Abstract
BACKGROUND
The current study was conducted to analyze the association between cigarette smoking and metastasis (the primary outcome) as well as time to biochemical disease recurrence (BCR), metastasis, castration-resistant prostate cancer (CRPC), and prostate cancer-specific and overall mortality (secondary outcomes) after radical prostatectomy among men from the Shared Equal Access Regional Cancer Hospital cohort.
METHODS
A retrospective analysis was performed of 1450 subjects for whom smoking status was available from preoperative notes. Analysis of baseline characteristics by smoking status was performed using the chi-square and rank sum tests. The association between smoking status and time to the event was analyzed using Kaplan-Meier plots, the log-rank test, and Cox and competing risk models.
RESULTS
A total of 549 men (33%) men were active smokers and 1121 (67%) were nonsmokers at the time of surgery. Current smokers were younger and had a lower body mass index, higher prostate-specific antigen level, and more extracapsular extension and seminal vesicle invasion (all P <.05). A total of 509 patients, 26 patients, 30 patients, 18 patients, and 217 patients, respectively, experienced BCR, metastasis, CRPC, prostate cancer-related death, and any-cause death over a median follow-up of 62 months, 75 months, 61 months, 78 months, and 78 months, respectively. After adjusting for preoperative features, active smoking was found to be associated with an increased risk of BCR (hazards ratio [HR], 1.25; P =.024), metastasis (HR, 2.64; P =.026), CRPC (HR, 2.62; P =.021), and overall mortality (HR, 2.14; P <.001). Similar results were noted after further adjustment for postoperative features, with the exception of BCR (HR, 1.10; P =.335), metastasis (HR, 2.51; P =.044), CRPC (HR, 2.67; P =.015), and death (HR, 2.03; P <.001).
CONCLUSIONS
Among patients undergoing radical prostatectomy, cigarette smoking was associated with an increased risk of metastasis. In addition, smoking was associated with a higher risk of BCR, CRPC, and overall mortality. If confirmed, these data suggest that smoking is a modifiable risk factor in patients with aggressive prostate cancer.
Keywords: disease-free survival, metastasis, mortality, prostate cancer, prostatectomy, prostate-specific antigen, smoking, tobacco
INTRODUCTION
Cigarette smoking is the number one cause of preventable morbidity and mortality in the United States.1 Although smoking is associated with an increased incidence of several cancers, including lung, kidney, and bladder cancers, its association with prostate cancer (PCa) remains unclear. Moreover, the effect of smoking on response to PCa treatment is still a matter of much debate. A recent meta-analysis found cigarette smoking was associated with worse prognosis and higher PCa-specific mortality regardless of treatment approach.2 This is corroborated by several studies demonstrating worse cancer outcomes among smokers.3–6 For example, data from the Health Professionals Follow-Up Study showed smoking at the time of PCa diagnosis was associated with higher rates of disease recurrence and increased disease-specific and overall mortality regardless of the treatment approach used.7 Likewise, we previously reported smoking was associated with more advanced disease at the time of radical prostatectomy (RP).8 With regard to outcomes after specific treatments, one study found higher biochemical disease recurrence (BCR) after RP among smokers.9 In addition, smoking was found to correlate with a shorter time to castration-resistant PCa (CRPC) in patients receiving androgen deprivation therapy (ADT).10 In addition, 2 studies indicated higher PCa progression rates after radiotherapy for localized tumors.11,12 However, in our prior study, we found smoking was not associated with BCR after RP. Thus, although much of the accumulated evidence suggests smoking could be related to more aggressive tumors and/or a suboptimal response to treatment, not all studies agree. To the best of our knowledge, the effects of smoking on long-term outcomes (eg, metastasis and mortality) specifically after RP have not been evaluated to date. Thus, the primary objective of the current study was to analyze the association between cigarette smoking and time to metastasis after RP among men from the Shared Equal Access Regional Cancer Hospital (SEARCH) cohort. The secondary objective was to evaluate the association between cigarette smoking and time to BCR, CRPC, PCa-specific mortality (PCSM), and overall mortality in the same sample. The hypothesis in the current study was that cigarette smoking is associated with worse BCR-free, metastasis-free, CRPC-free, and overall mortality.
