Abstract
2-Alkylthio analogues of adenosine 5'-triphosphate were synthesized and evaluated as P2y purinoceptor agonists. ATP and analogues transiently increased intracellular Ca2+ levels in C6 glioma cells and in skeletal muscle derived myotubes in culture. Most derivatives were resistant to stepwise dephosphorylation by ecto-ATPases.
INTRODUCTION
P2-purinergic receptors (P2R) on cell membranes are characterized by their natural agonists, i.e. by extracellularly acting adenosine 5'-triphosphate (ATP) or adenosine 5'-diphosphate (ADP), by synthetic derivatives of these and by effector systems triggered by the receptor activation. Recent conferences have collected available information on the subject1,2. Synthesis of specific ATP and ADP analogues that are only slowly broken down by relevant ectoenzymes is of considerable scientific and potentially therapeutic interest, since many tissues carry P2R that mediate excitatory or inhibitory physiological responses to ATP and its analogues. These responses are mediated by a number of P2-purinergic receptor subtypes3 that are coupled to ion channels4 or phosphoinositide metabolism5–8 as second messenger systems, possibly some mediate inhibition of adenylate cyclase activity9. In spite of a considerable effort to produce new ATP derivatives10 and, in particular, to produce antagonists to P2R, not one selective P2R subtype antagonist has so far been obtained, nor has any P2R so far been isolated or cloned.
A programme has been developed by our groups encompassing synthesis of new analogues of ATP (and ADP), with the main aim to obtain subtype-specific P2R antagonists and agonists. The biological activity of the new substances has so far been judged from measurements of ectoenzyme-induced32 (phosphatases, nucleotidases, phosphodiesterases) breakdown, studies of P2R ligand (agonist, antagonist) properties, second messenger formation8 and resultant biological effects.
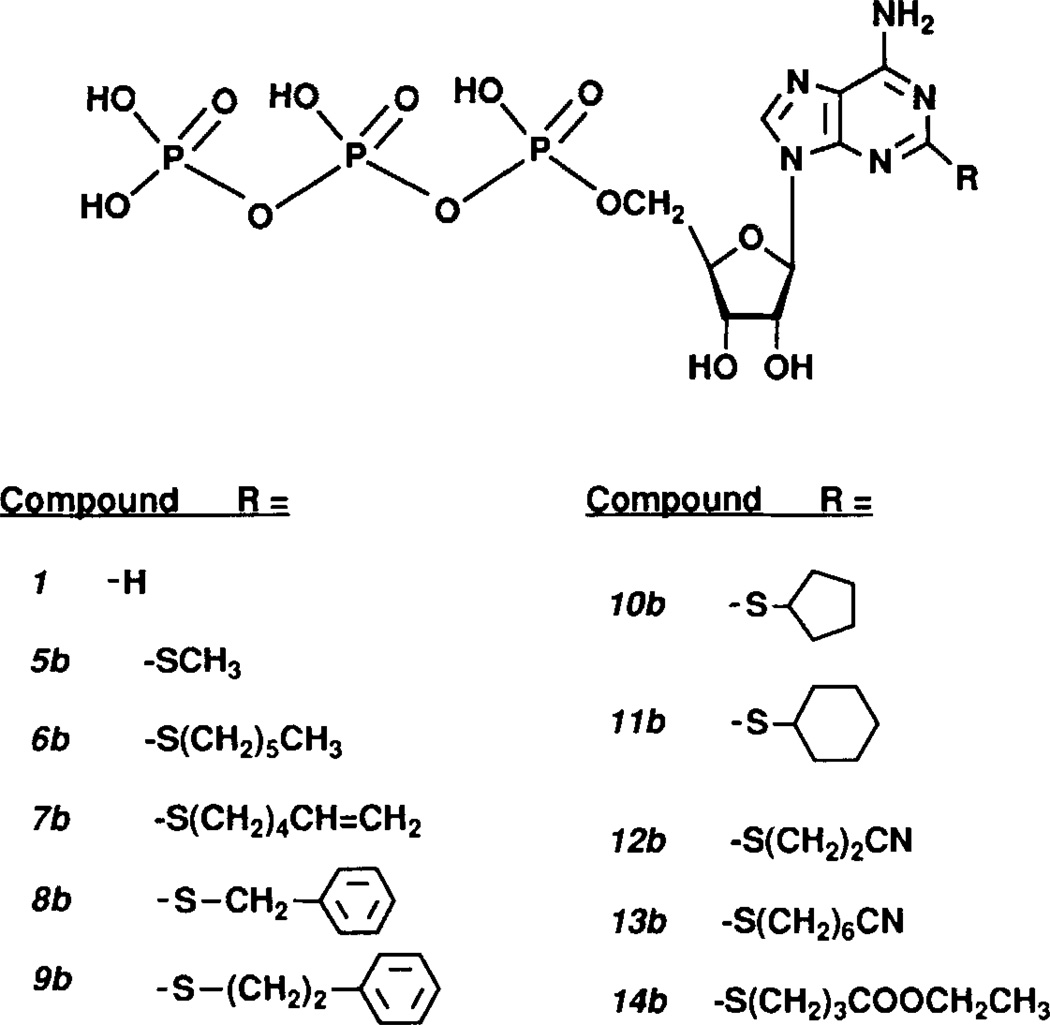
In this paper, we describe the synthesis of substituted 2-alkylthio-ATP derivatives (FIGS 1 and 2; see Material and methods). These compounds were designed to explore the structure-activity relationships at purinergic receptors, especially the P2y subtype, because 2-methylthio-ATP previously has been found to be the most potent substrate7 for this receptor. The receptor subtype seems to be present in the cell membrane of vascular endothelial cell4,11, smooth and striated muscle8,10, erythrocytes6, C6 glioma cells13 and others, though this classification is open to some doubt14. One reason for this is that skeletal muscle-derived myotubes in culture, used e.g. in this paper, were shown to carry excitatory P2Rs activated by P2yR agonists but not17,18 by adenosine 5' - (2-fluorodiphosphate), ADPβF, described by Hourani et a1.16 to be able to specifically activate another P2yR, that of smooth muscle. The observation may be related to the fact that the two "P2yR" have very different biological functions and that results from pharmacological and biochemical studies alone are insufficient as tools for the characterization of P2-purinergic receptors.
