Abstract
Purpose
We sought to predict renal growth based on clinical and metabolic parameters in patients with isolated methylmalonic acidemia (MMA), a group of disorders associated with chronic kidney disease.
Methods
Fifty MMA patients, followed from 2004 to 2011, were classified by molecular genetics and studied using a combined cross-sectional and longitudinal design that included renal ultrasound examinations, anthropometric measurements, and metabolic phenotyping. Renal length was compared to healthy controls and modeled to other clinical parameters using multiple regression analyses.
Results
Comparisons with age-matched controls showed that renal length in MMA subjects was significantly decreased (p < 0.05). Stepwise regression modeling found that combinations of height, serum cystatin C, and serum methymalonic acid concentrations best predicted kidney size. The regression equations used to generate MMA kidney nomograms were: renal length (cm) = 6.79 + 0.22 * age for the controls and 6.80 + 0.09 * age for the MMA cohort (p < 0.001; constant and slope).
Conclusions
Renal length, reflective of kidney growth, significantly decreased in MMA patients over time compared to controls and was predictable with select clinical parameters. Cystatin C and serum methylmalonic acid concentrations were highly correlated with smaller kidneys and decreased renal function in this patient population.
Keywords: isolated methylmalonic acidemia, renal growth, renal ultrasound, chronic kidney disease, cystatin C, methylmalonyl-CoA mutase, vitamin B12
Background
Methylmalonic acidemia (MMA) is an inborn error of metabolism caused by deficiency of the mitochondrial enzyme, methylmalonyl-CoA mutase (MUT). This vitamin B12 dependent enzyme catalyzes the isomerization of L-methylmalonyl-CoA into succinyl-CoA at the terminal step of propionyl-CoA oxidation.1 Isolated MMA exhibits genetic heterogeneity and most commonly is caused by mutations in the MUT gene, resulting in either complete (mut0) or partial (mut−) loss of enzymatic activity, but can also be caused by cblA (MMAA), cblB (MMAB), and the much rarer cblD variant 2 (MMADHC) complementation classes. The latter forms of MMA are the result of enzymopathies that impair the intracellular transport and metabolism of cobalamin.1 Unlike mut MMA, most patients with cblA and 50% of cblB forms of MMA are responsive to parenteral vitamin B12, a clinical and biochemical determinant that has been associated with improved survival and milder disease.2-7
MMA patients exhibit a well-recognized phenotype that features massive elevations of disease-related metabolites in the body fluids and multisystemic complications such as growth impairment, pancreatitis, metabolic stroke of the basal ganglia, intellectual disability and end stage kidney disease.1 Although this disorder has historically had a poor prognosis, with most vitamin B12-nonresponsive patients dying in the first decade of life2-4, earlier diagnosis through newborn screening and more aggressive treatment has afforded increased survival.7,8 This highlights the need to understand the natural history of MMA and to develop improved therapies.
Chronic kidney disease is a serious complication of MMA, developing in most mut and cblB patients by the age of 8 years.5,6 Although more commonly associated with vitamin B12 non-responsive forms of MMA, patients with mut− and cblA subtypes also can develop renal insufficiency and ultimately require kidney transplantation.7,9,10 While the mechanisms of renal injury in MMA have not been elucidated, the few reported kidney biopsies have consistently demonstrated tubular injury and interstitial fibrosis with secondary glomerulosclerosis.11-14 The primary pathological manifestation of kidney injury in MMA may be mediated through mitochondrial dysfunction in the proximal renal tubule, as has been described in a Mut mouse model.15
The most common marker of renal function, serum creatinine, correlates poorly with glomerular function in patients with MMA. Walter et al. documented that some MMA patients had a markedly depressed glomerular filtration rate (GFR) in the setting of normal serum concentrations of creatinine.13 Cystatin C based estimates of GFR, which are less dependent upon muscle mass, have not yet been evaluated in the MMA population.16-20 While the direct measurement of glomerular filtration function with agents such as inulin and iohexol is more accurate than GFR estimates, such testing is not routinely performed in MMA patients even though a small study conducted in the late 1990s documented reduced Cr-EDTA based GFR measurements in very young children with MMA. Although these authors did not describe other biochemical, imaging or enzymatic studies in the patient cohort, their observations highlight the need to develop better disease-associated biomarkers and to assess the utility of non-invasive imaging techniques to monitor and predict kidney growth in affected children7.
Renal size has been proven to be a valuable and useful parameter in evaluating renal growth in normal populations and those with genetic disorders.21,22 Furthermore, studies in healthy controls and patients with kidney disease have repeatedly documented the correlation between renal length and other markers of renal size such as renal volume, and GFR.23-25 Using ultrasonographic measurements derived from a large single center cohort of patients with MMA, we examined kidney growth and assessed the contribution of selected clinical and laboratory variables to renal size using regression analyses. The predictive equations and kidney size nomograms we have developed should provide a clinical tool to help define parameters associated with disease progression in MMA, afford prognostication for long-term management and help assess renoprotective interventions in the future.