MATERIALS AND METHODS
Study Population
After obtaining Institutional Review Board approval from each institution, data from patients undergoing RP between 1995 and 2010 at 4 Veteran Affairs medical centers (West Los Angeles, Calif; Augusta, Ga; and Durham and Asheville, NC) for whom smoking data were available were combined into the SEARCH database.13 Patients treated with preoperative ADT or radiotherapy were excluded. Of 2353 patients in the SEARCH database from sites with available smoking data, we excluded 486 (21%) due to missing smoking status at the time of RP and 197 (8%) due to missing data regarding ≥1 of the covariates in the study. This resulted in 1670 subjects (71%) versus 1267 in our initial report.8 We performed a comparison between patients for whom smoking status was available and those without it, adjusting for year of surgery and VA center. Of all demographic, biochemical, and pathological variables, the only statistically significant difference was a higher rate of seminal vesicle invasion noted among patients with missing smoking status (P =.049). Smoking status at the time of RP (yes or no) was determined by retrospective chart review of preoperative surgical and anesthesia notes. Information regarding smoking of other tobacco products (eg, cigars or pipes) and other tobacco exposures (eg, secondhand smoke, tobacco chewing) was not available. All patients were followed with serial prostate-specific antigen (PSA) determinations and clinical visits at intervals determined according to the discretion of the attending physician. BCR was defined as a single PSA level >0.2 ng/mL, 2 concentrations at 0.2 ng/mL, or secondary treatment for elevated PSA.14 Metastases were determined by review of radiology reports (including computed tomography, bone scan, radiograms, and magnetic resonance imaging) and clinical notes. CRPC was defined as elevated PSA and/or metastatic disease progression despite receipt of adequate ADT.15 Death and cause of death were determined by chart review of Veteran Affairs electronic medical records, which are linked with the Social Security Death Index. Additional treatment after RP was performed based on the judgment of the patient and treating physician.
Statistical Analysis
Patients were divided into 2 groups based on their smoking status at the time of RP, namely active smokers and nonsmokers (never-smokers and former smokers combined, given that former smoking history was not available for all patients). Baseline characteristic comparisons between smokers and nonsmokers were performed using chi-square tests for categorical data and rank sum tests for continuous variables. We also tested whether smoking was independently associated with the binary adverse pathological features of positive surgical margins, extracapsular extension, and seminal vesicle invasion using multivariable logistic regression analysis mutually adjusted for the preoperative characteristics of race (white, African American, or other), body mass index (BMI) (continuous, log-transformed, in kg/m2), age (continuous, in years), surgery year (continuous, in years), surgical center (4), preoperative PSA (continuous, log-transformed, in ng/mL), and biopsy Gleason score (2–6, 7, and 8–10). Univariable time to BCR; time to metastasis; and time to CRPC-specific, cancer-specific, and overall survival analyses were performed using Kaplan-Meier plots and log-rank tests. To test whether smoking was independently predictive of outcomes, we used a proportional hazards model adjusting initially only for preoperative characteristics (age, race, BMI, biopsy Gleason score, preoperative PSA level, surgical center, and surgery year) and then adjusting for both preoperative and postoperative features (age, race, BMI, preoperative PSA level, surgical center, surgery year, pathological Gleason score, surgical margin status, extracapsular extension, and seminal vesicle invasion). Given that smoking is associated with cardiovascular and other diseases that can, in turn, lead to non-PCa death, we performed competing risks analysis using death as a competing risk for BCR, metastasis, and CRPC. Competing risk analysis followed the method described by Fine and Gray.16 Our primary outcome was time to metastases because to the best of our knowledge this has not been reported previously and is an objective measure of advanced prostate cancer. All other outcomes were considered to be secondary. The proportional hazards assumption was addressed by examining Schoenfeld residuals of each variable and was tested with the statistic of Grambsch and Therneau.17 All statistical analyses were performed using Stata 11.2 statistical software (StataCorp, College Station, Tex). A P level <.05 was considered to be statistically significant.