FIGURE 1.
Structures of ATP analogues.
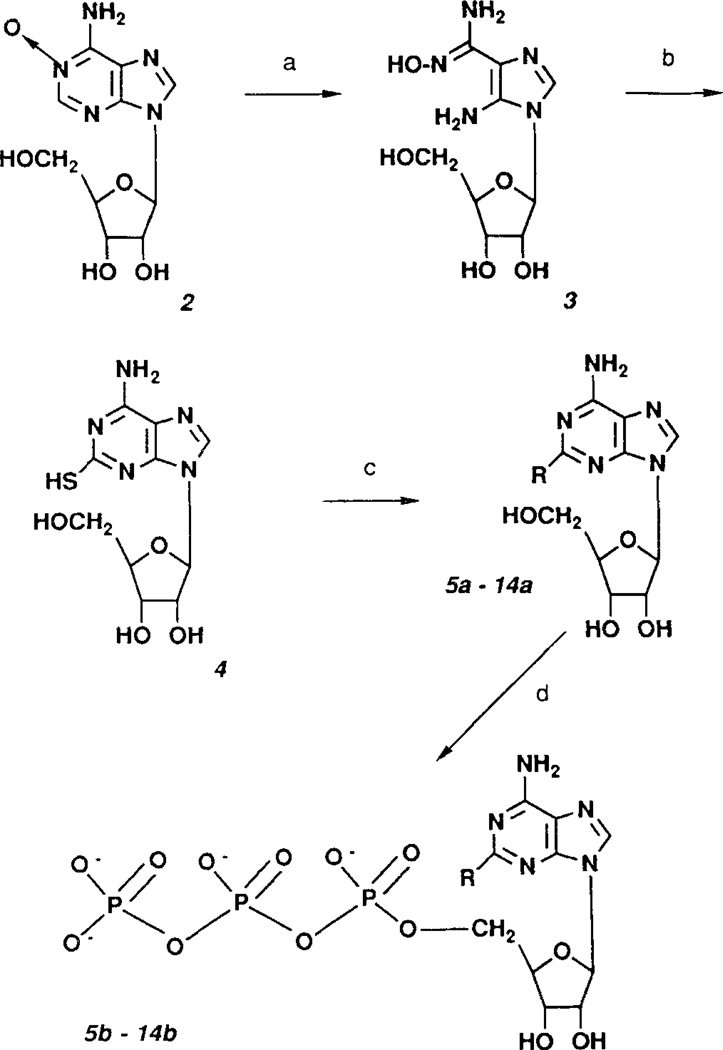
Figure 2.
Synthesis of 2-alkylthio-ATP derivatives. Reagents: a. NaOH; b, CS2; c, alkyl halide/NaOH; d, POCl3, tributylammonium pyrophosphate.
MATERIAL AND METHODS
Synthesis of 2-alkylthio adenosine 5'-triphosphate analogues19–21
Bold, italicized numbers refer to structures shown in FIGS 1 and 2.
5-Amino-1-β-D-ribofuranosylimidazole-4-carboxidoxime19, 3
The monohydrate of adenosine N-oxide was prepared in 71% yield, as described in ref 19. The product was evaluated by TLC (silica, CH3CN/H2O 85:15).
8.0g of adenosine N-oxide were added to a solution of 75ml 5M NaOH and refluxed on an oil bath for 15min. The flask was removed from the oil bath and rapidly cooled in ice-water, then in dry ice-acetone. The solution was adjusted to pH9 with conc. HC1 (approx. 30ml) and evaporated on a rotary evaporator. The residue was taken up in methanol, and precipitated NaCl was removed by pressure filtration through fritted glass and then washed several times with methanol. The collected filtrate was reduced in volume by evaporation leaving the product as a thick syrup or gum.
2-Thioadenosine, 4
The entire yield of the previous reaction was dissolved in water to a final volume of 25ml. This solution was mixed with 175ml of methanol and 50ml of carbon disulfide and heated in a pressure- and heat-resistant vessel at 110°C for 5h. After reaching room temperature, the vessel was refrigerated before opening. Yellow crystals of 2-thioadenosine were collected and washed on a Buchner funnel with H2O, then ethanol. After drying, 4.31g of 2-thio-adenosine remained (54% yield based on adenosine N-oxide).
General synthesis of 2-alkylthioadenosine derivatives, 6a–14a
200mg (0.63mmol) of 2-thioadenosine was added to 8.1ml (2.0mmol) of 0.25M NaOH and stirred until dissolved. To this was added 16ml of ethanol and 10 equivalents (6.3mmol) of alkyl halide (fewer equivalent amounts with more reactive alkyl halides). For primary alkyl halides the reaction was complete after stirring at room temperature overnight. Secondary halides and more sterically hindered primary halides required warming to 50°C for 1 to 2 days. The reactions were evaluated for completeness by TLC (silica, CHCl3/methanol 85:15 or CHCl3/methanol/acetic acid 85:10:5). Once complete, they were neutralized with HCl and evaporated in vacuo. The product crystallized during evaporation and was removed by filtration, washed sequentially with water and ethanol, and dried. If TLC indicated the presence of impurities, or if no crystals were obtained during evaporation of the reaction mixture, the residue was taken up in a small quantity of methanol and applied to a column of silica gel packed in CHCl3/methanol (85:15, v/v). Evaporation of the pure fractions usually yielded crystals. Otherwise the residue was recrystallized from ethanol/H2O to yield pure 2-alkylthio-adenosines. Melting points were as follows: 8a, benzylthio-, 129–130°C; 10a, cyclopentylthio-, 123–124°C; 11a, cyclohexylthio-, 153–154°C; 12a, 2-cyanoethylthio-, 117–119°C. Representative 1H-NMR spectra: compound 11a in d6-DMSO δ 8.20 (s, 1H, arom., C-8); 7.33 (s, 1H, NH); 5.78 (d, 1H, J=5.7 Hz, ribose C1'); 5.42 (d, 1H, J=6.1 Hz, 2'-OH); 5.14 (d, 1H, J=5.0 Hz, 3'-OH); 4.99 (t, 1H, 5'-OH); 4.66, 4.13 and 3.90 (each m, 1H, ribose CH); 3.72 (m, 1H, cyclohex.); 3.63, 3.55 (each m, 1H, CH of C-5'); 2.04 (m, 4H, cyclohex.); 1.2–1.7 (6H, cyclohex.). Compound 12a in d6-DMSO δ 8.26 (s, 1H, purine C-8); 7.48 (s, 1H, NH); 5.82 (d, 1H, J=5.9 Hz, ribose C1'); 5.43 (d, 1H, J=6.2 Hz, 2'-OH); 5.17 (d, 1H, J=4.8 Hz, 3'-OH); 5.03 (t, 1H, J=5.3 HZ, 5'-OH); 4.56, 4.12 and 3.92 (each m, 1H, ribose CH); 3.62, 3.56 (each m, 1H, CH of C-5'); 3.3 (2H, CH2, α-to CN); 3.00 (t, 2H, CH2, β-to CN).
