Summary
Horse anti-thymocyte globulin (h-ATG) and cyclosporine are the initial therapy for most patients with severe aplastic anaemia (SAA), but there is no practical and reliable method to predict response to this treatment. To determine whether pre-treatment blood counts discriminate patients with SAA who have a higher likelihood of haematological response at 6 months to immunosuppressive therapy (IST), we conducted a single institution retrospective analysis on 316 SAA patients treated with h-ATG-based IST from 1989 to 2005. In multivariate analysis, younger age, higher baseline absolute reticulocyte count (ARC), and absolute lymphocyte count (ALC) were highly predictive of response at 6 months. Patients with baseline ARC ≥ 25 × 109 /l and ALC ≥ 1 × 109 /l had a much greater probability of response at 6 months following IST compared to those with lower ARC and ALC (83% vs. 41%, respectively; p < 0.001). This higher likelihood of response translated to greater rate of 5-year survival in patients in the high ARC/ALC group (92%) compared to those with a low ARC/ALC (53%). In the era of IST, the baseline ARC and ALC together serve as a simple predictor of response following IST, which should guide in risk stratification among patients with SAA.
Keywords: Aplastic anaemia, antibody therapy, bone marrow failure
Introduction
Severe aplastic anaemia (SAA) can be successfully treated with haematopoietic stem cell transplantation (HSCT) or anti-thymocyte globulin (ATG)-based immunosuppression. Prior to the introduction of immunosuppressive treatment (IST), non-transplant options for SAA included androgens and transfusions (Furuhjelm and Eklund 1966, Sanchez-Medal, et al 1969). HSCT could be performed in patients with a human leucocyte antigen (HLA)-matched sibling donor. The introduction of ATG in the late 1970's and the addition of cyclosporine (CsA) to ATG in the 1980's led to a significant improvement in haematopoietic recovery and better survival in patients with SAA (Frickhofen, et al 1991, Gluckman, et al 1978). In the majority of cases, IST is often used first since most patients are not suitable candidates for HSCT due to age, comorbidities, or lack of a histocompatible sibling donor. The current standard immunosuppressive regimen is the combination of horse ATG (h-ATG) + CsA (Frickhofen and Rosenfeld 2000).
SAA has been pathophysiologically characterized as T-cell mediated organ-specific destruction of the haematopoietic stem cell compartment (Young, et al 2006). Early experiments showed that patient's lymphocytes suppressed haematopoiesis by activation of cytotoxic T cells expressing TH1 cytokines (interferon-γ and tumour necrosis factor) resulting in apoptosis of CD34+ progenitor cells, at least partially by expression of the Fas receptor and resulting in immune-mediated cytotoxicity (Maciejewski, et al 1995a, Maciejewski, et al 1995b). More recently, oligoclonal T-cell expansions have been described in SAA patients that diminish or disappear following successful IST (Risitano, et al 2004). However, despite the better understanding of the immune pathophysiology of SAA, response to IST cannot be routinely predicted.
We conducted a retrospective analysis in 316 patients with SAA who were treated with h-ATG-based IST at the National Institutes of Health (NIH) since 1989. Here we report for the first time that the combination of pre-treatment absolute reticulocyte count (ARC) and absolute lymphocyte count (ALC) is highly predictive of response and survival in SAA patients treated with a h-ATG based regimen.
Patient and methods
Patients who fulfilled entry criteria for SAA were enrolled in four different treatment protocols from November 1989 to April 2005 at the Warren Grant Magnuson Clinical Center and the Mark O. Hatfield Clinical Research Center at the National Institutes of Health in Bethesda, MD. Consecutive patients treated in these four sequential immunosuppression protocols were analyzed. All adult patients or parents (or legal guardian) of children (< 18 years of age) signed informed consent according the Institutional Review Board of the National, Heart, Lung, and Blood Institute. For protocol entry purposes, SAA was defined as a bone marrow cellularity of less than 30% and severe pancytopenia with at least two of the following peripheral blood count criteria: 1) absolute neutrophil count (ANC) < 0.5 × 109 /l; 2) ARC < 60 × 109 /l; 3) platelet count < 20 × 109 /l (Rosenfeld, et al 2003, Rosenfeld, et al 1995). Response was defined as no longer meeting criteria for SAA and was determined at 3 and 6 months following ATG (Rosenfeld, et al 2003, Rosenfeld, et al 1995, Scheinberg, et al 2006a). In this paper the haematological response at 6 months following initial h-ATG was adopted as the criteria for haematological recovery.
Bone marrow biopsy and aspiration, for morphology and cytogenetics, were performed before enrolment. Children and young adults (< 40 years of age) had chromosomes assayed after in vitro exposure of peripheral blood lymphocytes to diepoxybutane and in some cases also to mitomycin C to exclude Fanconi anaemia. All patients were tested for paroxysmal nocturnal haemoglobinuria (PNH) with the Ham test to 2000, when it was replaced by a flow cytometric assay (Dunn, et al 1999). The presence of a clone using flow cytometry was defined as the absence of glycosylphosphatidylinositol (GPI)-anchored surface proteins greater than 1% of neutrophils or red cells, and the size of the clone was defined by the highest level of red blood cells or neutrophils lacking GPI-anchored proteins.
Study design and treatment regimens
Three h-ATG-based regimens were included in the analysis: standard h-ATG/CsA, h-ATG/CsA/mycophenolate mofetil (MMF) and h-ATG/CsA/sirolimus. H-ATG (ATGAM, Pharmacia & Upjohn Company, Kalamazoo, Mich), CsA, and prednisone (the last for serum sickness prophylaxis) administration was the same for the 3 regimens and has been described previously (Rosenfeld, et al 1995, Scheinberg, et al 2006a). CsA was discontinued after 6 months in all but 37 patients who received h-ATG/CsA, which was followed by a slow CsA taper in the subsequent 18 months. MMF was dosed on the first day of ATG at 600 mg/m2 twice daily for children 11 years and younger and at 1 g twice daily for children 12 or over and adults, for a total of 18 months (Scheinberg, et al 2006a). Sirolimus was initiated on day 1 at 2 mg/d in adults and 1 mg/m2/d in children (< 40 kg) and administered for 6 months to a trough between 5 – 15 ng/ml.
Baseline counts
The variables included in the analysis were age, PNH and results from an automated complete haemogram. The haemoglobin level (and the mean corpuscular volume) were found to be unreliable due to variability in the threshold for packed red blood cell transfusion, the individual response to transfusion, and the influence of concomitant PNH clone. In order to account for biological and instrument variations and to minimize the effects of haematopoietic growth factors and transfusions, the lowest value during the 4 weeks preceding h-ATG were defined as “baseline” and the variables included were the ARC, ANC, ALC and the platelet count.