Materials and Methods
Clinical studies
Clinical and laboratory investigations were conducted through NIH study 04-HG-0127 “Clinical and Basic Investigations of Methylmalonic Acidemia and Related Disorders” (clinicaltrials.gov identifier: NCT00078078) in compliance with the Helsinki Declaration. All subjects or their guardians gave informed assent or consent. Subjects were evaluated during the period 2004 to 2011. Analysis included 84 renal ultrasound studies, each imaging the right and left kidney, from 50 non-transplanted patients aged 2.2 years to 36.3 with enzymatic and mutation confirmed forms of MMA. We treated each ultrasound from subjects with consecutive studies as a separate data point instead of averaging each patient's data because of the significant time interval, typically greater than one year, between visits. Patients were excluded after they had undergone a kidney or combined liver-kidney transplantation. The MMA patients were compared to a published control group of 209 patients from U.S. medical centers aged 0 to 19 years with indications for ultrasound that included urinary tract infections, enuresis, aniridia, scoliosis, and undescended testicle; this approach has been used previously.26
Clinical and laboratory measurements
Data included height, weight, age as well as concentrations of serum creatinine, serum methylmalonic acid, cystatin C, and urine methylmalonic acid. All laboratory testing was done by standard measures through either the Department of Laboratory Medicine at NIH or Mayo Medical laboratories. Patients were subdivided into mut, cblA, and cblB groups based on cellular complementation and molecular testing. Body surface area (BSA) was calculated using the formula:27 BSA(m2) = ([Height(cm) * Weight(kg)]/3600)1/2. Estimated glomerular filtration rate (eGFR) for children <18 years of age was calculated using updated Schwartz equations that include serum creatinine, or serum creatinine and cystatin C, respectively. 16 For adults, the CKD-EPI Creatinine and CKD-Epi Creatinine–Cystatin C eGFR equations were used.17
Ultrasonography and laboratory measurements
The Philips iU22 xMATRIX Ultrasound system with the C5-1 1-5 MHz bandwidth transducer and the General Electric LOGIQ E9 with the C-1-5-D 1-5 MHz bandwidth transducer were used to obtain abdominal ultrasound studies for all study participants. Renal length was measured in the longitudinal axis. The original measurements were made in the context of research, and read by one of three radiologists.
Statistical analyses
Demographic variables included age, genotype, and gender. The paired t-test was used to compare right and left kidney length. Pearson's correlation was used to determine variation between independent variables. Published data from age-matched controls26 were compared with MMA study patients using a two-sample t-test of the means assuming unequal variances and by a linear regression model that included the average of the right and left kidneys as the dependent variable and age as the independent variable.
In addition to variation with age, we examined the correlation of renal length with height because MMA patients can be shorter than healthy individuals. A second linear regression model was created with height as the independent variable. This linear model was compared to a different control group that created a nomogram of right and left renal length versus height using measurements from 325 German children ages 3 days to 16 years without known kidney disease.28
Using renal length as the dependent variable, the following independent variables were included in a multiple regression equation: age, height, weight, body surface area (BSA), body mass index (BMI), serum methylmalonic acid (MMAS), urine methylmalonic acid (MMAU), serum creatinine, cystatin C, eGFR creatinine (creatinine based), eGFR creatinine - cystatin C (creatinine and cystatin C based), gender and enzymatic subtype (complementation class).
The best model, as determined by the highest correlation coefficient (R2), was developed using stepwise regression analyses. Individual predictor variables were eliminated if the regression coefficient (β) had a statistical significance of p > 0.05 using the Wald test. Beta weights were calculated to assess the unique contribution of independent variables relative to the regression model (beta weight is the change in renal length when an independent variable increases one standard deviation while other independent variables are held constant). Stata version 11 (StataCorp LP, College Station, TX) was used for data manipulation and analysis.
When two or more variables were closely correlated (multicollinearity), such as MMAS and serum creatinine (Pearson coefficient of correlation 0.808 (p < 0.0001); See Table S1), they were not included in same multiple regression model.29 Multicollinear relationships were observed between the variable group of creatinine, cystatin C and MMAS, and when controlling for height, the R2 values for these predictors were similar (Table S2). Height, BSA, and weight also demonstrated multicollinearity when controlling for height, with R2 values of 0.767, 0.757, and 0.712, respectively.
Results
Demographics and MMA subtypes
Ultrasound measurements were available for 58 patients with isolated MMA who participated in our study. The average age of patients was 11.9 years and the age range was 2.2 years to 36.3 years. After excluding 7 patients who had kidney transplants and one patient with distorted renal anatomy due to severe scoliosis, 50 patients (86.2 %) were evaluated for further study. Of these patients, 35 were mut subtype yielding 63 ultrasound studies, 9 cblA subtype (14 ultrasound studies), and 6 cblB subtype (7 studies). A single kidney study includes an ultrasound of both right and left kidneys. Eighteen patients had sequential ultrasound studies yielding an additional 33 kidney studies.
Differences in renal length by anatomy, subtype, and gender
In the entire study group, the mean left kidney length was 0.37 cm greater than the right (p< 0.001); similar differences were noted in studies of healthy children.30 Based on these findings, the data were divided into left and right kidneys for subsequent multiple regression analyses. The mut patients had slightly but not significantly smaller kidneys on average compared to the cblA and cblB subtypes, and the enzymatic subtype was insignificant when added into the regression analysis (controlling for height and cystatin C): mut (p = 0.487), cblA (p = 0.208), and cblB (p = 0.485). Therefore, renal data from all subtypes was pooled. Likewise, gender was not a significant variable (p = 0.554).