Nucleoside 5'-triphosphates, 6b–14b
The procedure for nucleoside 5'-triphosphate synthesis was adapted from Kovacs and Ötvös20 and Moffat21.
Preparation of tri-n-butylammonium pyrophosphate solution for triphosphate synthesis: 6.69g of sodium pyrophosphate decahydrate (15mmol) was dissolved in 150ml of water. An excess of Dowex ion exchange resin, 50×8, 20–50 mesh, proton form, was placed in the solution of sodium pyrophosphate, and the mixture was stirred gently for 20min. A mixture of 60ml of ethanol and 7.14ml of tributylamine was placed in an ice-water bath, and the pyrophosphate solution was filtered directly into the flask. The resin was repeatedly washed with water until the filtrate was no longer acidic. The solvent was then evaporated under vacuum at 35°C, yielding a thick, nearly colorless syrup. This residue was twice treated with 90ml of ethanol and evaporated. The residue was taken up in 50ml of dimethylformamide (DMF, anhydrous grade from Aldrich Chemical Co., Milwaukee, WI, or distilled under vacuum and stored over 10Å molecular sieves) and evaporated once again. The residue was taken up in 10ml of anhydrous DMF. DMF-washes of the flask were added, finally yielding 30ml of 0.5M tri-n-butyl ammonium pyrophosphate in DMF. The preparation was stored cold over 10Å molecular sieves.
Preparation of triethylammonium bicarbonate (TEAB) buffer
A 1M solution was prepared by bubbling CO2 through 1M triethylamine (Aldrich, pure grade) for several hours (pH approx. 7.5).
Phosphorylation
0.3mmol dry nucleoside was placed in a conical flask together with 0.75ml of dry trimethyl phosphate (vacuum distilled from BaO) and the mixture was stirred until solution was attained. The solution was cooled in ice-water and dry 1,8-bis(dimethylamino) naphthalene (proton sponge, 0.45mmol, 96mg) was added. After 30min at 0°C, 60µl of POCl3 (0.64mmol, distilled) was added. After 2h of stirring at 0–4°C, a mixture of 0.5M tri-n-butylammonium pyrophosphate in anhydrous DMF (3ml, 5 equivalents) and tributylamine (0.3ml) was quickly added. After 1 minute 0.2M aqueous triethylammonium bicarbonate (TEAB, 30ml), pH7.5, was added to the reaction, and stirring was continued at room temperature for 30min. The mixture was then evaporated to dryness under high vacuum.
DEAE Sephadex purification
The crude nucleoside 5'-triphosphates were purified by ion-exchange chromatography (DEAE-Sephadex A-25, swelled in 1.0M NaHCO3) using a Pharmacia 20×1.6cm column at 4°C. After equilibrating the column with deionized water, the residue of the reaction mixture dissolved in water was applied. The column was washed with deionized water, followed by a solvent gradient of 0–650mM TEAB buffer in 1000ml to elute the triphosphates. Collected fractions were analyzed by TLC (silica, 1-propanol/NH4OH/H2O 11:7:2) for presence of triphosphates. The appropriate fractions were pooled and lyophilized. Successive lyophilization to remove TEAB yielded a product with a 'clear glass' appearance.
Preparative HPLC of adenosine 5'-triphosphates
A preparative HPLC method for the adenosine 5'-triphosphates was worked out using the following equipment: Pharmacia 2249 gradient pump, 2141 wavelength monitor set at 254nm, and 2221 integrator, Axxiom Chromatography ODS 5µ, 20cm semi-preparative column. Following ion-exchange chromatography and subsequent lyophilization, the product was taken up in a small volume of water, An analytical quantity of this solution was next injected to determine retention time and degree of separation from contaminants. A gradient from 0 to 15% acetonitrile in 50mM ammonium formate was applied over 15min. 2-Substituted adenosine 5'-triphosphates had retention times ranging from 7 to 14min.
Successful DEAE chromatography yielded purities from roughly 80 to 94%. The triphosphate peaks from repeated injections were collected and lyophilized several times.
Analysis of 2-alkylthio-adenosine 5'-triphosphates
New compounds were characterized (and resonance assigned) by 300MHz protonnuclear magnetic resonance spectroscopy using a Varian XL300 FT-NMR spectrometer. FAB mass spectrometry was performed on a JEOL SX102 high resolution mass spectrometer. 2-Methylthio-ATP was obtained from Research Biochemicals Inc., Natick, MA. Representative 1H-NMR spectra: Compound 5b (Na4 salt) in D2O: δ 8.37 (s, 1H, purine C-8); 6.14 (d, 1H, J=6.1Hz, ribose C1'); 4.8, 4.61 and 4.38 (each m, 1H, ribose CH); 4.23 (m, 2H, ribose C-5'); 2.60 (s, 3H, SCH3). Compound 9b (di-Et3N salt) in D2O: δ 8.46 (s, 1H, purine C-8); 7.2–7.3 (m, 5H, arom); 6.12 (d, 1H, J=5.5Hz, ribose C1'); 4.8, 4.58 and 4.39 (each m, 1H, ribose CH); 4.25 (m, 2H, ribose C-5'); 3.47 and 3.07 (each t, 2H, CH2CH2ϕ); 3.19 (9, 12H, Et3N); 1.27 (t, 18H, Et3N). For comparison, ATP (Na2 salt) in D2O: δ 8.62 and 8.42 (each s, 1H, purine C-8 and C-2); 6.14 (d, 1H, J=5.4Hz, ribose C1'); 4.74 (t, 1H, J=5.3Hz, ribose C2'); 4.58 (t, 1H, J=4.4Hz, ribose C3'); 4.43 (m, 1H, ribose C4'); 4.32 (m, 2H, ribose C-5').