Statistical methods
Summary statistics including means, proportions and their corresponding standard deviations have been used to describe patients' age, sex, and other baseline characteristics. P-values based on multi-sample tests for proportions and the analysis of variance F-tests were used to compare patients' baseline characteristics across the treatment groups. Sample proportions and their 95% confidence intervals were used to describe the 6-month response rates for patients categorized by discrete risk factors. Multi-sample tests for proportions were employed to compare the 6-month response rates for patients in different risk groups. For the purpose of statistical analysis, patients who did not complete 6 months of initial IST due to death, HSCT or who underwent a second course of IST were counted as non-responders, and those who underwent a second course of IST or HSCT prior to 6 months following initial IST were counted as “dead” on the short term survival (≤ 6 months) analysis. Univariate and multivariate logistic regression models were used to evaluate the effects of continuous baseline risks on the response probabilities and the probabilities of overall survival at 6 months. Since many of these risk variables, such as ARC, ALC, and ANC, are highly skewed and may have values at zero, natural log transformations log (X+1) for the observed covariates X served as the independent variables for the logistic models. Important covariates in the multivariate logistic regression models were chosen by the stepwise variable selection procedures.
For the purpose of presenting a simple picture on the effects of these covariates on the probabilities of response at 6 months and survival, we also considered categorical variables for ARC (< 25 × 109 /l, ≥ 25 × 109 /l), ALC (< 1 × 109 /l, ≥ 1 × 109 /l), ANC (< 0.2 × 109 /l, ≥ 0.2 × 109 /l) and platelet count (< 10 × 109 /l, ≥ 10 × 109 /l), and evaluated the estimated probabilities of response at 6 months and survival probabilities under these categorical covariates. These threshold values were chosen after examining both the parametric and nonparametric estimated probability curves of 6-month response and survival with continuous covariates. The parametric and nonparametric regression analyses suggested that the selected threshold values, while subjective, represented a simple parameter to demonstrate the overall effects of these variables, and potentially useful to guide clinical practice. We also examined various other thresholds using classification-tree regression (S-PLUS 8, Insightful Inc., Seattle, WA, USA) and found that our threshold choices were reasonably balanced between statistical accuracy, clinical relevance and simplicity. Survival probabilities for all patients with discrete and continuous baseline risks were evaluated using the Kaplan-Meier estimates and the Cox proportional hazard models with patients who underwent HSCT or lost to follow-up counted as censored. A P-value < 0.05 was considered to be statistically significant. The numerical results were computed by S-PLUS statistical package (Insightful Inc.).
Results
A total of 346 patients received IST for SAA at our institution between 1989 and 2005 on four different treatment protocols where three regimens were studied: h-ATG/CsA (used in two separate protocols), h-ATG/CsA plus MMF (Scheinberg, et al 2006a), and h-ATG/CsA plus sirolimus [our unpublished data]. These regimens yielded virtually identical outcomes and were therefore combined for this analysis. Only patients who received a h-ATG/CsA based regimen as their initial therapy were included; patients who received h-ATG/CsA as a second course, h-ATG alone without CsA or other investigational agent were excluded. A total of 316 patients who were treated with an initial course of h-ATG/CsA were analyzed for predictive characteristics. At 6 months following IST, 286 patients were evaluable for response; 25 patients had died and 5 patients received alternative therapies prior to completing 6 months from initial h-ATG due to worsening pancytopenia and clinical deterioration (three received a second course of IST and two underwent HSCT). The number of patients in each treatment regimen and the baseline characteristics of the 316-patient cohort are shown in Table 1. A correlation analysis between all the variables showed that the correlation coefficient was less than 0.2 between all the parameters included in the analysis with the exception of a weak association between the baseline ANC and ARC (0.483).
Table 1. Patient characteristics.
| All patients | H-ATG/CsA | H-ATG/CsA/MMF | H-ATG/CsA/Rapa | F-test P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median (25-75 IQ) |
N (%) | Median (25-75 IQ) |
N (%) | Median (25-75 IQ) |
N (%) | Median (25-75 IQ) |
||
| Total | 316 | 177 | 104 | 35 | |||||
| Age | 31 (18, 52) | 31 (18, 53) | 30 (19, 36) | 26 (17, 45) | 0.532 | ||||
| Sex (%) | |||||||||
| Male | 185 (59) | ----- | 100 (57) | (56.5) | 64 (62) | (61.5) | 21 | (60.0) | 0.700 |
| Female | 131 (41) | ----- | 77 | (43.5) | 40 | (38.5) | 14 | (40.0) | |
| Aetiology (%) | |||||||||
| Idiopathic | 295 (93) | ----- | 165 | (93.2) | 95 | (91.3) | 35 | (100) | 0.206 |
| Post-hepatitis | 21 (7) | ----- | 12 | (6.8) | 9 | (8.7) | 0 | ------ | |
| Baseline (× 109 /l) | |||||||||
| ANC | 0.264 (0.088, 0.469) | 0.250 (0.080, 0.449) | 0.289 (0.087, 0.461) | 0.340 (0.122, 0.563) | 0.608 | ||||
| ALC | 1.224 (0.785, 1.632) |
1.224 (0.749, 1.633) |
1.248 (0.781, 1.643) |
1.211 (0.969, 1.441) |
0.563 | ||||
| ARC | 13.3 (4.915, 28.150) |
12.250 (5.200, 28.050) |
14.121 (4.882, 30) |
13.400 (3.750, 27) |
0.733 | ||||
| Platelet count | 9 (5, 14) | 9 (5, 14) | 9 (6, 13.250) | 7 (5, 11) | 0.141 | ||||
| PNH (%) | |||||||||
| <1% | 112 (63.3) | ----- | 25 (59.5) | ----- | 60 (60) | ----- | 27 (77.1) | ----- | 0.166 |
| ≥1% | 65 (36.7) | ----- | 17 (40.5) | ----- | 40 (40) | ----- | 8 (22.9) | ----- | |
H-ATG/CsA, horse anti-thymocyte globulin + cyclosporine; H-ATG/CsA/MMF, horse anti-thymocyte globulin + cyclosporine + mycophenolate mofetil; H-ATG/CsA/Rapa, horse anti-thymocyte globulin + cyclosporine + sirolimus; ARC, absolute reticulocyte count; ALC, absolute lymphocyte count, ANC, absolute neutrophil count; PNH, paroxysmal nocturnal hemoglobinuria; IST, immunosuppressive therapy.; SD, standard deviation; 25-75 IQ, 25-75% interquartile range. Results of Ham test prior to 2000 are not shown; only the detection of a PNH clone by flow cytometry as described in the Methods is shown. A PNH clone cut-off of 1% is shown; a lower cut-off value was not used since the majority of the patients in our cohort had a PNH clone by flow cytometry as the threshold of detection decreased to less than 1%.