Renal length: correlations with age and height
Figure 2(a) and Figure 2(b) are nomograms showing renal length variation with age and height, respectively. For more severely affected patients, with serum methylmalonic acid levels greater than 2000 micromolar, renal lengths were smaller across the age and height spectrum (Figure 2).
Figure 2.
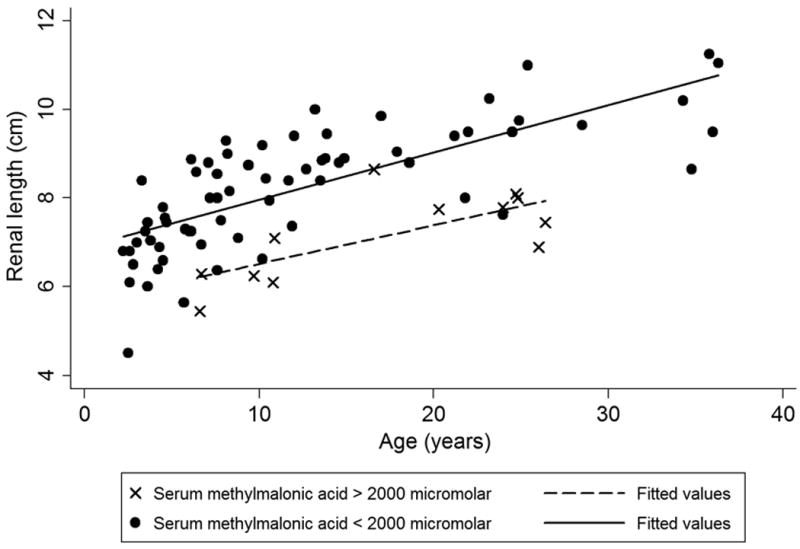
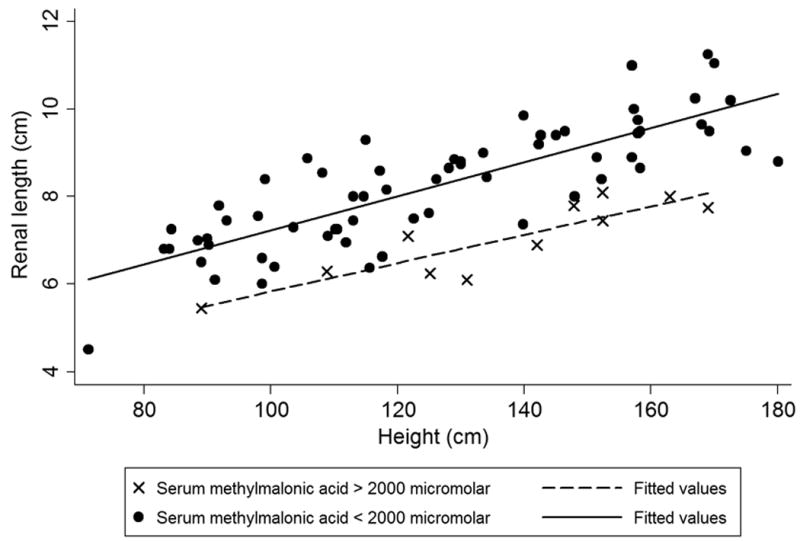
(a). Renal length vs. age, stratified by serum methylmalonic acid level. Patients with greater than 2000 micromolar serum methylmalonic acid have smaller kidneys. (b). Renal length vs. height, stratified by serum methylmalonic level. Similar to Figure 2(a), more severely affected patients with higher serum methylmalonic acid levels have smaller kidneys across height spectrum.
When comparing to a control group, MMA patients had a significant difference in average renal length (p < 0.05), as shown in Table 1 and Figure 1.26 The regression equation for the control group was renal length (cm) = 6.79 + 0.22 * age (years) for children older than 1 year. The linear regression equation for MMA subjects was 6.80 + 0.09 * age (years) with p < 0.001 for both the constant and βage coefficient. In addition to age (R2 = 0.40), renal length varied linearly with height (R2 = 0.51) [Figure 2 (b)] and BSA (R2 = 0.52). Because the published controls averaged right and left kidney lengths when developing their nomogram, we used an average of the right and left kidneys for comparison.26
Table 1. Renal Length of MMA subjects vs. Controls, by age.
The average and SD for renal length values for MMA patients (all enzymatic subtypes) and control subjects are presented in one year age intervals. Right and left renal lengths are averaged. Comparisons using the t test assumed unequal variances. Data from controls are from Rosenbaum et al.26 Asterisk denotes data not available.