Biochemical and pharmacological studies
Cell culturing
C6 rat glioma cell line22 was cultured in F-10 medium supplemented with L-glutamine, gentamycin, newborn calf serum and fetal calf serum.
Primary myotubes were prepared from skeletal muscle of chick embryos and maintained in culture as described previously23,24 They were used on day 7–9 after preparation.
Cell cultures were washed twice with Krebs Ringer Hepes buffer (KRH) before experiments.
Preparation of bovine brain membranes25
Bovine brain minus brainstem (PEL-Freez, Rogers, Arkansas; appr. 300g) was homogenised in an Osterizer blender (30s, liquefy setting) with 500ml HEPES (5mM)-Tris buffer (pH7.4). Further homogenisation was performed with a Brinkmann Polytron and a glass tube and tight-fitting teflon pestle. The homogenate was diluted with buffer to a total volume of 2500ml and centrifuged at 10000g for 10min. The pellet was discarded and the supernatant was centrifuged for 45min at 30000g. The supernatant was discarded and the pellet was taken up in 600ml of buffer, homogenized with a glass tube/teflon pestle and centrifuged at 30000g for 45min. The washing step was repeated and the pellet was taken up in 60ml buffer, homogenized, and stored at −80°C in 1ml aliquots for a maximum of 14 days. Protein content was assayed with the BCA method (Pierce Chemicals Co., Rockford, Illinois).
Enzyme-induced degradation of ATP and its derivatives
For a typical assay, enzyme-(ectophosphatases, nucleotidases and phosphodiesterases) induced dephosphorylation was studied at physiological pH and room temperature, unless otherwise stated. Triplicates of myotube culture (ca. 0.35mg prot/dish) and C6 glioma cells (0.65mg prot/dish) were incubated with the nucleotides (final conc. 10−4M) in KRH buffer and samples were withdrawn at selected time intervals, filtered through Millex-GS 0.22 µm (Millipore) or centrifuged and instantly frozen and stored at −80°C until analysis. As standard compounds, 2-methylthio-ATP and ATP were used. Products of enzyme-induced and spontaneous breakdown were separated by HPLC, identified and quantified.
The HPLC-system consisted of an Alltech Nucleotide/Nucleoside 7U column (250mm), equipped with an Alltech Adsorbosphere HS C18 5U guard column (20mm); eluant A consisted of 60mM ammonium phosphate and 5mM tetrabutyl-ammonium phosphate (TBAP) in 90% water/10% methanol; eluant B consisted of 5mM TBAP in methanol. A concentration gradient from 25% B to 75% B in 8min was applied. The flow rate was 1ml/min and the injection volume 10 or 20µ1. Peaks were detected by their UV absorption at 260nm, using a Hewlett-Packard diode array detector or a Pharmacia LKB 2141 wavelength monitor.
Origin and levels of cytoplasmic Ca2+
Cytoplasmic Ca2+ fluxes were demonstrated as previously described24,28 measuring fluorescence of fura-2 loaded cells at 510nm wavelength. A specially designed fluorimeter (Lindmark Innovations, Stockholm, Sweden), equipped with a deuterium lamp and a photon counter connected to a chart recorder (LKB; Bromma, Sweden) was used. Maximum and minimum fluorescence were determined after addition of 10µM ionomycin and 20mM MnCl2, respectively.
Cytoplasmic [Ca2+] was calculated as described by Grynkiewicz et al. (1985)26. In preliminary experiments aiming at the clarification of the origin of the increased [Ca2+]i, Ca2+ free, EGTA-supplied external medium as well as the voltage-sensitive Ca2+ channel blocker PN200-110 in the presence of high (50mM) external [K+] were used.
Receptor desensitization was demonstrated by repeated addition of ATP or analogue after the initial addition.
RESULTS
Synthesis and analysis
The structures of the 2-alkylthio analogues of ATP prepared in this study are shown in FIG. 1. Substitution at the 2-position was achieved (FIG. 2) by reaction of 2-thioadenosine with primary and secondary alkyl halides, followed by phosphorylation20,21 to obtain the 5'-triphosphate. General methods for this synthesis of 2-thioadenosine were modified from Kikugawa et al.19 Analytical data are found in TABLE 1.
Table 1.