Baseline blood count values correlate to response at 6 months
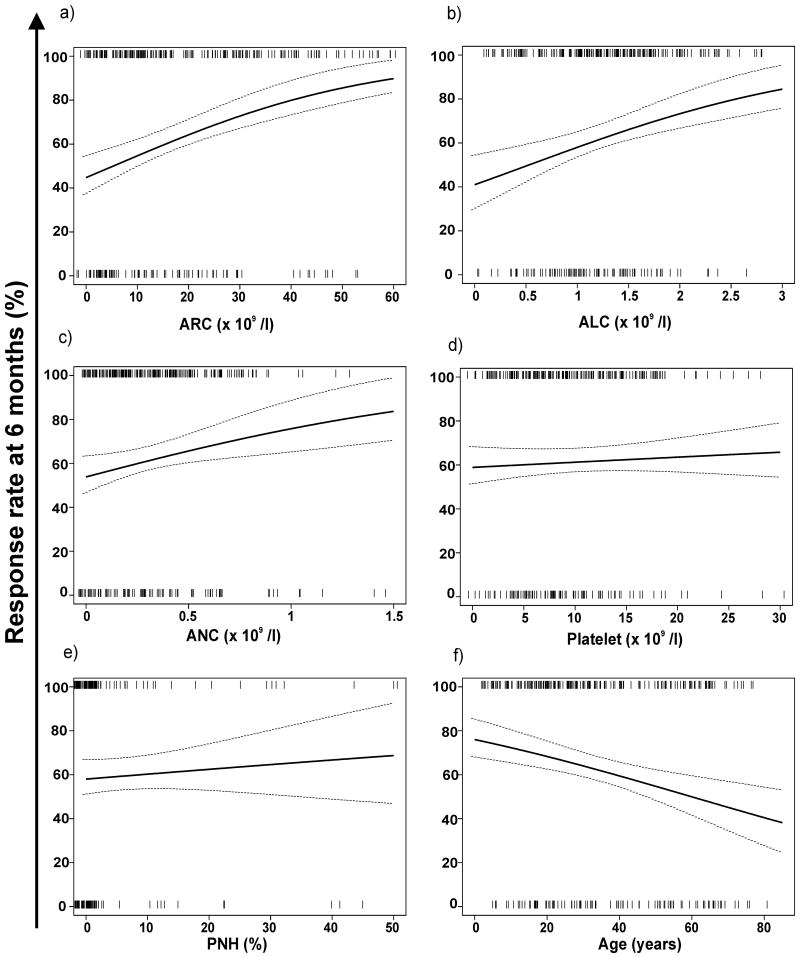
In univariate logistical regression analysis, younger age, higher ARC, ALC, and ANC correlated to response at 6 months. Neither the presence of a PNH clone nor the platelet count was predictive (Table 2). The continuous analysis of the graphical representation of the predicted 6-month response rate versus the baseline ARC, ALC, ANC, platelet count, and PNH is shown in Figure 1. Patients with a lower ARC and ALC had a lower probability of responding to IST compared to those with higher baseline values (Figs. 1A, B). The relationship between response and the baseline ANC was present but not as strong as with the ARC and ALC (Fig. 1C) and no significant relationship was observed with the baseline platelet count and the presence or size of the PNH clone (Figs. 1D, 1E). There was an inverse relationship between response and age, younger patients having a higher probability of response compared to older patients (Fig. 1F). A categorical risk factor analysis showed that patients with an ARC ≥ 25 × 109 /l, ALC ≥ 1 × 109 /l, ANC ≥ 0.2 × 109 /l and age younger than 18 had a higher probability of response at 6 months (Table 3).
Table 2. Univariate and multivariate logistic regression analysis of continuous risk factors on the response rate at 6 months.
| Univariate Logistic Model | Multivariate Logistic Model* | |||||
|---|---|---|---|---|---|---|
| Baseline risk | Coefficient (β) | SD | P-value | Coefficient (β) | SD | P-value |
| Log (ARC+1) | 0.4708 | 0.1077 | < 0.0001 | 0.4480 | 0.110 | < 0.0001 |
| Log (ALC+1) | 0.4852 | 0.1744 | 0.0054 | 0.3999 | 0.1870 | 0.0325 |
| Log(ANC+1) | 0.3559 | 0.0802 | < 0.0001 | ------ | ------ | ------ |
| Log (Plt+1) | 0.1083 | 0.1460 | 0.4581 | ------ | ------ | ------ |
| Log (PNH+1) | 0.0888 | 0.1324 | 0.5022 | ------ | ------ | ------ |
| Log (Age+1) | -0.6571 | 0.1866 | 0.0004 | -0.7386 | 0.1968 | < 0.0001 |
Log transformed variables are used
Model with variables selected by the stepwise procedure.
---- Variable deleted by the stepwise procedure.
ARC, absolute reticulocyte count; ALC, absolute lymphocyte count, ANC, absolute neutrophil count; PNH, paroxysmal nocturnal haemoglobinuria
Figure 1.
Baseline peripheral blood count values are plotted against the estimated probability of response based on univariate logistic regression. A positive correlation was observed between the absolute reticulocyte count (ARC), absolute lymphocyte count (ALC), absolute neutrophil count (ANC) and response at 6 months. There was no significant correlation between the baseline platelet count or the PNH clone size and response. Age correlated inversely with the probability of response at 6 months. The vertical bars “|” represent the covariate values (jittered for PNH to separate multiple subjects with the same values) for responders (top) and nonresponders (bottom). The dotted lines represent the 95% pointwise confidence intervals.