| MMA Patients | Controls | ||||
|---|---|---|---|---|---|
|
| |||||
| Age (years) | Observations | Renal Length (cm) | Observations | Renal Length (cm) | p-value |
| 2 to 3 | 6 | 6.22 ± 0.88 | 12 | 7.36 ± 0.54 | 0.01 |
| 3 to 4 | 6 | 7.19 ± 0.78 | 30 | 7.36 ± 0.64 | 0.32 |
| 4 to 5 | 7 | 6.88 ± 0.80 | 26 | 7.87 ± 0.50 | 0.013 |
| 5 to 6 | 2 | 6.48 ± 1.17 | 30 | 8.09 ± 0.54 | 0.009 |
| 6 to 7 | 7 | 7.24 ±1.2 | 14 | 7.83 ± 0.72 | 0.13 |
| 7 to 8 | 6 | 7.87 ± 0.86 | 18 | 8.33 ± 0.51 | 0.13 |
| 8 to 9 | 4 | 8.39 ± 0.99 | 18 | 8.90 ± 0.88 | 0.19 |
| 9 to 10 | 2 | 7.5 ± 1.77 | 14 | 9.20 ± 0.90 | 0.19 |
| 10 to 11 | 6 | 7.57 ±1.17 | 28 | 9.17 ± 0.82 | 0.008 |
| 11 to 12 | 3 | 7.64 ± 0.67 | 22 | 9.60 ± 0.64 | 0.008 |
| 12 to 13 | 2 | 9.03 ± 0.53 | 18 | 10.42 ± 0.87 | 0.023 |
| 13 to 14 | 5 | 9.12 ± 0.62 | 14 | 9.79 ± 0.75 | 0.039 |
| 14 to 15 | 2 | 8.85 ± 0.07 | 14 | 10.05 ± 0.62 | <0.001 |
| 15 to 16 | * | * | 6 | 10.93 ± 0.76 | * |
| 16 to 17 | 1 | 8.65 | 10 | 10.04 ± 0.86 | * |
| 17 to 18 | 3 | 9.52 ± 0.42 | 4 | 10.53 ± 0.29 | 0.016 |
| 18 to 19 | 1 | 8.8 | 8 | 10.81 ± 1.13 | * |
| TOTAL | 62 | 768 | |||
Figure 1.
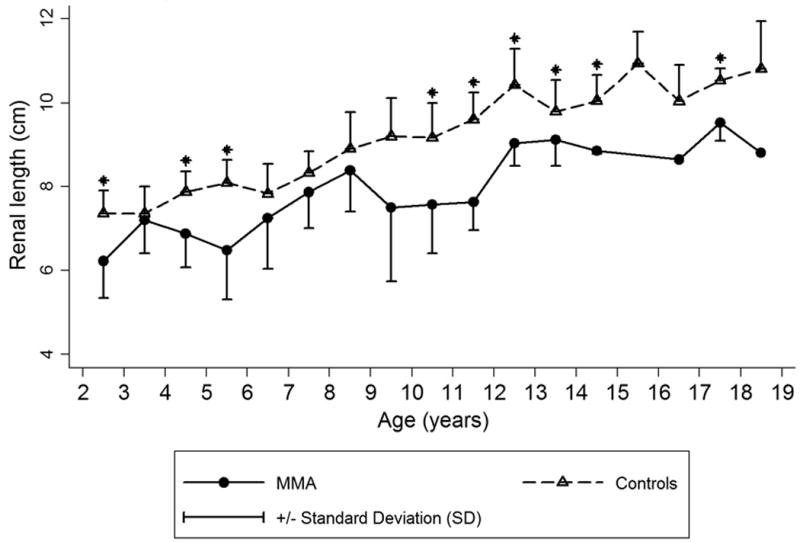
Renal length (average of left and right) vs. age in methylmalonic acidemia (MMA) and age-matched controls26. Mean ± SD. Asterisk denotes p < 0.05.
With height as the independent variable and renal length as the dependent variable, MMA patients had a diminished slope compared to historical controls, indicating that in MMA there is a decreased renal growth rate that is independent of height.28 For this group of published controls, renal length nomograms were developed for left and right kidneys separately.28 The control group equations were: left kidney length (cm) = 0.051 * height (cm) + 1.8, and right kidney length (cm) = 0.050 * height (cm) + 1.8. The MMA patient equations were left kidney length (cm) = 0.033 * height (cm) + 4.0, and right kidney length (cm) = 0.037 * height + 3.0 (p < 0.001 for β coefficients).
Renal length correlations with serum and urine laboratory values
Serum creatinine, MMAS and MMAU were analyzed according to enzymatic subtype and age (Table S3). For the mut and cblA groups, serum creatinine and MMAS increased with age compared to MMAU, which remained unchanged over time. The number of patients in the cblB group was too small to see trends similar to the mut and cblA groups. Table S1 shows that creatinine, MMAs and MMAU significantly (p < 0.05) correlated to age with Pearson's correlation coefficients of 0.503, 0.235, and −0.256, respectively for all enzymatic subtypes. Figure S1 shows serum creatinine, MMAs and MMAU variations with age. In more severely affected patients with a serum methylmalonic acid of 2000 micromolar or greater, serum creatinine rises faster with age [Figure S1(a)]; conversely, MMAs vs. age has a greater slope when correlated with eGFR creatinine greater than 60 mL/min/1.73m2 [Figure S1(b)].
Routine measurement of serum cystatin C began midway through the study and 33 of the 84 ultrasounds having matched serum cystatin C values. Cystatin C (Table S1) correlated closely with serum creatinine and MMAs and had Pearson correlation coefficients of 0.790 and 0.768, respectively. We also analyzed MMAS compared to eGFR creatinine - cystatin C and found that MMAS rises sharply when the eGFR creatinine - cystatin C is below 60 mL/min/1.73m2 (Figure 3).