FAB mass spectrala peaks for ATP and 2-thio-substituted analogues
| Compoundb | Formula | lntearal Massc | found: Low Resolutiond (unless noted) |
|---|---|---|---|
| 1 | C10H14N5O13P3Na2 | 551.0 | 572 (M−H2+Na), 550 (M−H), 528 (M-Na), 506 (M+H-Na2), 448 (M−H-PO3), 301, 279 |
| 5b | C11H16N5O13P3SNa2 | 596.9 | 596 (M−H), 574 (M-Na), 552 (M+H-Na2), 528, 494, 472 |
| 6b | C16H28N5O13P3S | 623.1 | 622 (M−H), 542 (M−H3-PO3) |
| Accurate mass calc: for C16H27N5O13P3S = 622.0539, found 622.0594 | |||
| 7b | C16H26N5O13P3S | 621.0 | 620 (M−H), 540 (M−H2-PO3) |
| 8b | C17H22N5O13P3S | 629.0 | 628 (M−H), 538 (M-Bz+) |
| 9b | C18H24N5O13P3S | 643.0 | 664, 642 (M−H), 562 (M−H2-PO3), 538 |
| 10b | C15H24N5O13P3S | 607.0 | 606 (M−H), 538, 526 (M−H2-PO3) |
| 11b | C16H26N5O13P3S | 621.0 | 642 (M−H2+Na), 620 (M−H), 540 (M−H2-PO3) |
| Accurate mass calc: for C16H25N5O13P3S = 620.0382, found 620.0384 | |||
| 12b | C13H19N6O13P3S | 592.0 | 591 (M−H), 538 |
| 13b | C17H27N6O13P3S | 648.1 | 669 (M−H2+Na), 647 (M−H), 567 (M−H2-PO3), 538 |
| Accurate mass calc: for C17H26N6O13P3S = 647.0491, found 647.0486 | |||
| 14b | C16H26N5O15P3S | 653.0 | 674 (M−H2+Na), 652 (M−H), 572 (M−H2-PO3), 538 |
Accurate mass (using fast atom bombardment in the negative ion mode) was measured on a JEOL SX102 high resolution mass spectrometer, using a glycerol matrix
Compound provided as triethylamine salts, unless noted. The triethylamine salt form was not observed in m. s. Where applicable, the 2-substituent is indicated in brackets.
The formula given as free acid (H4), unless noted.
Most intense peaks are in bold type. The fragment peak at mass 538 corresponds to 2-thiolate-ATP.
Disodium salt.
Metabolic stability and biological activity
Metabolic stability
Studies on enzyme-induced dephosphorylation of substituted adenosine 5'-triphosphates have been carried out using 1) a bovine brain membrane preparation presumably carrying both endo- and ecto-phosphohydrolases 2) primary myotubes from chick skeletal muscle in culture, and 3) C6 glioma cells from rat in culture (both in presence and absence of extracellular Ca2+), at physiological pH and 30°, 20°C and 37°C, respectively. From TABLE 2 it is seen that compounds 8b, 9b, 11b, 13b and 14b are resistent to enzyme-catalysed metabolism, while ATP, 2-MeS-ATP and compounds 6b, 7b and 12b seem subject to stepwise dephosphorylation during a 30 or 40 minute incubation. Dephosphorylation to the monophosphate was observed only in the case of ATP. There is a progression towards greater stability as the substituent group size increases e.g. 2-cyanoethylthio ATP, 12b, vs. 2-cyanohexyl- thio ATP, 13b. Increased stability is also observed as the side chain is aromatized or cyclized: e.g. 2-hexylthio ATP, 6b, vs. 2-benzylthio ATP, 8b, or 2-cyclohexylthio ATP, 11b. Spontaneous degradation could not be detected with any compound under the conditions used. The glioma cell line used was found to express hardly any ecto-ATPase activity.
TABLE 2. Test of metabolic stability.
The different compounds (10−4 M) were incubated with bovine brain membranes (40 min, 30°C), chick skeletal muscle myotubes (30 min, 20°C) or C6 glioma cells (30 min, 37°C). Results from HPLC analysis of the metabolites are presented as percentage of remaining mono-, di- and triphosphate.
| Compound | trip | dip | monoP | |
|---|---|---|---|---|
| Bovine 'brain membrane' preparation | ||||
| 1 | ATP | - | - | 100 |
| 5b | 2-MeS-ATP | 56 | 44 | - |
| 6b | Hexylthio- | 63 | 37 | - |
| 7b | Hexenylthio- | 48 | 52 | - |
| 9b | Phenylethylthio- | 100 | - | - |
| 11b | Cyclohexylthio- | 100 | - | - |
| Chick skeletal muscle derived myotubes | ||||
| 1 | ATP | 42 | 31 | 27 |
| 6b | Hexylthio- | n.d. | n.d. | n.d. |
| 7b | Hexenylthio- | 80a | 20 | - |
| 8b | Benzylthio- | 100 | - | - |
| 9b | Phenylethylthio-b | 100 | - | - |
| 11b | Cyclohexylthio- | 100 | - | - |
| 12b | Cyanoethylthio- | 48b | 40 | - |
| 13b | Cyanohexylthio- | 100 | - | - |
| 14b | (Ethyloxycarbony1)propylthio- | 100 | - | - |
| C6 glioma cells | ||||
| 1 | ATP (+Ca2+) | 93 | 3 | 4 |
| 1 | ATP (−Ca2+) | 87 | 8 | 5 |
| 9b | Phenylethylthio- | 100 | - | - |
| 11b | Cyclohexylthio- | 100 | - | - |
| 13b | Cyanohexylthio- | 100 | - | - |
Initial purity was 91% and 88%, respectively. No spontaneous hydrolysis was observed with any of the compounds under the assay conditions used.