Table 3. Univariate analysis of response rate at 6 months.
| Number of patients (%) | Response at 6 months | P-value | |||
|---|---|---|---|---|---|
| Number | Percent | 95% CI | |||
| H-ATG* | 316 (100) | 194 | 61.4 | (56.0, 66.8) | ------- |
|
| |||||
| Univariate Baseline Risk | |||||
|
| |||||
| ARC (× 109 /l) | |||||
|
| |||||
| ≥ 25 | 96 (30) | 77 | 80.2 | (72.1, 88.3) | < 0.0001 |
|
| |||||
| < 25 | 220 (70) | 117 | 53.2 | (46.5, 59.8) | |
|
| |||||
| ALC (× 109 /l) | |||||
|
| |||||
| ≥ 1 | 200 (63) | 139 | 69.5 | (63.1, 75.9) | 0.0001 |
|
| |||||
| < 1 | 116 (37) | 55 | 47.4 | (38.2, 56.6) | |
|
| |||||
| ANC (× 109 /l) | |||||
| ≥ 0.2 | 188 (59) | 128 | 68.1 | (61.4, 74.8) | 0.0030 |
|
| |||||
| < 0.2 | 128 (41) | 66 | 51.6 | (42.8, 60.3) | |
|
| |||||
| Platelet (× 109 /l) | |||||
|
| |||||
| ≥ 10 | 130 (41) | 85 | 65.4 | (57.1, 73.7) | 0.2243 |
|
| |||||
| < 10 | 186 (59) | 109 | 58.6 | (51.5, 65.7) | |
|
| |||||
| PNH | |||||
|
| |||||
| ≥ 1% | 65 | 39 | 60.6 | (47.8, 72.2) | 0.8895 |
|
| |||||
| < 1% | 112 | 66 | 58.9 | (49.7, 68.2) | |
|
| |||||
| Age (years) | |||||
|
| |||||
| < 18 | 78 (25) | 58 | 74.4 | (64.5, 84.3) | 0.0199 |
|
| |||||
| 18 to 60 | 187 (59) | 109 | 58.3 | (51.2, 65.4) | |
|
| |||||
| > 60 | 51 (16) | 27 | 52.9 | (38.8, 67.1) | |
Includes all regimens which are based on horse anti-thymocyte globulin (H-ATG)
ARC, absolute reticulocyte count; ALC, absolute lymphocyte count, ANC, absolute neutrophil count; PNH, paroxysmal nocturnal haemoglobinuria
In multivariate analysis, only younger age, ARC, and ALC were predictive of response at 6 months (Table 2). The contribution of the baseline ANC to lack of response primarily resulted from more early deaths (counted as non-responders) following ATG in patients with a very low ANC: 23 out of 25 patients (82%) who died during this period had a baseline ANC < 0.2 × 109 /l. When data from the 286 patients who were evaluable at 6 months were subjected to multivariate analysis, only pre-treatment baseline ARC and ALC (p=0.005 and 0.036, respectively) were predictive of response, along with younger age (p=0.018). Baseline ANC did not predict response in these 286 patients, consistent with the expected impact of low pre-treatment ANC on short-term survival (≤ 6 months).
When the two predictive baseline blood count parameters of ARC and ALC were combined in multivariate analysis, patients with an ARC ≥ 25 × 109 /l and an ALC ≥ 1 × 109 /l had a response rate 40% higher compared to those with baseline ARC < 25 × 109 /l and ALC < 1 × 109 /l (83% vs. 41%, respectively) (Table 4) (The increase in likelihood of response was observed at several other cut-off values other than 25 × 109 /l for ARC and 1 × 109 /l for the ALC). Overall, probability of response was higher in patients with an ARC ≥ 25 × 109 /l regardless of the baseline ALC, indicating the predominance of reticulocytes as a predictive parameter (Table 4). Among patients with ARC < 25 × 109 /l, the level of the baseline ALC yielded 2 distinct groups: those with an ALC ≥ 1 × 109 /l, which accounted for the majority of patients in whom the response rate was 62%; and those with an ALC < 1 × 109 /l, in whom the response rate was 41%. These data suggest that 3 groups (about 1/3 of patients in each) in our cohort have a distinct prognosis: those with a high baseline ARC (regardless of the ALC) who have about an 80% response rate (about 20% higher than the entire cohort); those with an ARC < 25 × 109 /l and an ALC ≥ 1 × 109 /l with a response rate of 62% (the overall response rate for the entire cohort); and those with both an ARC < 25 × 109 /l and an ALC < 1 × 109 /l with a response rate of 41% (about 20% lower than the entire cohort) (Table 4). When only adults (18 years and older) were analyzed, the baseline ARC and ALC remained predictive of response to IST (Supplementary Table SI).
Table 4. Multivariate analysis of response rate at 6 months.
| Number of patients (%) | Response at 6 months | P-value | |||
|---|---|---|---|---|---|
| Number | Percent | 95% CI | |||
| H-ATG* | 316 (100) | 194 | 61.4 | (56.0, 66.8) | ------- |
|
| |||||
| Multivariate Baseline Risk (× 109 /l) | |||||
|
| |||||
| ARC ≥ 25 & ALC ≥ 1 | 71 (22) | 59 | 83.1 | (74.2, 92.0) | |
|
| |||||
| ARC ≥ 25 & ALC < 1 | 25 (8) | 18 | 72.0 | (53.1, 90.9) | 0.2354** |
|
| |||||
| ARC < 25 & ALC ≥ 1 | 129 (41) | 80 | 62.0 | (53.5, 70.5) | 0.0018** |
|
| |||||
| ARC < 25 & ALC < 1 | 91 (29) | 37 | 40.7 | (30.4, 50.9) | < 0.0001** |
Includes all regimens which are based on horse anti-thymocyte globulin (H-ATG)
P-value for testing equal 6-month response rate with patients in the ARC ≥ 25 × 109 /l and ALC ≥ 1× 109 /l group. ARC, absolute reticulocyte count; ALC, absolute lymphocyte count, ANC, absolute neutrophil count; PNH, paroxysmal nocturnal haemoglobinuria
About 25% of patients treated with a h-ATG-based regimen were children under the age of 18 years (Scheinberg, et al, in press). As a younger age alone is in itself a good predictor of haematological response to IST, an analysis for other predictors in children may lack power due to the smaller sample size in our cohort, therefore, a separate analysis of the paediatric patients was not performed. However, when the above predictive criteria of the ARC and ALC were applied to paediatrics patients, the ALC was not predictive, but a pre-treatment ARC ≥ 25 × 109 /l remained a significant predictor of response in the paediatric cohort, with a response rate of 90% (26/29) compared to 65% (31/48) in those with an ARC < 25 × 109 /l (p=0.02).
The significance of the baseline counts was tested in patients treated in different time periods and both counts remained predictive with those with a baseline ARC ≥ 25 × 109 /l and ALC ≥ 1 × 109 /l having a greater response rate compared to those with an ARC < 25 × 109 /l and ALC < 1 × 109 /l: 1989-1995 (75% vs 43%, P=0.035), 1996-2001 (88% vs 55%, P=0.003) and 2002-2005 (83% vs 23%, P < 0.0001), respectively. This suggests that the influence of the ARC and ALC in predicting response was present despite the time period when treatment was administered. Similar analyses were performed for patients treated with the different h-ATG regimens and the ARC and ALC was predictive in those treated with h-ATG + CsA only.