RESULTS
Median patient age, BMI, and PSA level at the time of RP were 61 years, 27.9 kg/m2, and 6.2 ng/dL, respectively. A total of 678 men (41%) were African American. Positive surgical margins, extracapsular extension, seminal vesicle invasion, and lymph node involvement were detected in 716 men (43%), 312 men (19%), 123 men (7%) and 22 men (1%), respectively.
Of the 1670 men, 549 (33%) were active smokers and 951 (57%) were nonsmokers at the time RP was performed. Current smokers were younger (P <.001) and more likely to be African American (P <.001) compared with nonsmokers. Smokers also had a significantly lower BMI (P <.001), a higher preoperative PSA level (P =.030), and a higher incidence of extracapsular extension (P =.028) and seminal vesicle invasion (P =.012). There was a trend toward higher pathological Gleason scores and positive surgical margins among smokers; however, none of these associations reached statistical significance (Table 1). In multivariable analysis adjusted for preoperative variables, smoking was found to be independently associated with extracapsular extension (P =.012) but not positive surgical margins (P =.189) or seminal vesicle invasion (P =.099).
TABLE 1.
Baseline Patient and Disease Characteristics
| Variables | Smokers No. (%) | Nonsmokers No. (%) | Pa |
|---|---|---|---|
| Total no. of patients | 549 (33) | 1121 (67) | — |
| Age at surgery, y | <.001 | ||
| Median (IQR) | 60 (56–63) | 62 (58–66) | |
| Ethnic group | <.001 | ||
| White | 265 (48) | 641 (57) | |
| Black | 264 (48) | 414 (37) | |
| Other | 20 (4) | 66 (6) | |
| BMI, kg/m2 | <.001 | ||
| Median (IQR) | 26.9 (23.7–29.4) | 28.4 (26.0–31.8) | |
| Year of surgery | .591 | ||
| Median (IQR) | 2004 (2002–2007) | 2005 (2002–2007) | |
| Biopsy Gleason score | .196 | ||
| 2–6 | 306 (56) | 655 (59) | |
| 3+4 | 193 (35) | 345 (31) | |
| 4+3–10 | 47 (9) | 110 (10) | |
| Preoperative PSA, ng/mL | .030 | ||
| Median (IQR) | 6.5 (4.7–10.3) | 6.1 (4.6–8.8) | |
| Postoperative Gleason score | .074 | ||
| 2–6 | 197 (36) | 466 (41) | |
| 3+4 | 285 (52) | 552 (47) | |
| 4+3–10 | 67 (12) | 133 (12) | |
| Extracapsular extension | 119 (22) | 193 (17) | .028 |
| Positive surgical margins | 253 (46) | 463 (41) | .064 |
| Seminal vesicle invasion | 53 (10) | 70 (6) | .012 |
| Lymph node status | .471b | ||
| Positive | 9 (2) | 13 (1) | |
| Negative | 336 (61) | 665 (59) | |
| Not sampled | 204 (37) | 443 (40) |
Abbreviations: BMI, body mass index; IQR, interquartile range; PSA, prostate-specific antigen.
The chi-square test was used for categorical variables and analysis of variance was used for continuous variables.
Patients with unknown lymph node status were excluded from this analysis.
