Abstract
While traditional cell culture methods have relied on growing cells as monolayers, three-dimensional (3D) culture systems can provide a convenient in vitro model for the study of complex cell–cell and cell–matrix interactions in the absence of exogenous substrates and may benefit the development of regenerative medicine strategies. In this study, mesenchymal stem cell (MSC) spheroids, or “mesenspheres”, of different sizes, were formed using a forced aggregation technique and maintained in suspension culture for extended periods of time thereafter. Cell proliferation and differentiation potential within mesenspheres and dissociated cells retrieved from spheroids were compared to conventional adherent monolayer cultures. Mesenspheres maintained in growth medium exhibited no evidence of cell necrosis or differentiation, while mesenspheres in differentiation media exhibited differentiation similar to conventional 2D culture methods based on histological markers of osteogenic and adipogenic commitment. Furthermore, when plated onto tissue culture plates, cells that had been cultured within mesenspheres in growth medium recovered morphology typical of cells cultured continuously in adherent monolayers and retained their capacity for multilineage differentiation potential. In fact, more robust matrix mineralization and lipid vacuole content were evident in recovered MSCs when compared to monolayers, suggesting enhanced differentiation by cells cultured as 3D spheroids. Thus, this study demonstrates the development of a 3D culture system for mesenchymal stem cells that may circumvent limitations associated with conventional monolayer cultures and enhance the differentiation potential of multipotent cells.
Keywords: Mesenchymal stem cell, Suspension culture, Scaffold-free culture, Tissue engineering
Introduction
Over the past several decades, mesenchymal stem cells (MSCs) have emerged as an attractive cell source for the treatment of a variety of traumatic injuries and chronic diseases. Originally isolated simply on the basis of their adherence to tissue-culture polystyrene (TCPS), MSCs have been further characterized to be included within the CD105+, CD73+, CD90+, CD34−, CD45− cell fraction of the bone marrow population (Dominici et al. 2006). More recently, MSCs and similar progenitor cells have been found and isolated from a number of other tissue sources including adipose tissue and umbilical cords. Notably, MSCs possess the ability to self-renew and differentiate to a number of mesenchymal lineages in vitro and in vivo, including bone, fat, cartilage and skeletal muscle (Pittenger 2008; Pittenger et al. 1999). However, in addition to their multipotent differentiation capacity, MSCs secrete a cadre of potent trophic factors that contribute to tissue remodeling and are largely thought to be immunoprivileged cells, thereby providing for allogeneic, off-the-shelf administration of these cells or more differentiated progeny for regenerative medicine therapies (Baraniak and McDevitt 2010).
Despite their regenerative potency, a paucity of MSCs exist in most adult tissues and as a consequence, MSCs are often expanded to large numbers (upwards of 109 cells) in vitro prior to in vivo transplantation. Conventional in vitro MSC culture techniques have involved expansion of the cells in an adherent fashion on TCPS substrates. Although MSCs maintain their plasticity for several passages on TCPS, it has been demonstrated that these cells eventually lose their ability to self-renew, replicate, form clonal colonies and differentiate to a number of lineages with successive passages, often long before adequate cell numbers for transplantation can be obtained (Bonab et al. 2006; Bork et al. 2010; Stolzing et al. 2006; Wagner et al. 2008). Such challenges surrounding MSC culture currently limit their therapeutic potential and therefore, improved methods for MSC maintenance and directed differentiation in vitro are needed.
Three-dimensional (3D) aggregates of cells in suspension culture, that commonly adopt a spheroid shape, have been widely used in cancer research (to mimic the tumor microenvironment) (Ivascu and Kubbies 2006; Marrero et al. 2009; Ong et al. 2010; Timmins and Nielsen 2007) and in stem cell cultures to emulate embryogenesis (Kurosawa 2007; Shukla et al. 2010; Carpenedo et al. 2009) and achieve clonal propagation and spontaneous differentiation (Yang et al. 2009; Wan et al. 2010; Ahlenius and Kokaia 2010). More recently, “microtissues” and “macrotissues” have been formed in vitro from the re-assembly of cells dissociated from tissues, from cell lines and from stem cells (Garzoni et al. 2009; Kelm et al. 2010; Kelm et al. 2006; Langenbach et al. 2010). Such scaffold-free, 3D cell culture systems are thought to more closely resemble the physiological tissue environment by enabling greater cell–cell and cell–matrix interactions than conventional monolayer culture techniques and provide a platform for the study of cell behaviors such as proliferation, differentiation, apoptosis and extracellular matrix (ECM) and biomolecule production in the absence of exogenous, artificial matrices that may impede intrinsic cell behaviors.
