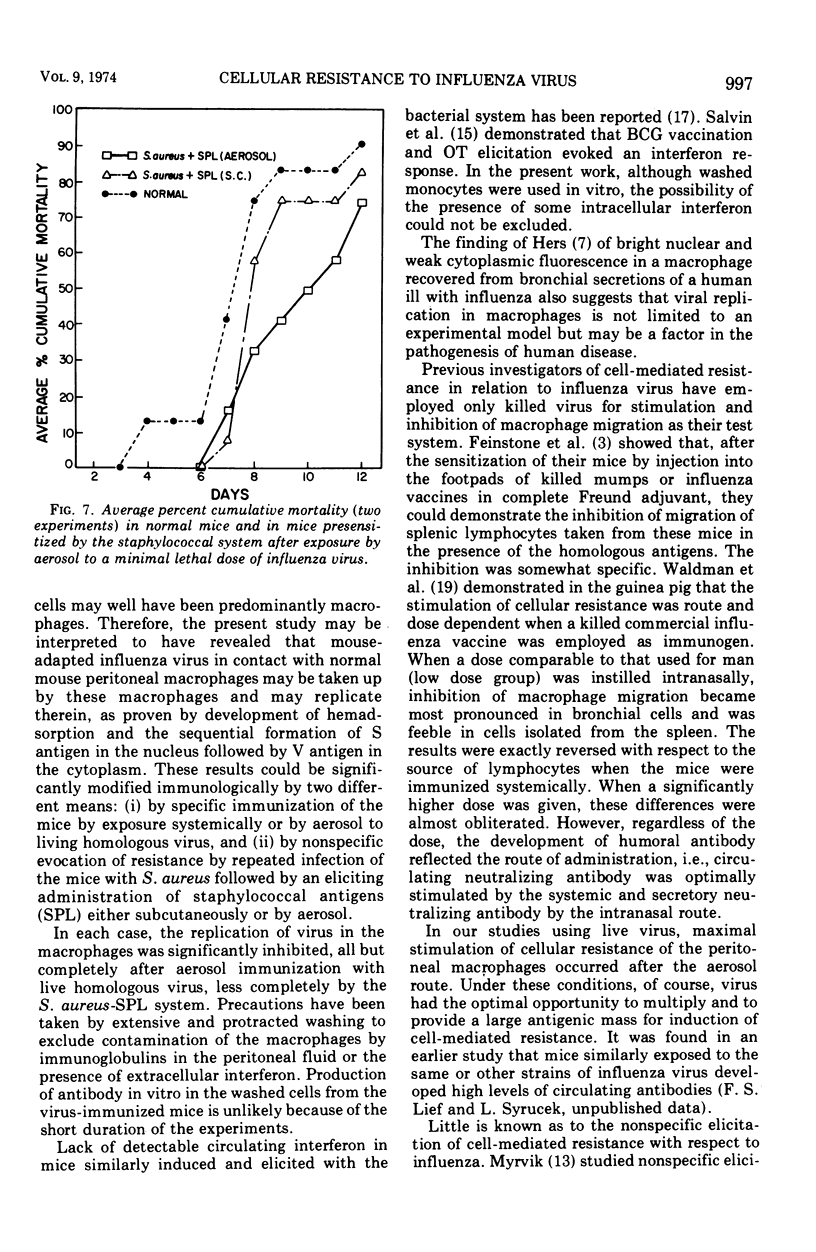
Abstract
We found that influenza virus had the capacity to replicate in the peritoneal macrophages of normal mice, as revealed by the development of hemadsorption and the appearance intracellularly of S and V antigens. Cell-mediated resistance was studied in mice infected with influenza virus or a bacterial sytem of induction-elicitation. In the homologous system, mice were injected intraperitoneally or exposed by aerosol to a sublethal dose of an egg-adapted swine strain of influenza virus. In the heterologous system, they were infected repeatedly with Staphylococcus aureus and elicited by subcutaneous or aerosol administration of staphylococcal antigens. The peritoneal macrophages from mice specifically or nonspecifically immunized were significantly more resistant than those from normal mice. Also longer survival to in vivo challenge by the mouse-adapted virus, as compared with normal mice, was indicated in bacterially stimulated mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. G., Mudd S. Protection of mice against vaccinia virus by bacterial infection and sustained stimulation with specific bacterial antigens. Infect Immun. 1973 Jan;7(1):62–67. doi: 10.1128/iai.7.1.62-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstone S. M., Beachey E. H., Rytel M. W. Induction of delayed hypersensitivity to influenza and mumps viruses in mice. J Immunol. 1969 Oct;103(4):844–849. [PubMed] [Google Scholar]
- Glasgow L. A. Cellular immunity in host resistance to viral infections. Arch Intern Med. 1970 Jul;126(1):125–134. [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- HERS J. F. FLUORESCENT ANTIBODY TECHNIQUE IN RESPIRATORY VIRAL DISEASES. Am Rev Respir Dis. 1963 Sep;88:SUPPL–338. doi: 10.1164/arrd.1963.88.3P2.316. [DOI] [PubMed] [Google Scholar]
- KAPRAL F. A., SHAYEGANI M. G. Intracellular survival of staphylococci. J Exp Med. 1959 Jul 1;110(1):123–138. doi: 10.1084/jem.110.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIFE F. S., HENLE W. Methods and procedures for use of complement-fixation technique in type- and strain-specific diagnosis of influenza. Bull World Health Organ. 1959;20(2-3):411–420. [PMC free article] [PubMed] [Google Scholar]
- LOEFFLER H., HENLE G., HENLE W. Attempts to influence the incomplete reproductive cycle of influenza virus in HeLa cells by antibodies. J Immunol. 1962 Jun;88:763–776. [PubMed] [Google Scholar]
- Myrvik Q. N. Function of the alveolar macrophage in immunity. J Reticuloendothel Soc. 1972 May;11(5):459–468. [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., De Courcy S. J., Jr, Mudd S. Cell-mediated immunity in mice infected with S. aureus and elicited with specific bacterial antigens. J Reticuloendothel Soc. 1973 Jul;14(1):44–51. [PubMed] [Google Scholar]
- Shayegani M., Mudd S. Lack of detectable circulating interferon in mice protected against vaccinia virus by induction and elicitation with bacterial systems. Infect Immun. 1973 Jan;7(1):117–118. doi: 10.1128/iai.7.1.117-118.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Dannenberg A. M., Jr, Ando M., Chandrasekhar S., Seluzicki J. A., Fabrikant J. I. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. I. Studies involving their incorporation of tritiated thymidine and their content of lysosomal enzymes and bacilli. Am J Pathol. 1972 Apr;67(1):159–180. [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H., COONS A. H. Fluorescent antibody studies with agents of varicella and herpes zoster propagated in vitro. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):789–794. doi: 10.3181/00379727-86-21235. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]