Figure 3.
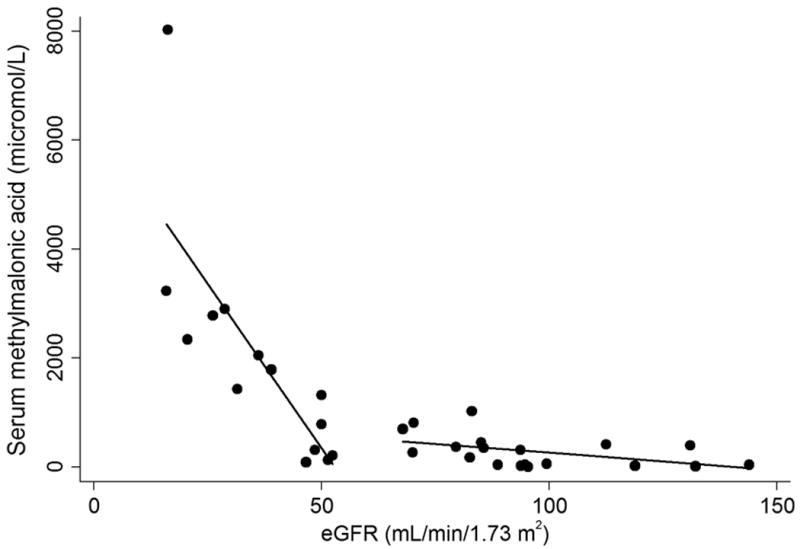
Serum methylmalonic acid (MMAS) for all enzymatic subtypes vs. eGFR creatinine - cystatin C. Best fitted linear regression lines are shown for eGFR ≤ 60 and eGFR > 60. MMAS increases markedly for eGFR values below 60 mL/min/1.73 m2.
Multiple regression modeling
The most predictive multiple regression model for renal length found cystatin C and height to be the optimal independent variables with beta weights of −0.51 and 0.75, respectively. Figure S2 is a representation of this regression model showing that renal length is inversely proportional to cystatin C when stratified by height. The final predictive equation for left kidney length (cm) = 4.9 – 0.92*cystatin C + 0.036*height; for the right kidney, predicted length (cm) = 3.6 – 0.92*cystatin C + 0.043*height. Table S2 compares MMAS, cystatin C, creatinine, and eGFR based multiple regression models stratified by different creatinine levels. The regression model using MMAS was found to be highly predictive of renal length when serum creatinine was below 1.5 (Table S2).
Discussion
Chronic kidney disease due to progressive tubulointerstitial injury is emerging as a frequent manifestation in MMA and eventually results in the need for dialysis or kidney transplantation: half of our cohort of MMA patients of all ages and 100% of our mut0 patients over age 15 either have been transplanted or have an eGFR below 60 mL/min/1.73 m2. Kidney disease in this population is notoriously difficult to diagnose and monitor early in life, as reliance on serum creatinine-based eGFR estimates may over-state the true GFR due to low muscle mass13 and kidney biopsy, the most accurate test for determining the nature and extent of renal disease, is invasive and is rarely offered to MMA patients. Once clinically manifest, renal disease in MMA patients has a relentless progression to kidney failure as is illustrated by the linear decline of eGFR in the patient population, with a faster rate of decline apparent in those with more severe metabolic parameters (Figure 4). The failure to detect chronic kidney disease early is certain to delay the implementation of renoprotective strategies in affected infants and children. Whereas blockade of the renin-angiotensin system (RAS) is the current standard of care for adults with chronic kidney disease, experience with renoprotective measures in the pediatric population has not been as thoroughly studied.3 For example, there has only been one published article addressing renoprotection with RAS blockade in MMA31 despite the formal recognition that such studies should be pursued.32 The successful small molecule treatments of related metabolic disorders that feature tubulointerstitial disease and progressive kidney failure, such as nephropathic cystinosis and tyrosinemia type I,32 and striking preclinical efficacy of systemic AAV gene delivery to treat the kidney disease of MMA in a mouse model provides strong practical and theoretical support for the study of renoprotective interventions in MMA patients.33
Figure 4.
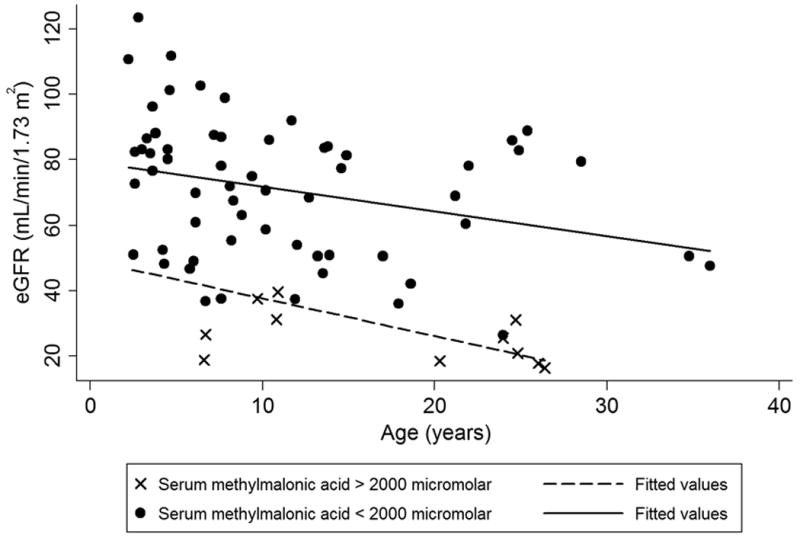
eGFR creatinine for all enzymatic subtype patients declines with age, reflective of a progressive chronic kidney disease. eGFR calculated using the updated Schwartz equation for children.16 For adults, the CKD-EPI creatinine equation was used.17 The solid line represents linear regression for patients with serum methylmalonic acid less than 2000 micromolar and dashed lines represent a linear regression for serum methylmalonic acid greater than 2000. The patients with a higher serum methylmalonic acid show a lower eGFR.