Baseline blood count values predict 6-month (short term) and long-term survival
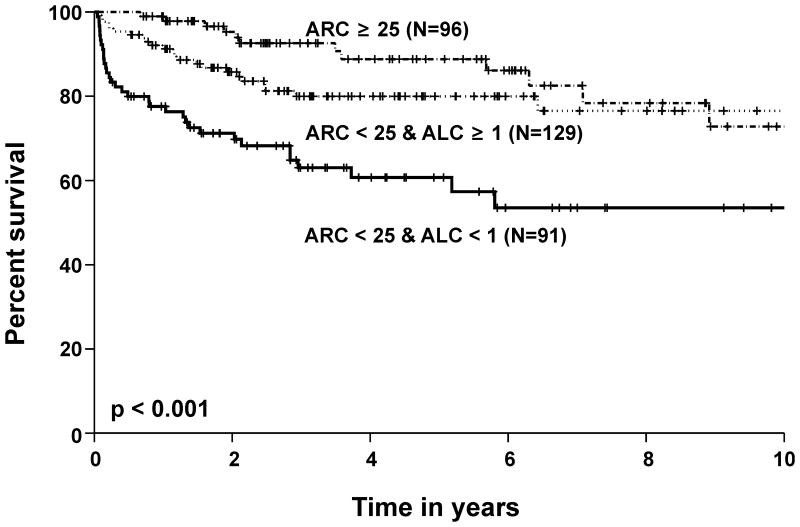
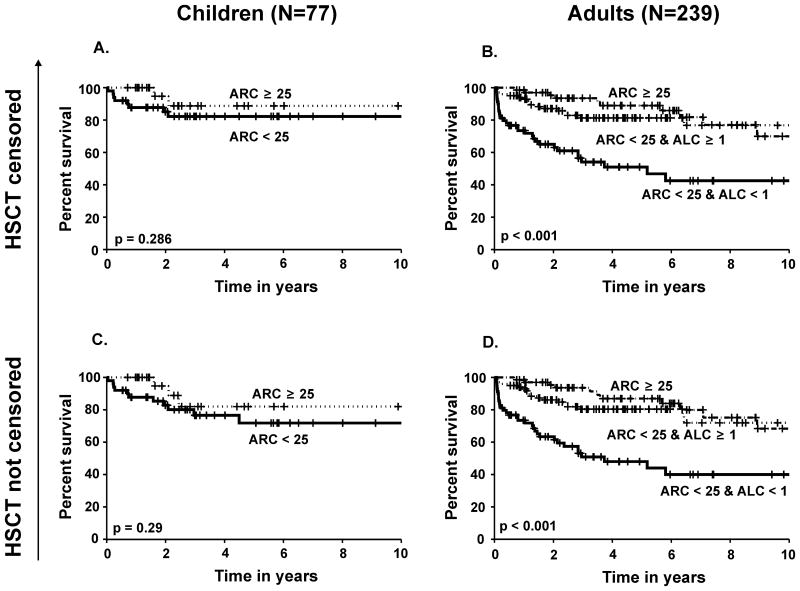
The two baseline parameters that were predictive of response (ARC and ALC) also predicted survival at 6 months following ATG by univariate analysis; however, multivariate analysis showed that baseline ANC was a more significant contributor to short term survival than either the ARC or ALC (Table 5). In multivariate analysis with categorical risk factors, patients with a higher ARC and ALC had a 20% higher short-term survival rate when compared to those with a lower ARC and ALC (100% vs. 80% respectively, p < 0.01). The same result was also found for long term survival: patients with a higher baseline ARC and ALC had a much higher probability of 5-year survival (92%) when compared to patients with a lower ARC and ALC baseline blood count (53%, p < 0.001) (Figure 2).). The overall survival according to baseline risk for children (age < 18) and adults are shown separately in Figure 3. A statistically significant higher probability of survival was observed in adults with a pre-treatment ARC ≥ 25 × 109 /l compared to those with an ARC < 25 × 109 /l and ALC < 1 × 109 /l. In children with an ARC ≥ 25 × 109 /l, a better survival probability was observed compared to those with an ARC < 25 × 109 /l, but this difference was not significant at α = 0.05 level, probably due to the smaller sample size and a better overall response rate observed in paediatric patients (Scheinberg et al, in press)
Table 5. Univariate and multivariate logistic regression analysis of continuous blood counts on the survival rate within the first 6 months.
| Univariate Logistic Model | Multivariate Logistic Model* | |||||
|---|---|---|---|---|---|---|
| Baseline risk | Coefficient (β) | SD | P-value | Coefficient (β) | SD | P-value |
| Log (ARC+1) | 0.4855 | 0.1410 | 0.0006 | -0.2022 | 0.1719 | < 0.2394 |
| Log (ALC+1) | 0.7711 | 0.2396 | 0.0013 | ------ | ------ | ------ |
| Log (ANC+1) | 0.7571 | 0.1152 | < 0.0001 | 0.8755 | 0.1586 | < 0.0001 |
| Log (Plt+1) | 0.1425 | 0.2375 | 0.5485 | ------ | ------ | ------ |
| Log (PNH+1) | 0.4362 | 0.4404 | 0.3219 | ------ | ------ | ------ |
| Log (Age+1) | -0.7244 | 0.3351 | 0.0306 | ------ | ------ | ------ |
Stepwise regression with multivariate logistic models returns three log transformed covariates: ANC, Plt and PNH.
------ Variable deleted by the stepwise procedure.
ARC, absolute reticulocyte count; ALC, absolute lymphocyte count, ANC, absolute neutrophil count; PNH, paroxysmal nocturnal haemoglobinuria; Plt, platelet count.
Figure 2.
Survival probability in all patients with a high baseline absolute reticulocyte count (ARC), low ARC and a high absolute lymphocyte count (ALC), and a low ARC and ALC (× 109 /l). Those who underwent haematopoietic stem cell transplantation were censored at the time of transplant.
Figure 3.
Survival probability in children less than 18 years (N=77) according to baseline ARC (× 109 /l) with haematopoietic stem cell transplantation (HSCT) censored (3A) and not censored (3C). Survival probability in adults only (N=239) according to baseline ARC and ALC (× 109 /l) with HSCT censored (3B) and not censored (3D).