This study demonstrates the successful use of a forced-aggregation technique to obtain homogeneous populations of MSC spheroids of controlled size and the subsequent maintenance of these spheroids, in a multipotent state, for extended periods of time in suspension culture. Additionally, MSCs from intact and dissociated spheroids could be differentiated to adipogenic and osteogenic lineages and differentiation was enhanced in MSCs following 3D culture as compared to cells cultured in parallel as adherent monolayers. Overall, these results describe a novel, 3D culture system for the extended maintenance of multipotent MSCs in the absence of artificial substrates and represent a new approach for enhancing MSC in vitro differentiation potential compared to conventional, adherent monolayer culture systems.
Materials and methods
MSC monolayer expansion
Murine bone marrow-derived MSCs (moMSCs, isolated from C57Bl/6J mice) were obtained from the Texas A&M College of Medicine Institute for Regenerative Medicine and expanded according to established protocols (Peister et al. 2004). Briefly cryopreserved moMSCs were thawed and plated onto a 15-cm tissue culture dish in 20 mL moMSC complete expansion medium (CEM, Iscove’s Modified Dulbecco’s Medium; Invitrogen/GIBCO) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals), 10% horse serum (HS; Hyclone), 2 mM L-glutamine (Invitrogen/GIBCO), 100U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B (Invitrogen/GIBCO). Following overnight incubation at 37°C, adherent moMSCs were washed with phosphate-buffered saline (PBS, Invitrogen/GIBCO) and detached from the tissue culture plate using 0.25% trypsin and 1 mM Ethylene Diamine Tetraacetic Acid (EDTA) in Hanks’ Balanced Salt Solution (Invitrogen/GIBCO). Cells were counted using a hemocytometer and plated onto 15-cm tissue culture dishes, at a density of 50 cells/cm2, in 20 mL CEM per dish. Cells were fed every 3–4 days with 20 mL of fresh CEM and maintained until they reached ~70% confluence, at which point cells were once again trypsinized, counted and either re-plated for monolayer cultures or used for spheroid formation.
Mesensphere formation and maintenance
MoMSC monolayers (passages 8–10) were dissociated with trypsin-EDTA and resuspended in CEM to obtain a single cell suspension. MSCs (1.8–6×106 cells/mL, corresponding to approximately 300, 600, or 1,000 cells/microwell) were added to Aggrewell™ 6-well inserts containing an array of 400×400 μm-sized microwells, centrifuged at 200g for 5 min to force cell aggregation into the wells and incubated at 37°C overnight (Ungrin et al. 2008). After 18 h of culture in microwells, MSC spheroids, hereafter referred to as “mesenspheres”, were removed from the wells using a wide-bore pipette, transferred to 100-mm bacteriological grade Petri dishes (~ 1,500 mesenspheres in 10 mL medium) and cultured in suspension on a rotary orbital shaker (Lab-Line Lab Rotator, Barnstead International) for up to 3 weeks at 45±2 rpm, similar to previously described methods for embryonic stem cell differentiation as embryoid bodies (Carpenedo et al. 2007). Media was exchanged every 3 days of suspension culture by allowing mesenspheres to sediment in 15-mL conical tubes, aspirating the old medium, re-suspending in 10 mL fresh medium and returning spheroids to Petri dishes on rotary orbital shakers.
