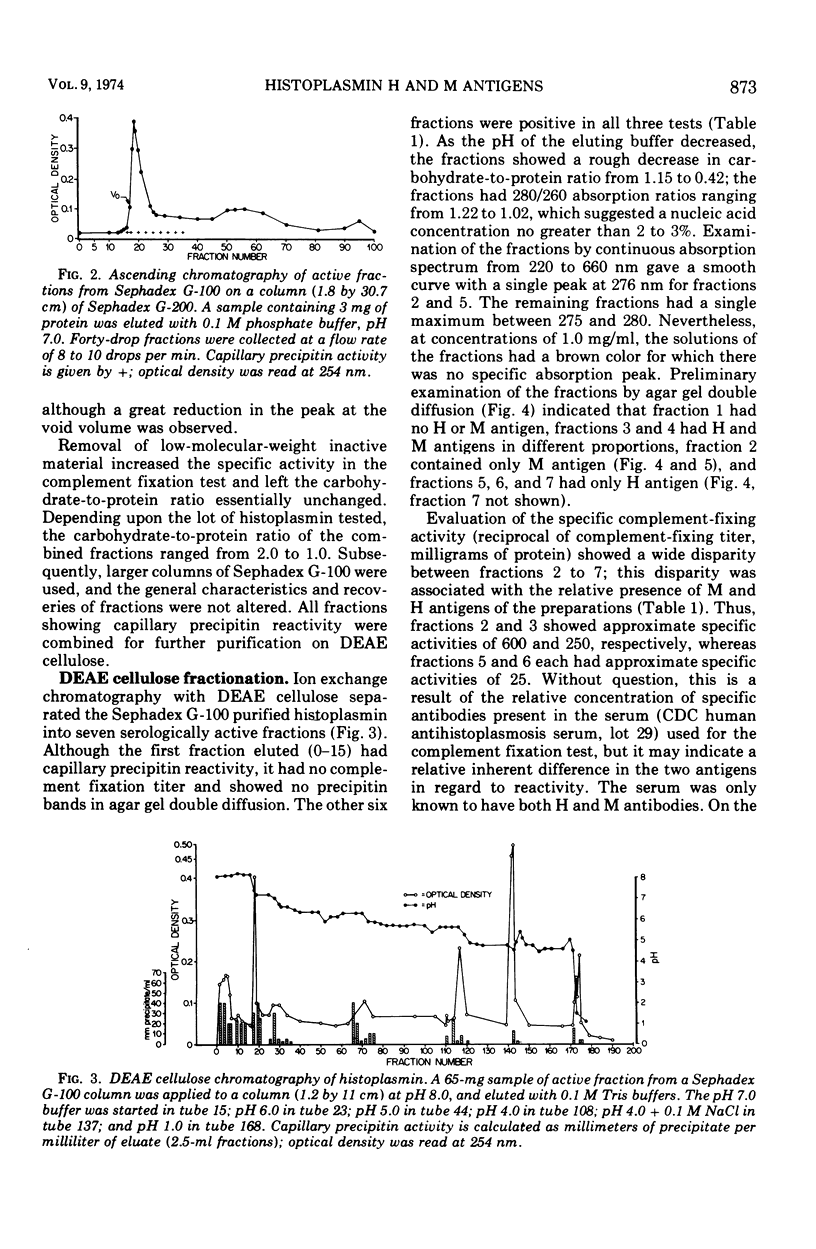


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besdine R. W., Pine L. Preparation and description of high-molecular-weight soluble surface antigens from a group A Streptococcus. J Bacteriol. 1968 Dec;96(6):1953–1960. doi: 10.1128/jb.96.6.1953-1960.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- Dickerson Q. H., Jr, Busey J. F. Chromatographic separation of h and m antigens from histoplasmin. Proc Soc Exp Biol Med. 1968 Jul;128(3):654–658. doi: 10.3181/00379727-128-33090. [DOI] [PubMed] [Google Scholar]
- Ehrhard H. B., Pine L. Factors influencing the production of H and M antigens by Histoplasma capsulatum: development and evaluation of a shake culture. Appl Microbiol. 1972 Feb;23(2):236–249. doi: 10.1128/am.23.2.236-249.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhard H. B., Pine L. Factors influencing the production of H and M antigens by Histoplasma capsulatum: effect of physical factors and composition of medium. Appl Microbiol. 1972 Feb;23(2):250–261. doi: 10.1128/am.23.2.250-261.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE C. H., DELALLA L. S., TOMPKINS V. N. Separation of specific antigens of Histoplasma capsulatum by ion-exchange chromatography. Proc Soc Exp Biol Med. 1960 Oct;105:140–141. doi: 10.3181/00379727-105-26037. [DOI] [PubMed] [Google Scholar]
- Gehreke C. W., Nakamoto H., Zumwalt R. W. Gas-liquid chromatography of protein amino acid trimethylsilyl derivatives. J Chromatogr. 1969 Nov 25;45(1):24–51. doi: 10.1016/s0021-9673(01)86179-3. [DOI] [PubMed] [Google Scholar]
- HEINER D. C. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics. 1958 Oct;22(4 Pt 1):616–627. [PubMed] [Google Scholar]
- Kaufman L. Value of immunodiffusion tests in the diagnosis of systemic mycotic diseases. Ann Clin Lab Sci. 1973 Mar-Apr;3(2):141–146. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Diaz F. J. Gas-liquid chromatography of twenty protein amino acids on a single column. J Chromatogr. 1971 Aug 5;60(1):134–136. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Phillips L. S., Pine L. Evaluation of methods used to purify acid-extracted group A streptococcal M protein. Appl Microbiol. 1971 Dec;22(6):963–973. doi: 10.1128/am.22.6.963-973.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUIRE P. G. A RELATIONSHIP BETWEEN THE MOLECULAR WEIGHTS OF MACROMOLECULES AND THEIR ELUTION VOLUMES BASED ON A MODEL FOR SEPHADEX GEL FILTRATION. Arch Biochem Biophys. 1964 Sep;107:471–478. doi: 10.1016/0003-9861(64)90303-0. [DOI] [PubMed] [Google Scholar]
- Schubert J. H., Wiggins G. L. Additional studies of histoplasmin formation. Mycopathol Mycol Appl. 1966 Nov 10;30(2):81–91. doi: 10.1007/BF02130354. [DOI] [PubMed] [Google Scholar]
- WIGGINS G. L., SCHUBERT J. H. RELATIONSHIP OF HISTOPLASMIN AGAR-GEL BANDS AND COMPLEMENT-FIXATION TITERS IN HISTOPLASMOSIS. J Bacteriol. 1965 Mar;89:589–596. doi: 10.1128/jb.89.3.589-596.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]