Biochemical Disease Recurrence
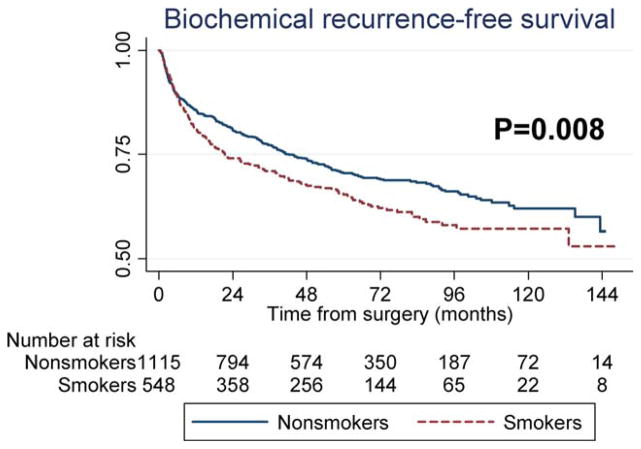
A total of 509 patients developed BCR, a secondary outcome, over a median follow-up of 62 months. Active smoking was found to be associated with increased BCR on univariable analysis (P =.008) (Fig. 1). After adjustments for preoperative characteristics, smoking retained an association with increased BCR risk (hazards ratio [HR], 1.25; P =.024). However, after further adjustments for postoperative characteristics, smoking was not found to be significantly related to BCR (HR, 1.10; P =.335) (Table 2). Similar HRs were found after including death as a competing risk (preoperative model: HR, 1.30 [P =.025] and postoperative model: HR, 1.23 [P =.086]).
Figure 1.
Recurrence-free survival is shown comparing smokers and nonsmokers.
TABLE 2.
Multivariable Association Between Cigarette Smoking and BCR-Free, Metastasis-Free, and OS
| Model | BCR-Free Survival
|
Metastasis-Free Survival
|
CRPC-Free Survival
|
PCSM
|
OS
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Univariable | 1.28 (1.07–1.53) | .008 | 2.14 (0.99–4.62) | .053 | 1.98 (0.96–4.06) | .063 | 2.16 (0.86–5.43) | .103 | 2.15 (1.65–2.80) | <.001 |
| Multivariable Preoperativea | 1.25 (1.03–1.51) | .024 | 2.64 (1.13–6.20) | .026 | 2.62 (1.16–5.93) | .021 | NA | — | 2.14 (1.61–2.83) | <.001 |
| Preoperative and postoperativeb | 1.10 (0.91–1.34) | .335 | 2.51 (1.03–6.11) | .044 | 2.67 (1.21–5.87) | .015 | NA | — | 2.03 (1.53–2.69) | <.001 |
Abbreviations: 95% CI, 95% confidence interval; BCR, biochemical disease recurrence; CRPC, castration-resistant prostate cancer; HR, hazards ratio; NA, not available; OS, overall survival; PCSM, prostate cancer-specific survival.
Model was adjusted for age, race, body mass index, biopsy Gleason score, preoperative prostate-specific antigen level, Veterans Affairs medical center, and year of surgery.
Model was adjusted for age, race, body mass index, preoperative prostate-specific antigen level, Veterans Affairs medical center, year of surgery, pathological Gleason score, surgical margin status, extracapsular extension, and seminal vesicle invasion.
Metastasis
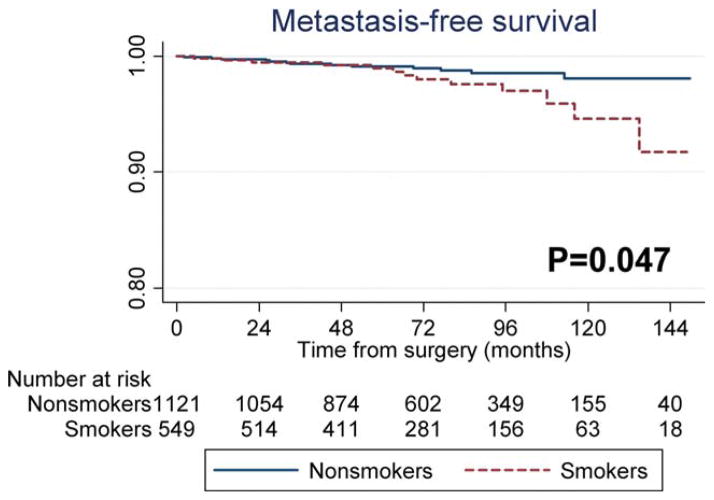
A total of 26 patients developed metastasis (the primary outcome of the study) over a median follow-up of 75 months. Active smoking was associated with an increased risk of metastasis on univariable analysis (P =.047) (Fig. 2). After adjustments for preoperative characteristics, smoking remained associated with an increased risk of metastasis (HR, 2.64; P =.026). After further adjustments for postoperative characteristics, we found similar HRs (HR, 2.51; P =.044) (Table 2). A model including death as a competing risk indicated comparable results (preoperative model: HR, 2.55 [P =.023] and postoperative model: HR, 2.42 [P =.066]).