Cell proliferation assay
Cell proliferation was assessed on the basis of 5-bromo-2′-deoxyuridine (BrdU) incorporation. MSC monolayers and mesenspheres at days 1, 2, 3, 4 and 7 of culture were pulsed with 10 μM BrdU (Molecular Probes) for 6 h. Monolayers and mesenspheres were washed twice with PBS, fixed in 10% formalin (10 min for monolayers, 30 min for mesenspheres) and washed three times with PBS to remove formalin. For immunofluorescence, monolayers and whole spheroids were permeabilized with 0.1% Triton X-100 in PBS for 10 min, followed by DNA denaturation with 2.3 N HCl for another 10 min at RT prior to incubation with an anti-BrdU antibody (1:200 dilution, Molecular Probes) for 1 h at RT. Samples were then washed with PBS, incubated with a fluorescently-conjugated secondary antibody (Alexa 488, 1:200 dilution; Molecular Probes) for 1 h at RT, washed again with PBS and nuclei were counterstained with Hoechst dye (1:100; Sigma Aldrich) for 5 min at RT. Samples were washed with PBS and deionized water prior to imaging. Monolayers were imaged using a Nikon TE 2000 inverted microscope (Nikon) and a SPOT Flex camera (Diagnostic Instruments). Whole mount spheroids were imaged using a Zeiss LSM 510 NLO Confocal Microscope (Carl Zeiss).
Mesensphere plating and dissociation
For experiments using plated or dissociated mesenspheres, spheroids were collected after 2, 4, or 7 days of suspension culture by gravity sedimentation. Mesenspheres were then plated onto tissue culture plates in CEM and cells were allowed to grow out from plated mesenspheres for 7 days with media exchanged every 3 days. Mesenspheres for dissociation were washed twice with PBS and incubated in 0.25% trypsin-EDTA at 37°C for 15–30 min (depending on the size of the spheroids) with mechanical agitation until a single-cell suspension was obtained. The resulting cell suspension was counted with a hemocytometer and cells were plated onto tissue culture multi-well plates (2,000 cells/cm2) for proliferation and differentiation assays.
In vitro MSC differentiation assays
Osteogenic and adipogenic differentiation assays were initiated once moMSC and dissociated spheroid monolayers (initially plated at 2,000 cells/cm2) reached ~70% confluence, after 48 h of rotary suspension culture of mesenspheres, or after 7 days of monolayer culture of plated mesenspheres. For adipogenic differentiation, cells were maintained in adipogenic differentiation medium (ADM, CEM supplemented with 5 μg/mL insulin, 50 μM indomethacin, 1 μM dexamethasone and 0.5 μM isobutylmethylxanthine). For osteogenic differentiation, cells were maintained in osteogenic differentiation medium (ODM, CEM supplemented with 1 nM dexamethasone, 20 mM β-glycerolphosphate, 50 μM L-ascorbic acid 2-phosphate and 50 ng/mL L-thyroxine sodium pentahydrate). All supplements were obtained from Sigma-Aldrich. Cells and mesenspheres were maintained in CEM, ADM, or ODM for 7–14 days from the initiation of differentiation (day 0) with media exchanged every 3 days.
Morphological examination
Phase contrast images of cell monolayers and plated and suspension mesenspheres were obtained at days 0, 2, 4, 7 and 14 of growth and differentiation using a Nikon TE 2000 inverted microscope. Monolayers and mesenspheres were assessed visually for differences in cell morphology and the cross-sectional area of mesenspheres was measured using NIH ImageJ image analysis software (http://rsb.info.nih.gov/ij). At least 250 distinct mesenspheres from each time point were used to calculate area measurements. Histogram plots of area values were generated by plotting the relative fraction of mesenspheres across a range of average cross-sectional areas.
Histological analyses
MSC monolayers and dissociated or plated spheroids were fixed with 10% formalin for 10 min and washed three times with PBS prior to staining. Mesenspheres were harvested for histology and fixed with 10% formalin for 30 min, paraffin processed, embedded and sectioned as described above. Prior to staining, all paraffin-processed slides were de-paraffinized. Slides were stained with hematoxylin and eosin (H&E) using a Leica Autostainer XL and coverslipped. Using established staining protocols, osteogenic differentiation in monolayers and spheroid samples was assessed by von Kossa and Alizarin Red staining (Dennis et al. 2002; Gregory et al. 2004) and adipogenic differentiation was assessed by Oil Red O staining (Bancroft 2002). ECM deposition by mesenspheres was analyzed using Masson’s trichrome and Safranin O staining for collagen and glycosaminoglycans, respectively. Brightfield images were captured using a Nikon 80i Upright Microscope and a SPOT Flex camera in conjunction with SPOT Advanced v.4.5 software (Diagnostic Instruments). Oil Red O and Alizarin Red staining were quantified using established protocols to extract stains from cell monolayers (Gregory et al. 2004; Rim et al. 2005) and by normalizing the amount of stain to the DNA content of samples (determined using the PicoGreen assay kit from Invitrogen). For whole-mount immunofluorescence, mesenspheres were washed with PBS, fixed with 10% formalin for 30 min, permeabilized with 2% Triton X-100 for 30 min, re-fixed with 10% formalin for 15 min and washed again with PBS. For Nile Red staining, samples were incubated with Nile Red solution (1:1000 in acetone/PBS) for 10 min in the dark at RT, washed with PBS and nuclei were counterstained with Hoechst dye (1:100) for 5 min. Samples were stored in PBS at 4°C and imaged in a small volume of PBS using a Zeiss LSM 510 NLO Confocal Microscope.