To predict renal length in MMA subjects, we first created a multivariate model and compared our data to healthy controls in the medical literature using age as the independent variable. Renal length was chosen as the dependent variable from other ultrasongraphic measurements such as renal volume and cortical thickness because renal length has the least amount of inter-observer variation and has been established to correlate with GFR.24,34 Compared to published healthy controls, most age groups in the MMA cohort had significantly smaller renal lengths (Table 1). The healthy control group and the MMA group both had similar constants (y-intercept), 6.79 and 6.80 respectively, suggesting that kidney size was normal at birth in the MMA patients; however, the slope (cm/year) of kidney length increase in the MMA group was less than half that of the controls: 0.09 cm/year versus 0.22 cm/year.26 In comparison to another large study of 778 healthy children less than 18 years of age, the MMA kidney growth slope was only a third of the controls: 0.09 cm/year versus 0.3 cm/year.35 These data provide a marker of the natural history of kidney growth in MMA, one that might be used in future studies to examine the effects of renoprotective regimens and perhaps gene therapy on the renal phenotype seen in the patients.
We were also interested in the comparison of MMA patients to healthy control subjects when height was the independent variable, especially because MMA patients have been shown to be shorter than age matched healthy subjects4,36. Although most of the standardized nomograms created for renal length use age as the independent variable, one study of healthy children developed a linear model with height as the independent variable.28 The slope of the nomogram for kidney length vs. height in the MMA patients was less than this particular control group, with right and left kidney MMA slopes of 0.037 cm (renal length)/cm (height) and 0.033 cm/cm, respectively, and control slopes of 0.050 cm/cm and 0.051 cm/cm, respectively. Thus, the comparison of MMA patients to yet another set of healthy children via linear modeling when controlling for height also demonstrated that MMA patients have slower renal growth, even when controlling for height.
Using multivariate modeling, we have found that over 70% of the variability (R2) in kidney length was captured by our model. In order to gauge how our multivariate predictive capability (R2) compared to those previously reported, we demonstrated a predictive capability of an independent multivariate model for a much larger study of 3031 healthy children under the age of 13 which found over 60% of variability was explained by their model (R2's of 0.696 and 0.712 for right and left kidney, respectively).37 Cystatin C and height were the most predictive independent variables in the multiple regression model. Table S2 shows that cystatin C, MMAS, or serum creatinine had similar R2 values when controlling for height and illustrates the significance of these variables as independent predictors of renal length.
Our results establish that for serum creatinine <1.5 mg/dL, MMAS is a stronger predictor of kidney length in the multivariate model than either cystatin C or eGFR creatinine - cystatin C (Table S2). When beta weights are included in the regression analysis, MMAs proves to be a highly significant independent variable with a value of −0.42. The proportional relationship between MMAS and smaller renal size in this study combined with a large beta weight for MMAS suggests that elevated MMAS is strongly associated with renal disease in methylmalonic acidemia when controlling for height by multiple regression. Additionally, Figure 2(a) and Figure 2(b) demonstrate smaller renal size versus age and height, respectively, in patients with serum methylmalonic acid greater than 2000 micromolar. The association between MMAS and renal failure is also evident in Figure 3 where MMAS is inversely related to eGFR creatinine - cystatin C below 60 mL/min/1.73 m2; additionally, all of our patients with a serum methylmalonic acid level above 1000 micromolar have an eGFR creatinine below 60 mL/min/1.73 m2. These observations would advocate for the routine measurement of serum methylmalonic acid concentrations in the longitudinal monitoring of patients with MMA and highlight the need for future studies to examine serum markers concomitant with a measured GFR.
To complement serum creatinine, we obtained cystatin C levels with 33 of the total 84 renal ultrasounds but added this measurement after the protocol began. Using the aggregate data, the multivariate model incorporating cystatin C emerged as the most precise model, explaining over 76% of renal length variation (R2 = 0.767) when controlling for height (see Figure S2). As expected, the regression model using cystatin C was more predictive than the model using GFR estimates calculated from serum creatinine and cystatin C (Table S2), which is most likely due to the muscle mass differences in MMA patients and the patients used to derive the eGFR equation.16,17 Our data suggests that serum cystatin C concentration should also be included in the routine monitoring of renal function in MMA patients and warrants further study, including validation against direct GFR assessments, especially because recent studies suggest that in the general population, GFR estimates that incorporate both serum creatinine and serum cystatin C appear to be the most accurate.18,19 In a population with reduced muscle mass such as MMA, the GFR equation using cystatin C or cystatin C and MMAs may be preferred.