Effects on Ca2+-homeostasis of cells in culture
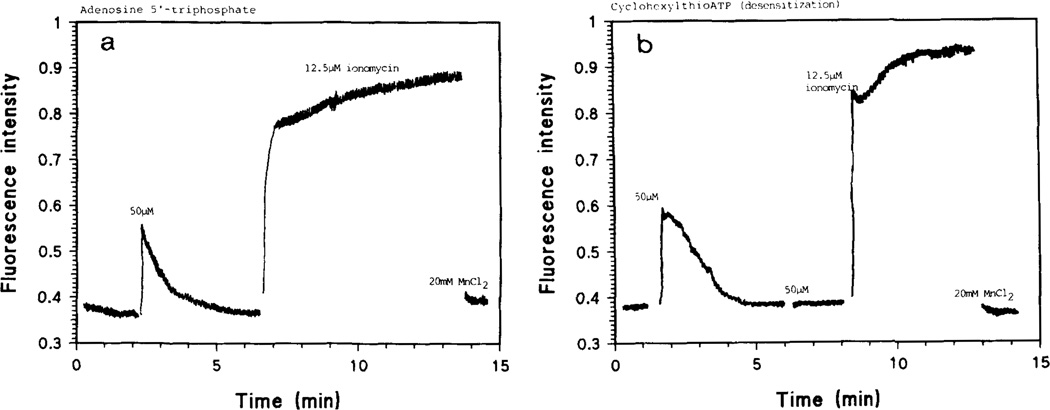
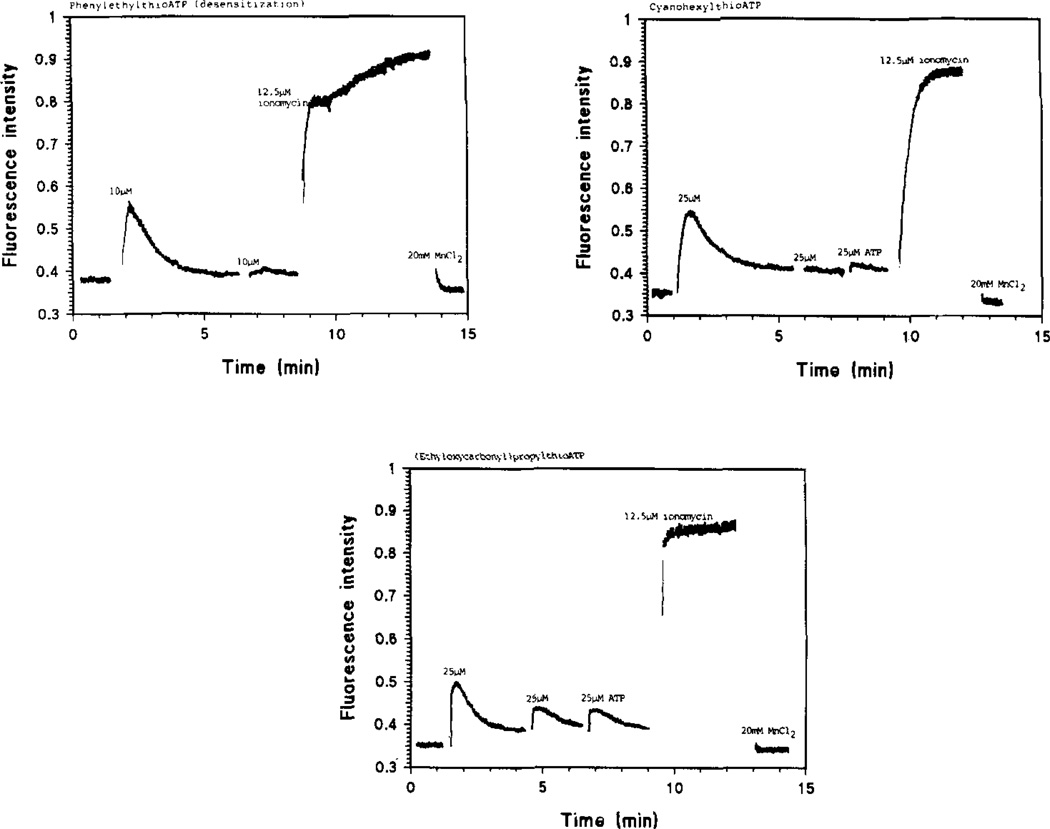
Heilbronn et al. have previously shown that ATP and some of its commercial analogues activate a P2R on the plasmalemma of skeletal muscle (chick24,28 and mouse29)-derived myotubes in culture, thus stimulating a G protein-phospholipase C cascade which results in a turnover of membrane phosphoinositides (PIS) and increases of intra-cellular inositol phosphates (IPS) and diacylglycerol (DAG). This is followed by transient increases in intra-cellular [Ca2+], earlier shown to depend on a two-step process, the first of which seems due to mobilization of intracellular Ca2+ and is followed by influx of Ca2+ through dihydropyridine-sensitive voltage-dependent channel17,24. Addition of micromolar concentrations of ATP or compounds 5b through 14b to the medium of fura-2-loaded myotubes in culture caused an immediate rise in fluorescence, followed by a gradual decrease to basal levels. FIG. 3 shows typical results and reveals that the rise in [Ca2+]i-induced fluorescence after addition of 2-(cyclohexylthio)-ATP, 11b, and the return to baseline has a different shape than that obtained with ATP. Especially for 2-(cyanohexyl)- and 2-([ethyloxycarbonyl]-propyl) thio ATP, 13b and 14b, the Ca2+-induced fluorescence decays slower; a similar effect was also seen with 2-(phenylethylthio)-ATP, 9b (FIG. 4).
FIGURE 3. Transient [Ca2+]i gradients evoked by ATP and 2-thio derivatives.
Fluorescence intensities are proportional to [Ca2+]i levels in the chick skeletal muscle derived myotubes. 2-Cyclohexylthio-ATP, 11b (B), produces a higher Ca2+ peak than ATP (A), and a differently shaped decay of the Ca2+-fura-2 signal, which my be due to different sensitivity to ATPase action. Receptor desensitization by compound 11b is also shown (B).
FIGURE 4.
The P2Y-purinoceptor of skeletal muscle myotubes is desenitized by repeated addition of compounds 9b, 13b and 14b. Subsequent stimulation of Ca2+-evoked fluorescene by ATP was also inhibited.
All compounds tested seem to desensitize the P2-receptor, since repeated addition of nucleotide analogues has very little effect on [Ca2+]i (FIGS 3 and 4).