Figure 2.
Metastasis-free survival is shown comparing smokers and nonsmokers.
Castration-Resistant Prostate Cancer
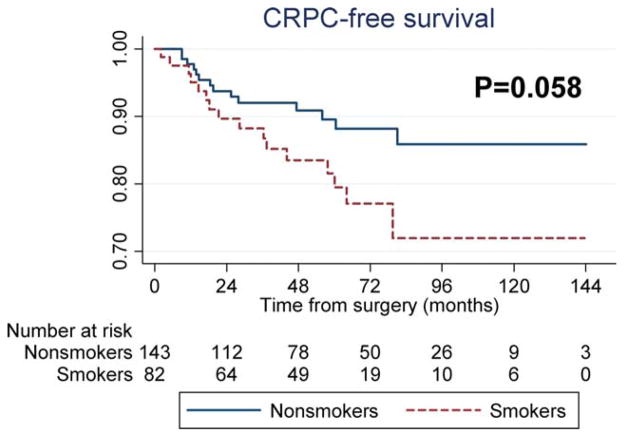
A total of 227 patients (14%) received ADT after RP, 30 of whom developed CRPC (a secondary outcome) over a median follow-up of 61 months after the initiation of ADT. In these patients, we found a trend toward increased CRPC risk among active smokers on univariable analysis (P =.058) (Fig. 3). After adjustments for preoperative and postoperative features, smoking retained an association with CRPC (preoperative model: HR, 2.62 [P =.021] and postoperative model: HR, 2.67 [P =.015]) (Table 2). Similar HRs were found after including death as a competing risk (preoperative model: HR, 2.50 [P =.026] and postoperative model: HR, 2.60 [P =.023]).
Figure 3.
Castration-resistant prostate cancer (CRPC) survival is shown comparing smokers and nonsmokers.
PCa-Specific Mortality
A total of 18 patients died of PCa (a secondary outcome) over a median follow-up of 78 months. On univariable analysis, there was a trend toward higher cancer mortality among active smokers, but it did not reach statistical significance (HR, 2.16; P =.103) (Table 2). Given the limited number of cancer deaths, we did not perform multivariable analyses.
Overall Mortality
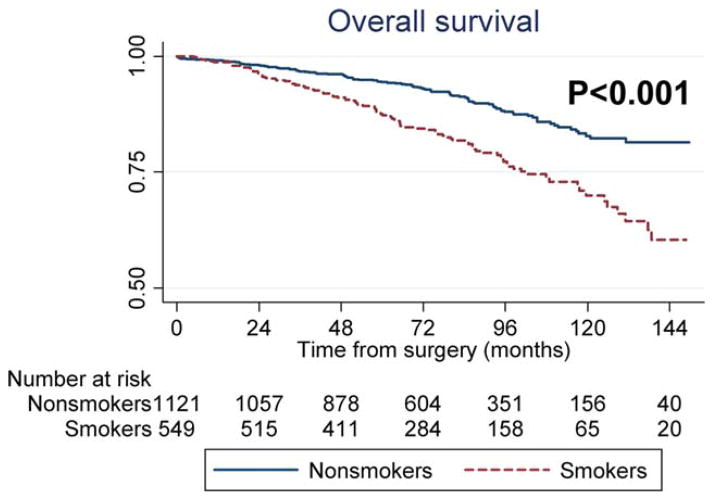
A total of 217 patients died of any cause (a secondary outcome) over a median follow-up of 78 months. Active smoking was associated with an increased risk of overall mortality on univariable analysis (P <.001) (Fig. 4). After adjustments for preoperative (HR, 2.14; P <.001) or postoperative characteristics, smoking remained significantly related to overall mortality (HR, 2.03; P <.001) (Table 2).
