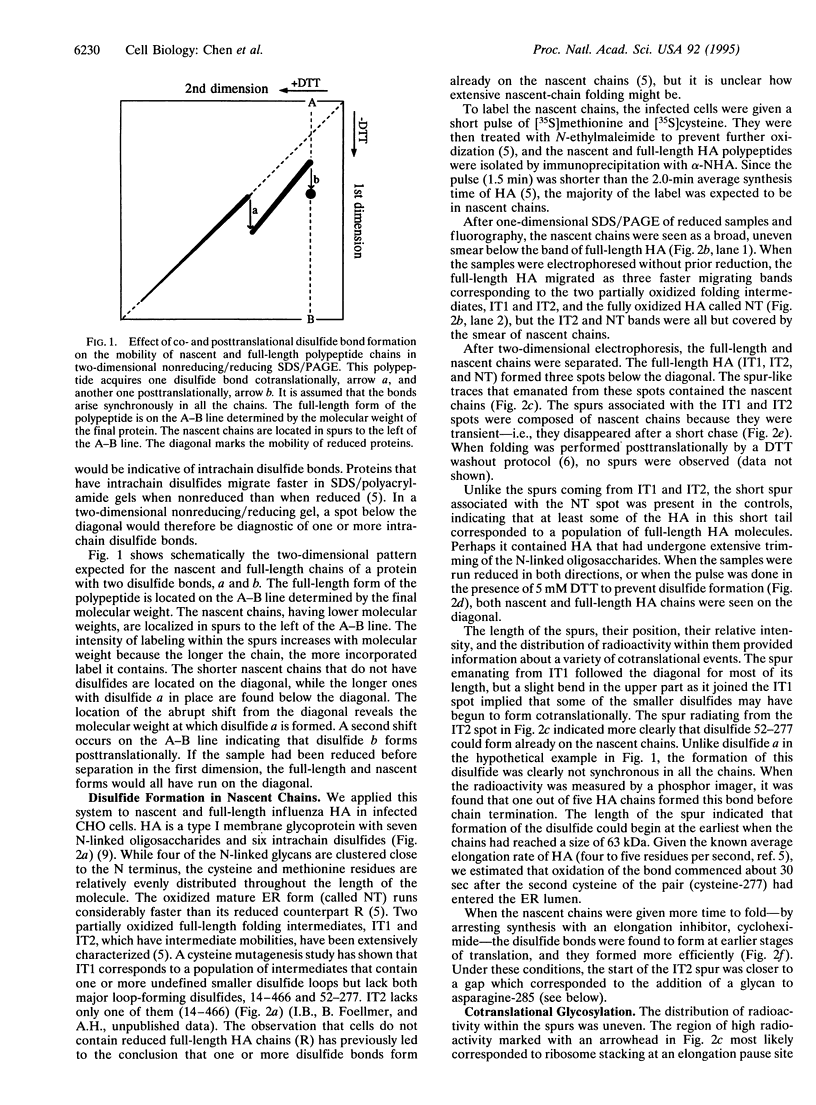
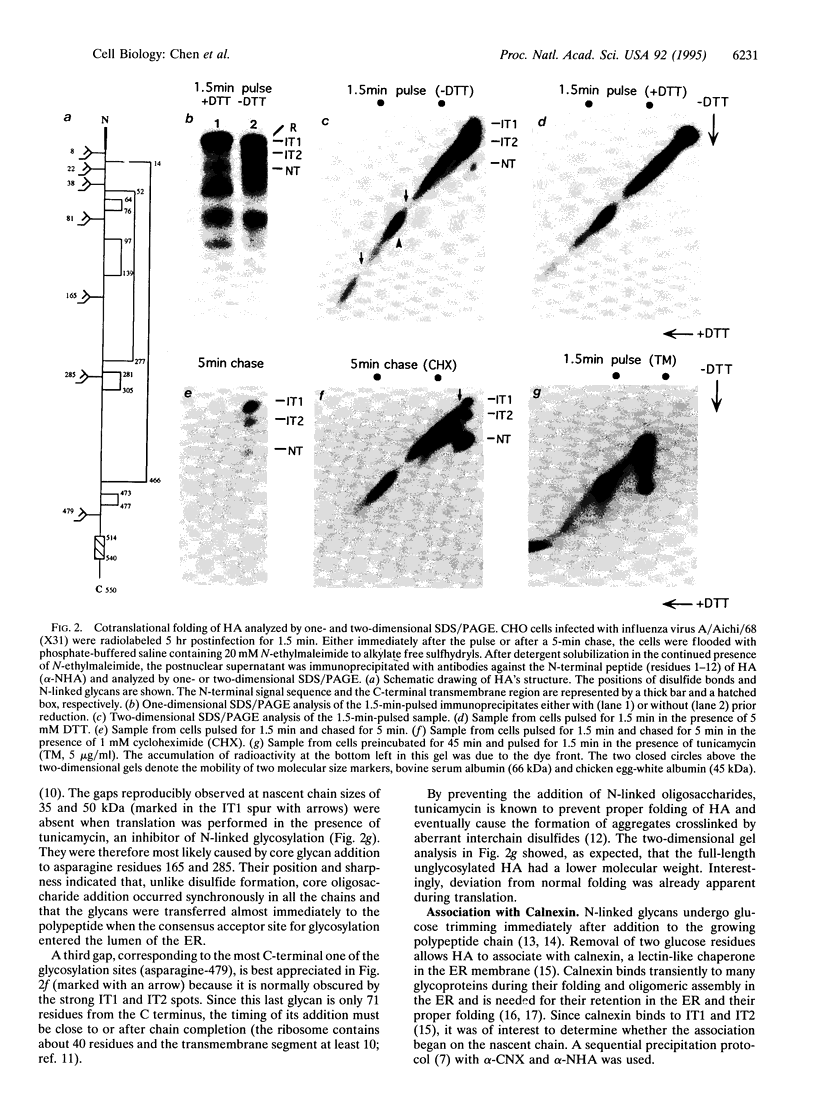
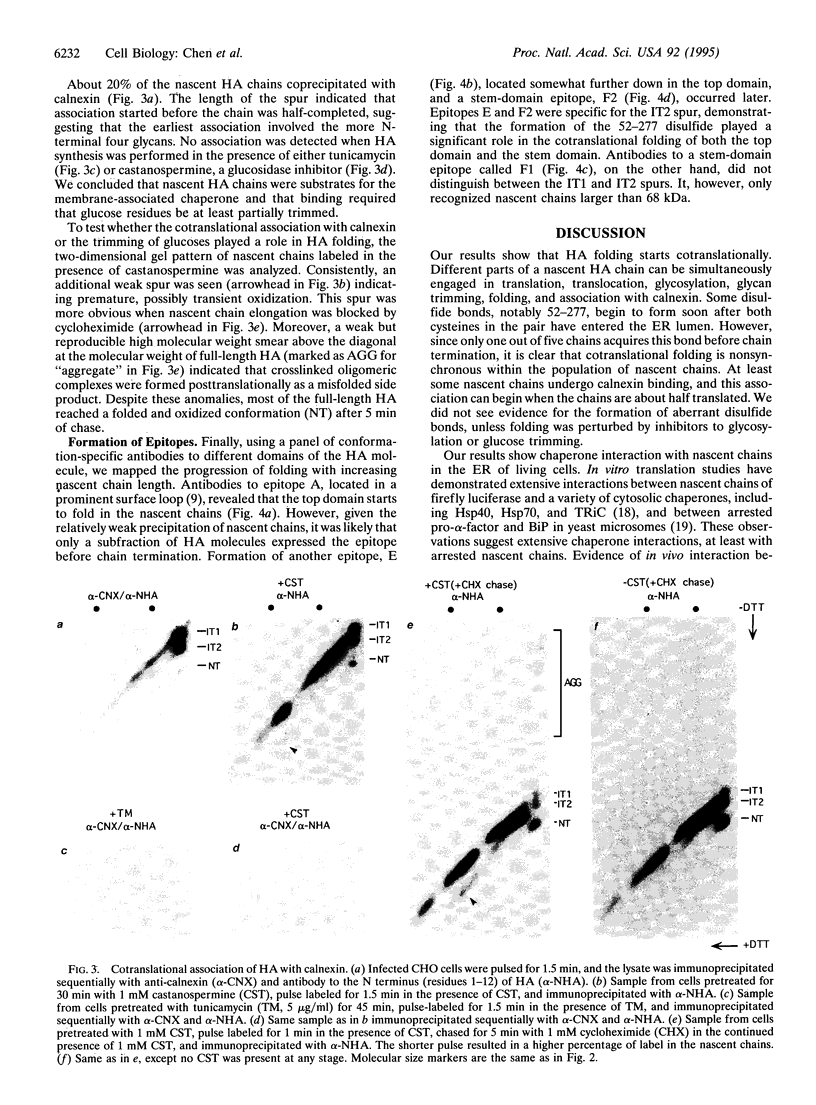
Abstract
To analyze cotranslational folding of influenza hemagglutinin in the endoplasmic reticulum of live cells, we used short pulses of radiolabeling followed by immunoprecipitation and analysis with a two-dimensional SDS/polyacrylamide gel system which was nonreducing in the first dimension and reducing in the second. It separated nascent glycopolypeptides of different length and oxidation state. Evidence was obtained for cotranslational disulfide formation, generation of conformational epitopes, N-linked glycosylation, and oligosaccharide-dependent binding of calnexin, a membrane-bound chaperone that binds to incompletely folded glycoproteins via partially glucose-trimmed oligosaccharides. When glycosylation or oligosaccharide trimming was inhibited, the folding pathway was perturbed, suggesting a role for N-linked oligosaccharides and calnexin during translation of hemagglutinin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Lee J. T. Co-translational excision of alpha-glucose and alpha-mannose in nascent vesicular stomatitis virus G protein. J Cell Biol. 1984 Jun;98(6):2245–2249. doi: 10.1083/jcb.98.