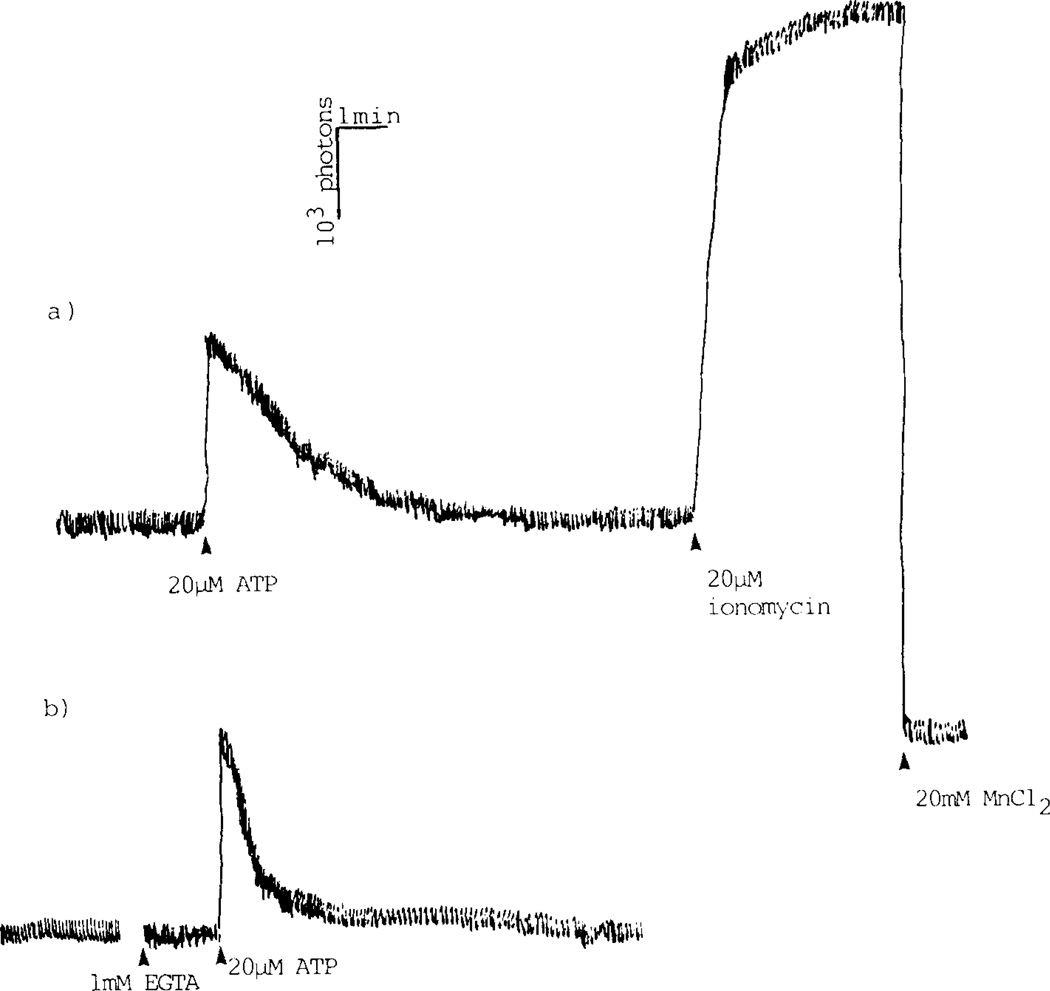
ATP and derivatives also induced [Ca2+]i increase in C6 glioma cells. Addition of EGTA to a Ca2+ free medium lowered this increase, suggesting a Ca2+ influx component. L-type voltage sensitive Ca2+ channels seem not involved, as addition of PN200-110 before ATP and in the presence of 50mM K+ was unable to block the increase and PN200-110 added after ATP or derivatives did not change the course of the Ca2+-evoked fluorescence decline to baseline (FIG. 5). In similarity with the myotubes, the P2-receptors on C6 glioma cells were also found to be desensitized by ATP as well as by the tested compounds 9b, 11b and 13b. Calculations using maximum increases in [Ca2+]i-fluorescence result in apparent EC50 values for ATP and 2-cyclohexylthio-ATP of 7.1µM and 3.2µM, respectively.
FIGURE 5. ATP stimulated increase of cytosolic Ca2+-concentration in C6 glioma cells.
Intracellular levels of free Ca2+, [Ca2+]i, were measured in a fluorimeter using the high affinity fluorescence probe fura-2. In the presence of external Ca2+ (A) the cytosolic Ca2+ increase displays a biphasic appearance interpreted as an initial rapid release of Ca2+ from intracellular stores, e g endoplasmic reticulum, followed by opening of Ca2+ channels in the plasma membrane, which results in influx of extracellular Ca2+.
In Ca2+-free buffer (B), agonist stimulation produces a more narrow peak of [Ca2+]i increase, i e the channel mediated portion of the Ca2+ signal is absent.
In preliminary experiments it was found that ATP as well as 9b, but not 11b, increase the intracellular levels of IPS of C6 glioma cells. Interestingly, the 2-cyanohexyl derivative, 13b, seems to inhibit IP formation.
DISCUSSION
The main aim of this work is to produce new non-hydrolyzable and subtype specific ligands for characterization of P2-purinergic receptors and their role in biological systems. 2-Methylthio-ATP is one of the most potent known agonists at P2y receptors7. Our design strategy was directed towards a functionalized congener approach27 in which the site of attachment of a chemically reactive chain is through the 2-thioether group.
The degradation studies carried out with primary myotubes in culture and with 2-alkylthio ATP analogues carrying bulky substituents demonstrated increased resistance of these compounds to ecto-ATPase-catalysed stepwise dephosphorylation, suggesting steric hindrance at the binding site of ATP hydrolysing enzymes. The brain membrane preparation was shown to dephosphorylate ATP and substances 5b–7b, but not 9b or 11b, probably suggesting that neither ecto- nor endo-ATPases metabolize the latter substances. The different enzymes involved have not yet been characterized. The C6 glioma cell line was shown to express only a very low ATPase activity compared to cultures described in the literature (C6 glioma cells30, primary astrocytes31); as ATP degradation is approximately 10% after 30min at 37°C in the presence as well as in the absence of Ca2+ in the incubation buffer. This makes our cell line very useful for P2R ligand activity studies.
All novel compounds acted as P2yR agonists, in the sense that their external application to either chick embryo skeletal muscle derived myotubes or rat C6 glioma cell line caused transient increases in cytoplasmic Ca2+ levels, as measured with fura-2-loaded cells. All substances desensitized their receptor. From the fluorescence studies on the new compounds a few things have been learned. Gross effects of ATP and 2-thio derivatives were similar. However, there are several interesting differences between the ATP and the 2-thio-derivatives which demand further investigation. Based on relative Ca2+ peak heights, the order of potency at the chick skeletal muscle myotube P2y receptor is as follows: 9b, 11b, ATP, 13b, 14b. However, the shape of the Ca2+-fura-2 signal differed for some derivatives from that caused by ATP. Differences in resistance to hydrolysis by ecto-phosphohydrolases may have influenced the rate and shape of the decay of the intracellular Ca2+ increases e.g. due to prolonged receptor occupancy and ongoing receptor desensitization. Another possibility is suggested by the observation that ATP-activated receptor causes increased IP3 levels while this seems not the case with at least some of the novel compounds. Thus, these compounds may increase intracellular Ca2+ levels by another route than an ATP-activated receptor.