Figure 4.
Overall survival is shown comparing smokers and nonsmokers.
DISCUSSION
Several studies have suggested that smoking may be related to more aggressive PCa and/or a suboptimal response to therapy. For example, studies have demonstrated an increased risk of BCR after RP among smokers.7,9 Others found smoking was associated with an increased risk of CRPC in patients with advanced PCa who were receiving ADT.10 Moreover, 2 studies demonstrated a higher rate of disease progression after radiotherapy for localized tumors.11,12 In addition, there is evidence suggesting smokers have higher PCa-specific mortality.7 However, to the best of our knowledge, the current study is the first to evaluate the effects of smoking on long-term oncologic outcomes (eg, metastasis and mortality) specifically after RP.
In the current study of 1670 patients with PCa undergoing RP, cigarette smoking was found to be associated with more aggressive disease at the time of surgery. Smokers had significantly higher preoperative PSA levels and more extracapsular extension and seminal vesicle invasion compared with nonsmokers. In addition, there was a trend toward a higher pathological grade and more positive surgical margins among smokers; however, these latter associations did not reach statistical significance. Similar studies of patients undergoing RP demonstrated comparable results. For example, Roberts et al found smokers had more extraprostatic disease extension and higher Gleason scores at the time of surgery.18 In a previous report using the same but slightly smaller data set with shorter follow-up, we showed smoking to be associated with higher PSA levels and more extracapsular extension and seminal vesicle invasion.8 Therefore, it appears that smokers undergoing RP have more advanced and possibly more aggressive disease at baseline.
Given the more advanced disease noted at baseline among smokers, it is not surprising that cigarette smoking has been associated with an increased risk of BCR after RP. It is interesting to note that this finding differs from our prior study in which smoking was not found to be significantly associated with BCR (HR, 1.37; P =.129 on univariable analysis). However, the current study differs in that we had a larger sample size (1670 patients vs 1267 patients) but, importantly, a much longer follow-up (62 months vs 37 months). This resulted in greater power to detect associations despite a slightly weaker association noted (univariable HR of 1.37 in our prior study vs 1.28 in the current study). The association in the current study remained statistically significant even after adjustments for preoperative characteristics. However, after adjustments for preoperative and postoperative features, the association was attenuated and not statistically significant, suggesting perhaps that the additional BCR risk was related, at least in part, to worse disease aggressiveness at baseline. In this hypothesis, worse disease at baseline is the mediator of the deleterious effects of smoking in cancer outcomes. Unfortunately, we were unable to determine whether this is true or the lack of a significant association is due to limited statistical power. However, when taking into account the greater risk of non-PCa death among smokers and using competing risks, smoking was suggestively associated with BCR even after adjusting for pathological features, although this association was not statistically significant (P =.086). Nonetheless, a similar study published by Joshu et al found that smokers were at an increased risk of BCR after RP independent of baseline and postoperative disease characteristics.9 This suggests that PCa occurring in smokers may have more aggressive behavior independent of baseline disease. Thus, more studies are still required to determine which factors are associated with worse disease-free survival among smokers after RP. Nevertheless, the combined evidence supports the finding that smoking is associated with a worse response to RP and an increased risk of BCR.
Beyond BCR, smokers had higher metastasis risk than nonsmokers (the primary outcome of the current study). Specifically, the association between smoking and metastasis was much stronger (2.5 times the risk of non-smokers) versus the excess BCR risk (only 28% excess BCR risk noted among smokers). Similar to the findings of the current study but in a different setting in which all patients were treated with radiotherapy, the study by Pantarotto et al found that smoking conferred a higher risk of metastasis in both current and former smokers.12 Similar to Oefelein et al, we found the risk of CRPC after ADT to be elevated among smokers.10 The hazards of developing CRPC among smokers were nearly 2.5 times greater than for nonsmokers. Thus, more advanced and possibly more aggressive disease at baseline, a shorter time from RP to BCR, and a higher risk of metastatic disease and progression to CRPC are likely reasons for why there was a trend toward a higher PCSM risk among smokers in the current study and in a separate study, although the association in the current study was not statistically significant, perhaps related to low power.2 Collectively, these studies all suggest that smoking is associated with worse outcomes after RP, as measured by several endpoints.