Discussion
Severity in AA is defined by revered criteria which derive from publications of Camitta and colleagues in the 1970s, mainly directed to the selection of patients for bone marrow transplantation, a hazardous undertaking at that time (Camitta et al, 1975, 1976). These authors astutely recognized from their own experience and the literature that the outcomes in aplastic anaemia could be described by biphasic curves, with patients with more mild disease surviving months to years compared to those with more severe pancytopenia who died within weeks to a few months of diagnosis (Li, et al 1972, Williams, et al 1973). However, discriminating between these two groups was not feasible, at least in part due to the heterogeneity of their clinical presentation, supportive care provided and the low numbers of cases. Although their report of a prospective randomized study comparing HLA-matched sibling donor HSCT to conventional treatment is most frequently cited to reference the “Camitta criteria” (Camitta, et al 1976), the first definition appeared earlier, and the absence of firm grounding was explicit: “Clinical classification of the patients was performed by means of arbitrary criteria” (Camitta, et al 1975). In this historic study, severity was defined as the presence of at least two of the following three peripheral blood count criteria: ANC < 0.5 × 109 /l, platelet count < 20 × 109 /l and reticulocytes < 1%. Camitta and co-authors cite other authors' work in their various discussions of outcomes, but these references are to relatively small numbers of patients: samples of 101 in Utah (Williams, et al 1973); 24 from Mt. Sinai Hospital in New York (Davis and Rubin 1972); 34 from Mexico City (Duarte, et al 1972) in various categories of aplastic anaemia treated under a variety of regimens of the times. Some predictive models were proposed to correlate with better survival in SAA patients who did not undergo HSCT and were treated with transfusions and/or androgens. The pre-treatment reticulocyte count was often included in these models, but none gained wide acceptance, in part because they were impractical (in including complex formulas) and relied on subjective parameters (such as bone marrow morphology and differential counts) (Hormann, et al 1984, Najean and Pecking 1979, Rozman, et al 1981, Te Velde and Haak 1977, Williams, et al 1978). In addition, manually determined blood counts were less accurate than the current highly precise and reproducible values achieved with laboratory automation.
Not only do the popular Camitta criteria lack substantive clinical evidence of utility, but they were established in the pre-ATG era and used to define patients who benefited from HSCT, at a time when long term survival in patients with SAA who were not treated by transplant was dismal. The introduction of IST about 30 years ago has dramatically changed the outcome for patients with SAA; its benefits (and limitations) have now been quantitated in systematic studies in the US, Japan and Europe (Young, et al 2006). Across many studies, haematological response correlated to improved long-term survival (Young, et al 2006). For the 1/3 of patients who do not achieve a haematological response, repeated courses of immunosuppression are often administered, with variable reported response rates (Di Bona, et al 1999, Means, et al 1988, Scheinberg, et al 2006b, Tichelli, et al 1998). In patients who lack a histocompatible sibling donor, alternative donor HSCT has achieved an overall long term survival of about 50%, with better results for young children (Deeg, et al 2006, Passweg, et al 2006). In retrospective surveys, IST overall rivals HSCT in providing long-term survival, although some categories of patients, defined by age and neutrophil count, do better with one or the other therapy (Locasciulli, et al 2007). Unfortunately, a favourable response to IST cannot be routinely or reliably predicted by the Camitta criteria or other clinical and/or laboratory parameters. The presence of a PNH clone at baseline has been suggested as a marker of a favourable response to IST in adults (Sugimori, et al 2006). In recent years, the detection of a small population of CD55-/CD59- cells by flow cytometry has greatly increased the sensitivity of detecting a small PNH clone compared to the more traditional Ham's test. A correlation with response, however, was not confirmed in our study or in a Japanese cohort of children (Yagasaki, et al 2006). The reason for this discrepancy may be due to the different methods of determining the presence of a PNH clone; Sugimori et al (2006) defined a PNH clone as GPI- neutrophils > 0.003% or GPI- RBCs > 0.005%; for Yagasaki et al (2006) a PNH clone was defined as GPI- RBCs > 0.037%, and in our cohort a PNH clone was considered present when either GPI- neutrophils or RBCs were ≥ 1%.
To minimize patient and treatment heterogeneity, our analysis was conducted only in SAA patients who received h-ATG + CsA-based regimens with very similar outcomes. Our data showed that younger patients have a higher likelihood of response following IST compared to older patients. This relationship between age and response to IST was not observed in a large retrospective European study, where older age was not found to negatively affect the probability of response; however, survival was worse in the older patients (the majority of patients in this retrospective study were treated in the 1980s with ATG alone, with many fewer patients treated with the combination of ATG + CsA as used in the current study) (Tichelli, et al 1999).
Patients with very severe neutropenia (ANC < 0.2 × 109 /l) represent a high-risk group in SAA due to their risk of life-threatening infections. In our study, the contribution of the ANC to response at 6 months was due only to the expected close relationship of ANC and short-term survival. When only survivors at 6 months were analyzed, the effect of ANC on response dissipated, while the effects of age, baseline ARC, and ALC remained predictive of haematological response to IST and survival.
Prior to ATG becoming standard IST in SAA, a low baseline ARC was reported to be associated to a higher short-term mortality in several small retrospective studies (Haak, et al 1977, Lohrmann, et al 1976, Rozman, et al 1981). In the era of IST, the baseline ARC has been reported to be predictive of response in a young female cohort who experienced a delay in recovery of bone marrow function following IST (Nissen, et al 1993). In our experience, robust recovery of the reticulocyte count following immunosuppressive treatment predicted long-term survival, again suggesting that this blood count parameter may help in clinically assessing bone marrow function (Rosenfeld, et al 2003). In general, recovery in patients with aplastic anaemia who are treated with IST involves elimination of autoreactive T cell clones that target progenitor cells in the bone marrow, which is then followed by recovery of haematopoiesis. A higher baseline ARC and ALC may indicate better residual marrow function and the presence of sufficient stem cells to support blood cell production after IST. The bone marrow targets of immune attack in SAA remain elusive, but it is possible that in patients with a low ARC and ALC a more pronounced destruction of elements in a more primitive haematopoietic stem cell compartment has occurred (affecting both myeloid and lymphoid haematopoietic) compared to when both the ARC and ALC are high; and in patients with a high ARC and low ALC and vice-versa, a more committed progenitor may be predominantly affected, leading to a better probability of recovery after the immunological insult is controlled.