Statistical analyses
Statistical analyses were performed using SPSS software. Comparisons across multiple experimental groups were conducted using a one-way analysis of variance (ANOVA) followed by Tukey post hoc analysis to determine significant differences (p<0.05).
Results
Mesensphere formation and maintenance
Following overnight incubation in Aggrewell™ inserts, mesenspheres of varying size, formed by incorporating 300, 600, or 1,000 cells per microwell, were observed (Fig. 1). Spheroids formed with 300 and 600 cells were visibly smaller than 1,000 cell spheroids both in microwells and upon transfer to suspension culture. However, uniform starting populations of spheroids were obtained at each of the cell densities examined and homogeneous populations of mesenspheres could be maintained for extended periods of time in suspension without significant agglomeration (Fig. 2a). By day 4 of culture, 300-cell spheroids had increased in size (from 19.6±2.2×103 μm2 to 21.2±0.9×103 μm2 average area) while the 600- and 1,000-cell spheroid average areas had decreased (from 23.3±5.1×103 μm2 to 12.6±8.0×103 μm2 for 600-cell and from 31.3±1.7×103 μm2 to 27.6±1.6×103 μm2 for 1,000-cell spheroids). By day 7 of culture, the 1,000-cell spheroid average area (24.7±4.8×103 μm2) was comparable to the 300-cell spheroid average area (19.3±0.6×103 μm2) while, surprisingly, 600-cell spheroids were noticeably smaller (7.9±5.6×103 μm2 average area). Independent of the starting density, each of the mesensphere populations exhibited fairly narrow size distributions by day 7 (Fig. 2b), indicating maintenance of homogeneous populations of spheroids through at least 7 days of suspension culture.
Fig. 1.
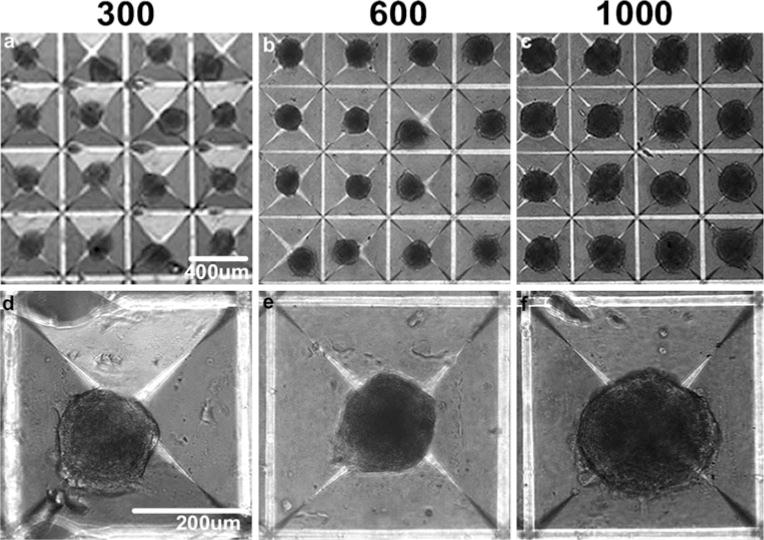
Mesensphere formation. Mesenspheres of controlled size, incorporating a 300, b 600, or c 1,000 cells each, were formed using forced-aggregation followed by MSC overnight incubation in Aggrewell™ inserts. d–f The relative size of the spheroids formed overnight correlated to the average number of cells entrapped within the individual wells. Scale bars (a–c) 400 μm, (d–f) 200 μm
Fig. 2.