We also demonstrated higher overall mortality among smokers, which can be explained by harms to overall health including cardiovascular and pulmonary diseases and perhaps by more PCa deaths. Indeed, the results of the current study demonstrate excess BCR, metastasis, and CRPC among smokers, which all are correlated with PCa mortality.19 Moreover, our competing risk analysis suggested that the risk of these endpoints is not significantly altered by competing death causes (eg, cardiovascular or pulmonary). Thus, just because smokers are more likely to die of causes other than PCa compared with non-smokers should not be reason to overlook the risk of adverse PCa outcomes in these patients.
Several potential biological mechanisms can explain how smoking affects PCa. Multiple specific genetic and epigenetic changes are more frequent in smokers. For example, mutations in genes such as K-RAS and p53 induced by polycyclic aromatic hydrocarbons and nitrosamines present in tobacco could potentially lead to worse cancer progression.20 Smoking also affects the immune system by concomitantly promoting inflammation and suppressing immune function such as reducing T-cell and natural killer cell activation.21 Both pathways (inflammation and decreased immune function) could facilitate tumor growth. In addition, smoking has an antiestrogenic effect, which could promote PCa growth.22 Moreover, there is evidence to suggest that nicotine promotes angiogenesis, leading to faster tumor growth and progression to metastatic disease.23,24 Finally, confounding lifestyle factors such as higher alcohol consumption, a lower rate of exercise, and an overall unhealthy lifestyle among smokers could play a role in explaining the association between smoking and cancer outcomes.25 Although to the best of our knowledge the association between lifestyle and PCa progression has not been fully determined, it is likely that a combination of biologic and behavioral factors is responsible for the association between smoking and aggressive PCa.8
The strengths of the current study include a mature, well-established, multicentric database with detailed outcome data. The main limitation of the current study is its retrospective design. In addition, smoking is associated with comorbidities that were not controlled in our analyses given that these data were not available for all patients. These comorbidities may influence a patient’s decision of whether to undergo RP. For example, patients at higher surgical risk with less aggressive disease may be treated with watchful waiting whereas those with a similar surgical risk and more aggressive disease may undergo RP. This would result in more aggressive disease in groups with high comorbidity (ie, smokers) versus those with low comorbidity (ie, nonsmokers). However, we did not find any significant preoperative evidence of more aggressive disease among smokers (eg, biopsy Gleason score or clinical stage) to support such a hypothesis. In addition, even after adjusting for baseline preoperative factors, smoking was associated with poor outcomes among patients with PCa. We did not analyze cumulative and current tobacco exposure given that the data were unavailable for most smokers. We also did not analyze other types of smoking (eg, cigars) and different tobacco exposures (eg, chewing tobacco) because the relevant data were not available, although the number of men in those categories was presumably low. In addition, the relatively low number of patients with metastasis (N =26) and CRPC (N =30) may have affected the accuracy of our statistical models. As noted, we were unable to analyze PCa-specific survival in multivariable models given the limited number of deaths from PCa. Finally, the length of the follow-up in the current study was modest and larger studies with longer follow-up are required to confirm these findings.
Among patients undergoing RP, cigarette smoking was found to be associated with more aggressive and advanced disease at the time of RP and an increased risk of metastasis (the primary outcome of the current study) and multiple secondary outcomes including BCR, CRPC, and overall mortality. If confirmed in other studies, this would establish smoking as a modifiable risk factor among patients with aggressive PCa. Whether smoking cessation after diagnosis can limit the adverse effects of smoking requires further study.
Acknowledgments
FUNDING SUPPORT
Research support was received from the Department of Veterans Affairs, the National Institutes of Health (NIH) (grant R01CA100938 to Dr. Aronson), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (to Dr. Aronson), the Georgia Cancer Coalition (to Dr. Terris), and NIH grant K24CA160653.