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Brenner M. B., Thomas D. Y., Williams D. B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994 Mar;19(3):124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Formation of an intrachain disulfide bond on nascent immunoglobulin light chains. J Biol Chem. 1979 Sep 25;254(18):8869–8876. [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Hoover-Litty H., Wagner K. R., Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991 Aug;114(3):401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Ohtsuka K., Hartl F. U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994 Jul 14;370(6485):111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R. Protein translocation across the endoplasmic reticulum: a tunnel with toll booths at entry and exit. Cell. 1993 Nov 19;75(4):589–592. doi: 10.1016/0092-8674(93)90476-7. [DOI] [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994 Oct 21;266(5184):456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Klausner R. D. Architectural editing: determining the fate of newly synthesized membrane proteins. New Biol. 1989 Oct;1(1):3–8. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Davidson L. K. The biosynthesis of rat serum albumin. In vivo studies on the formation of the disulfide bonds. J Biol Chem. 1982 Aug 10;257(15):8847–8853. [PubMed] [Google Scholar]
- Protzel A., Morris A. J. Gel chromatographic analysis of nascent globin chains. Evidence of nonuniform size distribution. J Biol Chem. 1974 Jul 25;249(14):4594–4600. [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Interaction of secreted nascent chains with surrounding membrane in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5922–5925. doi: 10.1073/pnas.75.12.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Diagonal polyacrylamide-dodecyl sulfate gel electrophoresis for the identification of ribosomal proteins crosslinked with methyl-4-mercaptobutyrimidate. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3946–3950. doi: 10.1073/pnas.71.10.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu U., Braakman I., Helenius A. Membrane glycoprotein folding, oligomerization and intracellular transport: effects of dithiothreitol in living cells. EMBO J. 1993 May;12(5):2151–2157. doi: 10.1002/j.1460-2075.1993.tb05863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]