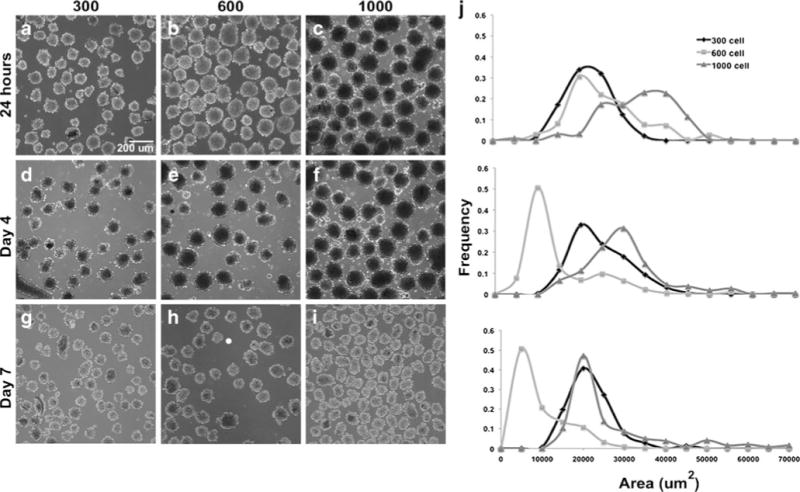
Mesensphere maintenance and size analysis. Following overnight mesensphere formation, spheroids were transferred to suspension culture on a rotary orbital shaker for subsequent analyses. a–i Uniform starting populations of spheroids were obtained at each of the cell densities examined and homogeneous populations were maintained for extended periods of time (up to 7 days shown) in suspension without significant agglomeration. j Morphometric size analyses confirmed that homogeneous mesensphere populations could be maintained through at least 7 days of suspension culture. The 300-cell spheroids initially increased in size while the 600- and 1,000-cell spheroid sizes decreased slightly. By day 7 of culture, the 1,000-cell spheroid average area was comparable to the 300-cell spheroid average area. Scale bar 200 μm
In order to assess cell proliferation and necrosis within mesenspheres, samples were collected for BrdU and H&E staining at various time points. An absence of necrotic core formation within spheroids, suggestive of cell viability in the interior of mesenspheres, was evident at all time points examined. Additionally, BrdU staining of mesenspheres demonstrated the presence of proliferating cells within spheroids at all time points up to day 7 (Fig. 3). However, BrdU-positive cells comprised only a small number of cells within spheroids, indicating that only a fraction of the cells (<5%) were actively proliferating in 3D culture.
Fig. 3.
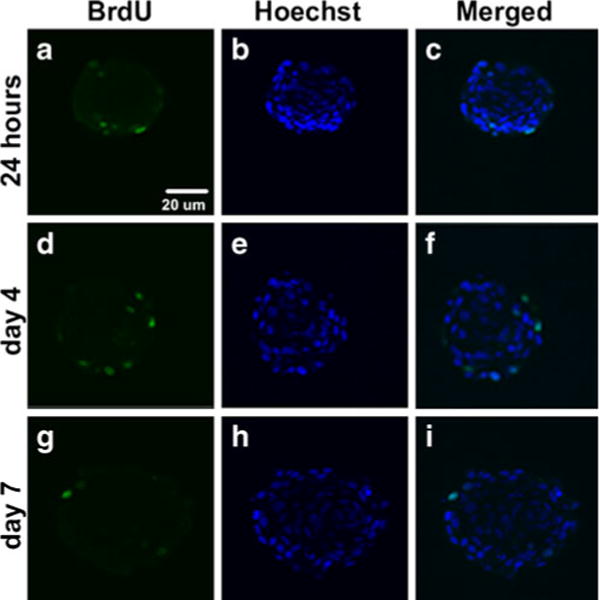
Cell proliferation within mesenspheres up to 7 days of culture. Mesenspheres in suspension culture were pulsed with BrdU to confirm MSC proliferation within spheroids. a–i BrdU staining indicated the presence of cycling cells within spheroids through 7 days of suspension culture. a, d, g However, BrdU+ cells were observed mainly at the periphery of spheroids and comprised only a small percentage (<5%) of the total number of cells. Scale bar 20 μm
Differentiation of MSCs in suspension culture
Phase contrast images comparing differentiated monolayers to 300-, 600- and 1,000-cell mesenspheres demonstrated differences in cell morphology as early as day 4 of osteogenic differentiation (not shown). Matrix mineralization was evident in monolayers and spheroids of all three sizes as early as day 4 and increased through day 14 of differentiation. Mineralization was not evident in cultures maintained in CEM (Fig. 4a–c), while mineral deposits within monolayers and mesenspheres in ODM appeared as opaque regions (Fig. 4i–k). Adipogenic differentiation, evidenced by lipid vacuole formation, was not apparent in monolayers at day 14 of differentiation (not shown) and was not evident until 21–28 days of differentiation (Fig. 4e). Little difference was seen in morphology between spheroids maintained in CEM and those maintained in ADM at all time points examined (Fig. 4b, c, f, g; day 14 shown).