The ARC and ALC are two simple values that are readily available in a routine complete haemogram and are widely performed as an automated and standardized test, which minimizes result variability between different days of testing or in different centres. Some research laboratory findings that reflect the pathophysiology of SAA, such as the increased ratio of activated T cells (Verma, et al 2002), increased interferon-γ expression in bone marrow and peripheral T cells (Sloand, et al 2002), increased expression of heat shock protein (Takami, et al 1999), telomere length and telomerase gene mutations (Calado and Young 2008) and the presence small numbers of aneuploid bone marrow cells (Sloand et al 2007) have been proposed as useful in prognosis, and while not currently either generally available or applied, these assays may eventually be combined with blood count criteria to individualize therapy in patients with SAA. As the indication for HSCT broadens the risk-benefit analysis of each treatment, modality can be better assessed when response to IST can be predicted in SAA. Although the administration of IST may not be precluded based on the likelihood of response, protocols might be designed to institute salvage therapies early post h-ATG/CsA. For example, in patients with very severe neutropenia (and therefore a higher immediate mortality risk) and a low probability of response who do not achieve a haematological response at 3 months, a matched sibling donor HSCT in older patients, an unrelated HSCT in younger patients or a repeat course of IST in those who lack a viable donor could be justified.
Our study is limited by its retrospective nature and the long period that was used for the analysis. However, during the 16-year period, the inclusion, diagnostic and response criteria for SAA at our institution were unchanged; IST was based on h-ATG and CsA; and the follow-up also remained consistent. A second possible limitation is the reliance on similar parameters, peripheral blood counts, for the determination of both a haematological response post-IST and pre-IST prognosis. Indeed, it is not unreasonable that marrow function, as reflected by the degree of cytopenia, should influence the likelihood of response to an intervention, with better preserved stem cell numbers correlating with better short- and long-term outcomes. However, while reasonable as a hypothesis, some paediatric studies, for example, have reported superior response rate and survival in children with very severe (ANC < 0.2 × 109 /l) compared to severe aplastic anaemia (Fuhrer, et al 2005). Methodologically, the prognostic utility of our threshold values seems unlikely to be secondary to their proximity to response criteria. Neither platelets nor granulocyte number was predictive, although they figure in response criteria. Furthermore, iterative statistical testing indicated that multiple reticulocyte and lymphocyte thresholds were similarly predictive as the numbers selected for our prognostic score.
Because SAA is a rare disease, prospective studies to confirm a predictive model could take over a decade to conclude even in large referral centres. As the Camitta criteria were asserted in the era pre-ATG and on little concrete basis, we believe they do not provide guidance to clinicians caring for SAA patients with regard to the likelihood of response to ATG + CsA. The predictive method described here will be important for the purpose of comparison between studies and, most importantly, in clinical decision making, particularly regarding timing of transplantation as the indication for matched sibling HSCT (now offered to older patients) and alternative donor HSCT (in patients who lack an HLA-matched sibling) broadens.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Heart, Lung and Blood Institute.
Footnotes
Authorship: P Scheinberg participated in the primary conception, data collection and analysis, and drafted the manuscript; CO Wu did all the statistical analysis and participated in interim discussions; O Nunez participated in the data collection; NS Young participated in the primary conception, data collection and analysis, interim analysis and discussions, and writing of the manuscript.
Conflict of Interest Statement: The authors have no conflict of interest to declare.
References
- Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008 doi: 10.1182/blood-2007-08-019729. blood-2007-2008-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camitta BM, Rappeport JM, Parkman R, Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45:355–363. [PubMed] [Google Scholar]
- Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, Rappeport JM, Storb R. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- Davis S, Rubin AD. Treatment and prognosis in aplastic an‘mia. Lancet. 1972;1:871–873. doi: 10.1016/s0140-6736(72)90740-4. [DOI] [PubMed] [Google Scholar]
- Deeg HJ, O'Donnell M, Tolar J, Agarwal R, Harris RE, Feig SA, Territo MC, Collins RH, McSweeney PA, Copelan EA, Khan SP, Woolfrey A, Storer B. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006;108:1485–1491. doi: 10.1182/blood-2006-03-005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bona E, Rodeghiero F, Bruno B, Gabbas A, Foa P, Locasciulli A, Rosanelli C, Camba L, Saracco P, Lippi A, Iori AP, Porta F, De Rossi G, Comotti B, Iacopino P, Dufour C, Bacigalupo A. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Br J Haematol. 1999;107:330–334. doi: 10.1046/j.1365-2141.1999.01693.x. [DOI] [PubMed] [Google Scholar]
- Duarte L, López Sandoval R, Esquivel F, Sánchez-Medal L. Androstane therapy of aplastic anaemia. Acta Haematol. 1972;47:140–145. doi: 10.1159/000208508. [DOI] [PubMed] [Google Scholar]
- Dunn DE, Tanawattanacharoen P, Boccuni P, Nagakura S, Green SW, Kirby MR, Kumar MS, Rosenfeld S, Young NS. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131:401–408. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- Frickhofen N, Rosenfeld SJ. Immunosuppressive treatment of aplastic anemia with antithymocyte globulin and cyclosporine. Semin Hematol. 2000;37:56–68. doi: 10.1016/s0037-1963(00)90030-1. [DOI] [PubMed] [Google Scholar]
- Frickhofen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Herrmann F, Freund M, Meusers P, Salama A, Heimpel H. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German Aplastic Anemia Study Group. N Engl J Med. 1991;324:1297–1304. doi: 10.1056/NEJM199105093241901. [DOI] [PubMed] [Google Scholar]
- Fuhrer M, Rampf U, Baumann I, Faldum A, Niemeyer C, Janka-Schaub G, Friedrich W, Ebell W, Borkhardt A, Bender-Goetze C German/Austrian Aplastic Anemia Working G. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106:2102–2104. doi: 10.1182/blood-2005-03-0874. [DOI] [PubMed] [Google Scholar]
- Furuhjelm U, Eklund J. Treatment of aplastic anemia with anabolic steroids and corticosteroids. Ann Paediatr Fenn. 1966;12:89–95. [PubMed] [Google Scholar]