Footnotes
The views and opinions of, and endorsements by, the author(s) do not reflect those of the US Army or the Department of Defense.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huncharek M, Haddock S, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zu K, Giovannucci E. Smoking and aggressive prostate cancer: a review of the epidemiologic evidence. Cancer Causes Control. 2009;20:1799–1810. doi: 10.1007/s10552-009-9387-y. [DOI] [PubMed] [Google Scholar]
- 4.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control. 2008;19:25–31. doi: 10.1007/s10552-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 5.Rohrmann S, Genkinger JM, Burke A, et al. Smoking and risk of fatal prostate cancer in a prospective U.S. study. Urology. 2007;69:721–725. doi: 10.1016/j.urology.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohrmann S, Linseisen J, Allen N, et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2013;108:708–714. doi: 10.1038/bjc.2012.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira DM, Antonelli JA, Presti JC, Jr, et al. Association of cigarette smoking with interval to biochemical recurrence after radical prostatectomy: results from the SEARCH database. Urology. 2010;76:1218–1223. doi: 10.1016/j.urology.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshu CE, Mondul AM, Meinhold CL, et al. Cigarette smoking and prostate cancer recurrence after prostatectomy. J Natl Cancer Inst. 2011;103:835–838. doi: 10.1093/jnci/djr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oefelein MG, Resnick MI. Association of tobacco use with hormone refractory disease and survival of patients with prostate cancer. J Urol. 2004;171:2281–2284. doi: 10.1097/01.ju.0000125123.46733.93. [DOI] [PubMed] [Google Scholar]
- 11.Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S. The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol. 2004;171:1543–1546. doi: 10.1097/01.ju.0000118292.25214.a4. [DOI] [PubMed] [Google Scholar]
- 12.Pantarotto J, Malone S, Dahrouge S, Gallant V, Eapen L. Smoking is associated with worse outcomes in patients with prostate cancer treated by radical radiotherapy. BJU Int. 2007;99:564–569. doi: 10.1111/j.1464-410X.2006.06656.x. [DOI] [PubMed] [Google Scholar]
- 13.Whitley BM, Moreira DM, Thomas JA, et al. SEARCH Database Study Group. Preoperative weight change and risk of adverse outcome following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. Prostate Cancer Prostatic Dis. 2011;14:361–366. doi: 10.1038/pcan.2011.42. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:365–369. doi: 10.1016/s0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29:3695–3704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:12. [Google Scholar]
- 18.Roberts WW, Platz EA, Walsh PC. Association of cigarette smoking with extraprostatic prostate cancer in young men. J Urol. 2003;169:512–516. doi: 10.1097/01.ju.0000046160.80804.7f. discussion 516. [DOI] [PubMed] [Google Scholar]
- 19.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–1184. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Kudahetti S, Fisher G, Ambroisine L, et al. p53 immunochemistry is an independent prognostic marker for outcome in conservatively treated prostate cancer. BJU Int. 2009;104:20–24. doi: 10.1111/j.1464-410X.2009.08407.x. [DOI] [PubMed] [Google Scholar]
- 21.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res. 2008;57:497–503. doi: 10.1007/s00011-008-8078-6. [DOI] [PubMed] [Google Scholar]
- 22.Hickey K, Do KA, Green A. Smoking and prostate cancer. Epidemiol Rev. 2001;23:115–125. doi: 10.1093/oxfordjournals.epirev.a000776. [DOI] [PubMed] [Google Scholar]
- 23.Conklin BS, Zhao W, Zhong DS, Chen C. Nicotine and cotinine up-regulate vascular endothelial growth factor expression in endothelial cells. Am J Pathol. 2002;160:413–418. doi: 10.1016/S0002-9440(10)64859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis R, Rizwani W, Banerjee S, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrucci L, Izmirlian G, Leveille S, et al. Smoking, physical activity, and active life expectancy. Am J Epidemiol. 1999;149:645–653. doi: 10.1093/oxfordjournals.aje.a009865. [DOI] [PubMed] [Google Scholar]