Fig. 4.
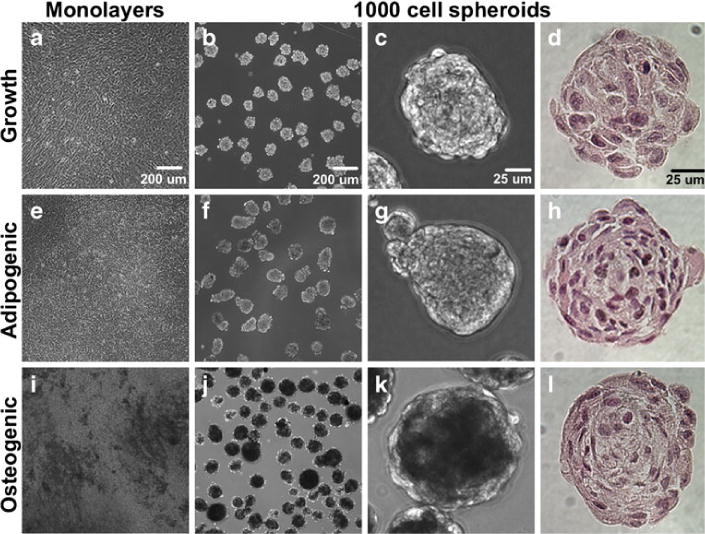
Maintenance of mesensphere differentiation potential in suspension culture. Monolayers and mesenspheres maintained under growth and differentiation conditions were examined using phase contrast microscopy for signs of differentiation. Mesenspheres were also histologically examined for differences in morphology. a–c Monolayers and mesenspheres maintained in growth medium (up to 16 days of culture) did not demonstrate lipid vacuole formation or matrix mineralization. e–g Lipid vacuole formation was evident in adipogenic monolayers by day 28 of differentiation but could not be discerned in adipogenic mesenspheres at day 14. b, c, f, g Mesenspheres maintained in adipogenic medium resembled those in growth medium upon microscopic examination. i–k Matrix mineralization was evident in both osteogenic monolayers and mesenspheres by day 14 of differentiation (opaque regions). d, h, l H&E staining of mesenspheres did not demonstrate any major differences in morphology between conditions. Scale bars (a, b, e, f, i, j) 200 μm, (c, d, g, h, k, l) 25 μm
Histological analyses of spheroid cross-sections revealed the absence of a necrotic core in all samples up to 14 days of differentiation (Fig. 4d, h, l; H&E images) and no obvious differences in differentiation potential or cell morphology were evident based on initial mesensphere size (not shown), hence 1,000-cell spheroids were used for all subsequent studies. Vacuole-like structures, commonly associated with adipogenic differentiation, were visible in some mesenspheres maintained in ADM (not shown), whereas such structures were not evident in mesenspheres maintained in CEM or ODM. However, drastic differences in H&E staining were not evident between any of the culture conditions up to day 14 of differentiation (Fig. 4d, h, l). Collagen deposition was not evident in mesenspheres maintained in CEM or ADM (Fig. 5a, d) as assessed by Masson’s trichrome staining. However, the presence of collagen was evident in spheroids maintained in ODM (highlighted by arrows in Fig. 5g), suggesting increased ECM production by osteogenic spheroids compared to those maintained under growth or adipogenic conditions. No positive GAG staining was evident in any of the mesensphere samples from any of the experimental groups (not shown). Matrix mineralization was not evident in any of the mesenspheres maintained in CEM or ADM (Fig. 5b, e) but Von Kossa staining of mesenspheres maintained in ODM for 14 days demonstrated the presence of calcium mineral deposits within spheroids (highlighted by arrows in Fig. 5h). Conversely, Nile Red staining demonstrated the presence of abundant lipid vacuoles throughout mesenspheres maintained in ADM (Fig. 5f and Supplemental Figure 1). Mesenspheres maintained in CEM also exhibited some lipid vacuole formation, although much less than those maintained in ADM (Fig. 5c and Supplemental Figure 1), while those in ODM were devoid of lipid vacuoles (Fig. 5i and Supplemental Figure 1).