ACKNOWLEDGEMENTS
We thank Ms. Homa Hasanvan for skillful technical assistance. This work was supported by the Swedish Medical Research Council, grants B91-04X-03907-19B and B92-04X-03907-20 to E. Heilbronn.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- 1.Burnstock G. In: Purines in Cellular Signalling: Targets for New Drugs. Jacobson KA, Daly JW, Manganiello V, editors. New York: Springer; 1990. pp. 241–253. [Google Scholar]
- 2.Dubyak GR, Fedan JS, editors. Biological Actions of Extracellular ATP. Ann. New York Acad. Sci. 1990;603 [PubMed] [Google Scholar]
- 3.Burnstock G, Kennedy C. Gen. Pharmacol. 1985;16:433. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 4.Nakazawa K, Matsuki N. Pflügers Archiv. 1987;409:644. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- 5.Dubyak GR. Arch. Biochem. Biophys. 1986;245:84. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper CL, Morris AJ, Harden TK. J. Biol. Chem. 1989;264:6202. [PubMed] [Google Scholar]
- 7.Pirotton S, Raspe E, Demolle C, Erneux C, Boeynaems JM. J. Biol. Chem. 1987;262:17461. [PubMed] [Google Scholar]
- 8.Häggblad J, Heilbronn E. Neurosci. Lett. 1987;74:199. doi: 10.1016/0304-3940(87)90149-2. [DOI] [PubMed] [Google Scholar]
- 9.Van Rhee AM, IJzerman AP, Soudijn W. Abst. P44; Int. Symp. on Pharmacology of Purinergic Receptors, IUPHAR Satellite Symposium; July 1990; Noordwijk, The Netherlands. [Google Scholar]
- 10.Cusack NJ, Hourani SMO. In: Purines in Cellular Signalling: Targets for New Drugs. Jacobson KA, Daly JW, Manganiello V, editors. New York: Springer; 1990. pp. 254–259. [Google Scholar]
- 11.Pearson JD, Gordon JL. Biochem. Pharmacol. 1989;38:4157. doi: 10.1016/0006-2952(89)90509-1. [DOI] [PubMed] [Google Scholar]
- 12.Sabbadini RA, Dabs SA. J. Bioenerg. Biomembr. 1989;21:163. doi: 10.1007/BF00812068. [DOI] [PubMed] [Google Scholar]
- 13.Theveniau M, Guo X-J, Rage P, Rougon G. J. Neurochem. 1991;57:67. doi: 10.1111/j.1471-4159.1991.tb02100.x. [DOI] [PubMed] [Google Scholar]
- 14.Allsup DJ, Boarder MR. Mol. Pharmacol. 1991;38:84. [PubMed] [Google Scholar]
- 15.Heilbronn E, Häggblad J. In: NATO AS1 Series. Zimmerman H, editor. H21. Heidelberg: Springer Verlag Berlin; 1988. p. 425. [Google Scholar]
- 16.Hourani SMO, Welford LA, Loizou GD, Cusack NJ. Eur. J. Pharmacol. 1988;147:131. doi: 10.1016/0014-2999(88)90642-5. [DOI] [PubMed] [Google Scholar]
- 17.Heilbronn E, Eriksson H. unpublished results. [Google Scholar]
- 18.Wood BE, Squire A, O'Connor SE, Leff P. Biological Actions of Extracellular ATP. In: Dubyak GR, Fedan JS, editors. Ann. New York Acad. Sci. Vol. 603. 1990. p. 461. [PubMed] [Google Scholar]
- 19.Kikugawa K, Suehiro H, Aoki A. Chem. Pharm. Bull. 1977;25:1959. doi: 10.1248/cpb.25.1959. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs T, Ötvös L. Tetrahedron Lett. 1988;29:4525. [Google Scholar]
- 21.Moffat JG. Can. J. Chem. 1964;42:599. [Google Scholar]
- 22.Benda P, Lightbody J, Sato G, Levine L, Sweet W. Science. 1968;161:370. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 23.Häggblad J, Eriksson H, Heilbronn E. Acta Physiol. Scand. 1985;125:389–393. doi: 10.1111/j.1748-1716.1985.tb07734.x. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson H, Heilbronn E. Biochem. Biophys. Res. Comm. 1989;159:878. doi: 10.1016/0006-291x(89)92190-6. [DOI] [PubMed] [Google Scholar]
- 25.Van Galen PJM, IJzerman AP, Soudijn W. FEBS Lett. 1987;223:197. doi: 10.1016/0014-5793(87)80535-5. [DOI] [PubMed] [Google Scholar]
- 26.Grynkiewicz G, Poenie M, Tsien RY. J. Biol. Chem. 1985;260:3440. [PubMed] [Google Scholar]
- 27.Jacobson KA. In: Comprehensive Medicinal Chemistry. Emmett JC, editor. Vol. 3. London: Pergamon Press; 1990. pp. 601–642. [Google Scholar]
- 28.Häggblad J, Heilbronn E. FEBS Lett. 1988;235:133. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- 29.Tassin AM, Häggblad J, Heilbronn E. Muscle Nerve. 1990;13:142. doi: 10.1002/mus.880130210. [DOI] [PubMed] [Google Scholar]
- 30.Pianet I, Merle M, Labouesse J. Biochem. Biophys. Res. Comm. 1989;163:1150. doi: 10.1016/0006-291x(89)92341-3. [DOI] [PubMed] [Google Scholar]
- 31.Lai K-M, Wong PCL. J. Neurochem. 1991;57:1510. doi: 10.1111/j.1471-4159.1991.tb06345.x. [DOI] [PubMed] [Google Scholar]
- 32.Pearson JD. Methods Pharmacol. 1985;6:83. [Google Scholar]