- Gluckman E, Devergie A, Faille A, Barrett AJ, Bonneau M, Boiron M, Bernard J. Treatment of severe aplastic anemia with antilymphocyte globulin and androgens. Exp Hematol. 1978;6:679–687. [PubMed] [Google Scholar]
- Haak HL, Hartgrink-Groenveld CA, Ernisse JG, Speck B, van Rood JJ. Acquired aplastic anaemia in adults. I. A retrospective study of 40 cases: single factors influencing prognosis. Acta Haematol. 1977;58:257–277. doi: 10.1159/000207837. [DOI] [PubMed] [Google Scholar]
- Hormann A, Berchthold W, Rhyner K, Wiedemann-Sigg C, Gmur J. Prognosis in acquired aplastic anemia. Acta Haematol. 1984;71:81–89. doi: 10.1159/000206562. [DOI] [PubMed] [Google Scholar]
- Li FP, Alter BP, Nathan DG. The mortality of acquired aplastic anemia in children. Blood. 1972;40:153–162. [PubMed] [Google Scholar]
- Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, Schrezenmeier H, Passweg J, Fuhrer M on the behalf of the Severe Aplastic Anemia Working Party of the European Group for, B. & Marrow T. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11–18. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- Lohrmann HP, Kern P, Niethammer D, Heimpel H. Identification of high-risk patients with aplastic anaemia in selection for allogeneic bone-marrow transplantation. Lancet. 1976;2:647–650. doi: 10.1016/s0140-6736(76)92462-4. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon-gamma and tumor necrosis factor-alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 1995a;85:3183–3190. [PubMed] [Google Scholar]
- Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anemia. Br J Haematol. 1995b;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- Means RT, Jr, Krantz SB, Dessypris EN, Lukens JN, Niblack GD, Greer JP, Flexner JM, Stein RS. Re-treatment of aplastic anemia with antithymocyte globulin or antilymphocyte serum. Am J Med. 1988;84:678–682. doi: 10.1016/0002-9343(88)90104-0. [DOI] [PubMed] [Google Scholar]
- Najean Y, Pecking A. Prognostic factors in acquired aplastic anemia. A study of 352 cases. American Journal of Medicine. 1979;67:564–571. doi: 10.1016/0002-9343(79)90226-2. [DOI] [PubMed] [Google Scholar]
- Nissen C, Gratwohl A, Tichelli A, Stebler C, Wursch A, Moser Y, Dalle Carbonare V, Signer E, Buser M, Ritz R, Speck B. Gender and response to antilymphocyte globulin (ALG) for severe aplastic anaemia. Br J Haematol. 1993;83:319–325. doi: 10.1111/j.1365-2141.1993.tb08288.x. [DOI] [PubMed] [Google Scholar]
- Passweg JR, Perez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W, Hows JM, Marsh JC, Pasquini R, Schrezenmeier H, Socie G, Zhang MJ, Bredeson C. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transplant. 2006;37:641–649. doi: 10.1038/sj.bmt.1705299. [DOI] [PubMed] [Google Scholar]
- Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR [beta]-CDR3 sequencing. The Lancet. 2004;364:355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. Jama. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- Rozman C, Marin P, Gra¤ena A, Nomdedu B, Montserrat E, Feliu E, Vives-Corron JL. Prognosis in acquired aplastic anaemia. A multivariate statistical analysis of 80 cases. Scandinavian Journal of Haematology. 1981;26:321–329. doi: 10.1111/j.1600-0609.1981.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Medal L, Gomez-Leal A, Duarte L, Guadalupe Rico M. Anabolic androgenic steroids in the treatment of acquired aplastic anemia. Blood. 1969;34:283–300. [PubMed] [Google Scholar]
- Scheinberg P, Nunez O, Wu C, Young NS. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006a;133:606–611. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. British Journal of Haematology. 2006b;133:622–627. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Wu CO, Nunez O, Young NS. Long-Term Outcome of Pediatric Patients with Severe Aplastic Anemia Treated with Antithymocyte Globulin and Cyclosporine. The Journal of Pediatrics. doi: 10.1016/j.jpeds.2008.06.004. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100:1185–1191. doi: 10.1182/blood-2002-01-0035. [DOI] [PubMed] [Google Scholar]
- Sloand EM, Scheinberg P, Fenlon E, Blancato J, Pfannes L, Young NS. Monosomy 7 detected by FISH at disease presentation is a marker for non-response to immunosuppression. Blood (ASH Annual Meeting Abstracts) 2007;110:506a. [Google Scholar]
- Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, Omine M, Nakao S. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–1314. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- Takami A, Nakao S, Tatsumi Y, Wang H, Weihua Z, Yamazaki H, Yasue S, Shiobara S, Matsuda T, Mizoguchi H. High inducibility of heat shock protein 72 (hsp72) in peripheral blood mononuclear cells of aplastic anaemia patients: a reliable marker of immune-mediated aplastic anaemia responsive to cyclosporine therapy. British Journal of Haematology. 1999;106:377–384. doi: 10.1046/j.1365-2141.1999.01540.x. [DOI] [PubMed] [Google Scholar]
- Te Velde J, Haak HL. Aplastic anaemia. Histological investigation of methacrylate embedded bone marrow biopsy specimens; correlation with survival after conventional treatment in 15 adult patients. Br J Haematol. 1977;35:61–69. doi: 10.1111/j.1365-2141.1977.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Tichelli A, Passweg J, Nissen C, Bargetzi M, Hoffmann T, Wodnar-Filipowicz A, Signer E, Speck B, Gratwohl A. Repeated treatment with horse antilymphocyte globulin for severe aplastic anaemia. Br J Haematol. 1998;100:393–400. doi: 10.1046/j.1365-2141.1998.00578.x. [DOI] [PubMed] [Google Scholar]
- Tichelli A, Soci G, Henry-Amar M, Marsh J, Passweg J, Schrezenmeier H, McCann S, Hows J, Ljungman P, Marin P, Raghavachar A, Locasciulli A, Gratwohl A, Bacigalupo A. Effectiveness of immunosuppressive therapy in older patients with aplastic anemia. The European Group for Blood and Marrow Transplantation Several Aplastic Anaemia Working Party. Ann Intern Med. 1999;130:193–201. doi: 10.7326/0003-4819-130-3-199902020-00004. [DOI] [PubMed] [Google Scholar]
- Verma A, Deb DK, Sassano A, Kambhampati S, Wickrema A, Uddin S, Mohindru M, Van Besien K, Platanias LC. Cutting Edge: Activation of the p38 Mitogen-Activated Protein Kinase Signaling Pathway Mediates Cytokine-Induced Hemopoietic Suppression in Aplastic Anemia. J Immunol. 2002;168:5984–5988. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- Williams DM, Lynch RE, Cartwright GE. Drug-induced aplastic anemia. Seminars in Hematology. 1973;10:195–223. [PubMed] [Google Scholar]
- Williams DM, Lynch RE, Cartwright GE. Prognostic factors in aplastic anaemia. Clinics in Haematology. 1978;7:467–474. [PubMed] [Google Scholar]
- Yagasaki H, Yoshida N, Hirano N, Nishio N, Hama A, Wang Y, Takahashi Y, Kojima S. Presence of HLA-DR15, a Minor PNH Clone, or an Aplastic Anemia - Associated Autoantibody Do Not Predict a Favorable Response to Immunosuppressive Therapy in Children with Aplastic Anemia. Blood (ASH Annual Meeting Abstracts) 2006;108:984. [Google Scholar]
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.