Prior investigations have not consistently demonstrated a relationship between MMAU and kidney disease.5,13 A multicenter, retrospective review of 35 patients suggested that MMAU predicted chronic kidney disease as determined from calculations using the Schwartz formula; however, inter- and intra-individual variations were claimed to contribute to overlap in the enzymatic subgroups of this study.5 In contrast, Walter et al. found no correlation between random urinary methylmalonic acid levels and reduction in GFR.13 We found that the urine methylmalonic acid concentration was not a significant predictor of renal length when controlling for height (p = 0.471): MMAU did not correlate with trends in serum creatinine as can be seen by the statistically insignificant Pearson's correlation in Table S1 (R = 0.0389). Given the poor correlation between MMAU, and renal length or trends in serum creatinine, our data advocates using MMAS as a predictor of renal disease and including this analyte in the routine monitoring of patients with MMA.
The mut patients had slightly smaller kidneys than the other subgroups; however, when controlling for cystatin C and height, we did not detect a significant difference between enzymatic subtypes on kidney length. Another study found an increase in chronic kidney disease in mut0 and cblB compared to other enzymatic subtypes causing isolated MMA, which would suggest that these individuals might have smaller kidneys.5 A possible explanation for this discrepancy between the studies may derive from the small number of participants with cblB; in both our study (n=6) and that of Horster et al. (n=9).5 In addition to MMA subtypes, we did not find gender to be a contributor to renal length in MMA patients. Boys' and girls' body sizes grow at different rates; however, multiple studies, including ours, have demonstrated that renal length is not significantly different between the sexes.30
Our study has a number of limitations. Despite using the largest single center MMA patient cohort described to date, the sample size was comparatively small and we were unable to use a discovery and replication design. Second, the majority of our data was cross-sectional, which may introduce increased variability in the nomograms compared to data derived from a purely longitudinal design. Third, the most severely affected patients may not have been fit for travel to NIH, and thus underestimated our effect size.
In summary, the rate of renal growth in the MMA subjects studied here was one half to one third that observed in controls.26,35 Renal length is likewise significantly decreased compared to normal controls and predicted by a multiple regression model that uses the readily available clinical variables of height and cystatin C. When serum creatinine values fall in the age normal range, MMAS and cystatin C are comparable predictors of renal length, suggesting that both should be included in the routine evaluation in MMA patients. In addition to generating a clinically useful nomogram to follow kidney growth in MMA, our results capture a marker of the natural history of the renal disease of MMA and should assist with the study of therapeutic interventions designed to target kidney disease in this population.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the NHGRI and NIDDK, NIH.
Footnotes
Supplementary information is available at the Genetics in Medicine website
References
- 1.Manoli I, Venditti CP. Methylmalonic Acidemia. 2005 Aug 16 [Updated 2010 Sep 28] In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews™ [Internet] Seattle (WA): University of Washington, Seattle; 1993. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1231/ [PubMed] [Google Scholar]
- 2.Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983 Apr 14;308(15):857–861. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaides P, Leonard J, Surtees R. Neurological outcome of methylmalonic acidaemia. Archives of disease in childhood. 1998 Jun;78(6):508–512. doi: 10.1136/adc.78.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Meer SB, Poggi F, Spada M, et al. Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr. 1994 Dec;125(6 Pt 1):903–908. doi: 10.1016/s0022-3476(05)82005-0. [DOI] [PubMed] [Google Scholar]
- 5.Horster F, Baumgartner MR, Viardot C, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB) Pediatr Res. 2007 Aug;62(2):225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- 6.Cosson MA, Benoist JF, Touati G, et al. Long-term outcome in methylmalonic aciduria: a series of 30 French patients. Mol Genet Metab. 2009 Jul;97(3):172–178. doi: 10.1016/j.ymgme.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Touati G, Valayannopoulos V, Mention K, et al. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. Journal of inherited metabolic disease. 2006 Apr-Jun;29(2-3):288–298. doi: 10.1007/s10545-006-0351-7. [DOI] [PubMed] [Google Scholar]
- 8.Dionisi-Vici C, Deodato F, Röschinger W, Rhead W, Wilcken B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. Journal of inherited metabolic disease. 2006;29(2-3):383–9. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- 9.Van Calcar SC, Harding CO, Lyne P, et al. Renal transplantation in a patient with methylmalonic acidaemia. Journal of inherited metabolic disease. 1998 Oct;21(7):729–737. doi: 10.1023/a:1005493015489. [DOI] [PubMed] [Google Scholar]
- 10.Coman D, Huang J, McTaggart S, et al. Renal transplantation in a 14-year-old girl with vitamin B12-responsive cblA-type methylmalonic acidaemia. Pediatric nephrology. 2006 Feb;21(2):270–273. doi: 10.1007/s00467-005-2071-x. [DOI] [PubMed] [Google Scholar]
- 11.Molteni KH, Oberley TD, Wolff JA, Friedman AL. Progressive renal insufficiency in methylmalonic acidemia. Pediatr Nephrol. 1991 May;5(3):323–326. doi: 10.1007/BF00867492. [DOI] [PubMed] [Google Scholar]
- 12.Rutledge SL, Geraghty M, Mroczek E, Rosenblatt D, Kohout E. Tubulointerstitial nephritis in methylmalonic acidemia. Pediatr Nephrol. 1993 Feb;7(1):81–82. doi: 10.1007/BF00861581. [DOI] [PubMed] [Google Scholar]
- 13.Walter JH, Michalski A, Wilson WM, Leonard JV, Barratt TM, Dillon MJ. Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr. 1989 Jan;148(4):344–348. doi: 10.1007/BF00444131. [DOI] [PubMed] [Google Scholar]
- 14.Oberholzer VG, Levin B, Burgess EA, Young WF. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967 Oct;42(225):492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler RJ, Zerfas PM, Shanske S, et al. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009 Apr;23(4):1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009 Mar;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C--a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101(5):875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 19.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem. 2007 Mar;40(5-6):383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Narvaez-Sanchez R, Gonzalez L, Salamanca A, et al. Cystatin C could be a replacement to serum creatinine for diagnosing and monitoring kidney function in children. Clin Biochem. 2008 May;41(7-8):498–503. doi: 10.1016/j.clinbiochem.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt IM, Main KM, Damgaard IN, et al. Kidney growth in 717 healthy children aged 0-18 months: a longitudinal cohort study. Pediatr Nephrol. 2004 Sep;19(9):992–1003. doi: 10.1007/s00467-004-1479-z. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz-Neira CL, Traubici J, Alan D, et al. Sonographic assessment of renal growth in patients with Beckwith-Wiedemann syndrome: the Beckwith-Wiedemann syndrome renal nomogram. Clinics (Sao Paulo) 2009;64(1):41–44. doi: 10.1590/S1807-59322009000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adibi A, Adibi I, Khosravi P. Do kidney sizes in ultrasonography correlate to glomerular filtration rate in healthy children? Australas Radiol. 2007 Dec;51(6):555–559. doi: 10.1111/j.1440-1673.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 24.Paleologo G, Abdelkawy H, Barsotti M, et al. Kidney dimensions at sonography are correlated with glomerular filtration rate in renal transplant recipients and in kidney donors. Transplant Proc. 2007 Jul-Aug;39(6):1779–1781. doi: 10.1016/j.transproceed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Di Zazzo G, Stringini G, Matteucci MC, Muraca M, Malena S, Emma F. Serum creatinine levels are significantly influenced by renal size in the normal pediatric population. Clin J Am Soc Nephrol. 2011 Jan;6(1):107–113. doi: 10.2215/CJN.00580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum DM, Korngold E, Teele RL. Sonographic assessment of renal length in normal children. AJR Am J Roentgenol. 1984 Mar;142(3):467–469. doi: 10.2214/ajr.142.3.467. [DOI] [PubMed] [Google Scholar]
- 27.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987 Oct 22;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 28.Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol. 1985;15(1):38–43. doi: 10.1007/BF02387851. [DOI] [PubMed] [Google Scholar]
- 29.Stoltzfus JC. Logistic regression: a brief primer. Acad Emerg Med. 2011 Oct;18(10):1099–1104. doi: 10.1111/j.1553-2712.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 30.Zerin JM, Blane CE. Sonographic assessment of renal length in children: a reappraisal. Pediatr Radiol. 1994;24(2):101–106. doi: 10.1007/BF02020164. [DOI] [PubMed] [Google Scholar]
- 31.Ha TS, Lee JS, Hong EJ. Delay of renal progression in methylmalonic acidemia using angiotensin II inhibition: a case report. J Nephrol. 2008 Sep-Oct;21(5):793–796. [PubMed] [Google Scholar]
- 32.Foreman JW. Cystinosis and Fanconi Syndrome. In: Avner ED, Harmon W, Niaudet P, editors. Pediatric nephrology. 5th. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 789–806. [Google Scholar]
- 33.Senac JS, Chandler RJ, Sysol JR, Li L, Venditti CP. Gene therapy in a murine model of methylmalonic acidemia using rAAV9-mediated gene delivery. Gene Ther. 2012 Apr;19(4):385–391. doi: 10.1038/gt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger AE, Hernandez RJ, Zerin JM, Marks TI, Kelsch RC. Interobserver and intraobserver variations in sonographic renal length measurements in children. AJR Am J Roentgenol. 1991 May;156(5):1029–1032. doi: 10.2214/ajr.156.5.2017927. [DOI] [PubMed] [Google Scholar]
- 35.Akhavan A, Brajtbord JS, McLeod DJ, Kabarriti AE, Rosenberg HK, Stock JA. Simple, age-based formula for predicting renal length in children. Urology. 2011 Aug;78(2):405–410. doi: 10.1016/j.urology.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Hauser NS, Manoli I, Graf JC, Sloan J, Venditti CP. Variable dietary management of methylmalonic acidemia: metabolic and energetic correlations. Am J Clin Nutr. 2011 Jan;93(1):47–56. doi: 10.3945/ajcn.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luk WH, Lo AX, Au-Yeung AW, et al. Renal length nomogram in Hong Kong Asian children: sonographic measurement and multivariable approach. J Paediatr Child Health. 2010 Jun;46(6):310–315. doi: 10.1111/j.1440-1754.2010.01714.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