Fig. 5.
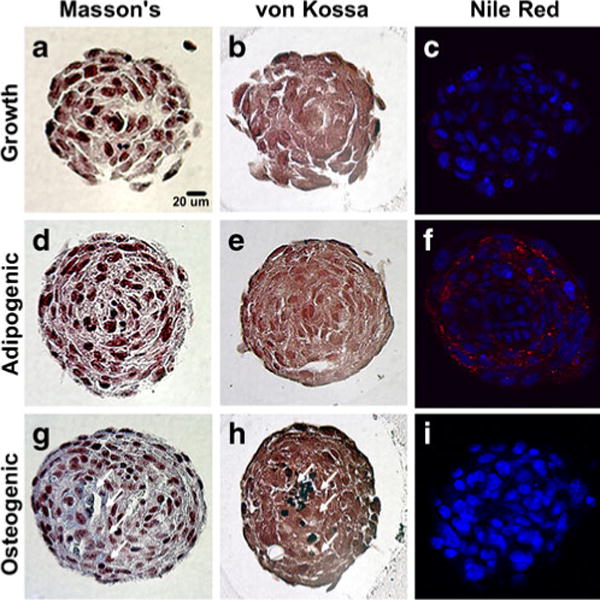
Mesensphere histological examination. Following suspension culture, mesenspheres were histologically examined for ECM deposition and markers of adipogenic and osteogenic differentiation. a, d Collagen deposition, assayed using Masson’s trichrome staining, was not evident in growth or adipogenic spheroids. g However, Masson’s trichrome staining indicated the presence of collagen (stained blue, arrows) within the ECM of osteogenic mesenspheres at day 14 of differentiation. b, e, h Positive von Kossa staining for calcium mineral deposits (stained black, arrows) was evident within osteogenic mesenspheres but not others following 14 days of differentiation. f Lipid vacuole formation (stained red) within adipogenic mesenspheres was confirmed by Nile Red staining at day 14 of differentiation. c, i Some lipid vacuole formation was evident in growth mesenspheres but none was evident in osteogenic mesenspheres. Scale bar 20 μm
In all spheroids, cells on the exterior were elongated in comparison to those on the interior and distinct differences in nuclear organization were evident within mesenspheres exposed to differentiation conditions (ADM and ODM) compared to spheroids in CEM (Figs. 4d, h, l and 5). Nuclei in adipogenic and osteogenic mesenspheres formed a radial pattern while nuclei in growth spheroids exhibited a more randomly organized multi-cellular pattern, suggesting differences in multi-cellular organization within mesenspheres that accompanied differentiation.
Maintenance of MSC plasticity following 3D culture
MSC outgrowths from plated mesenspheres and dissociated cells exhibited a typical spindle-shaped morphology resembling MSC monolayer cultures when maintained in CEM. However, dissociated cells immediately clustered together upon plating, aggregating into colony-like cell units (not shown). Such immediate “clustering” was not evident upon plating of conventional cell monolayers. BrdU staining confirmed the retention of MSC proliferative capacity following 3D suspension culture. Furthermore, proliferation in recovered cells was comparable to that of conventional monolayers as evidenced by the small proportion of cells (<5%) that incorporated BrdU within newly synthesized DNA (6-h pulse; Supplemental Figure 2).
Lipid vacuole formation and matrix mineralization were not evident in monolayers, plated mesenspheres, or dissociated cells maintained in CEM when examined by phase contrast or brightfield microscopy post-staining (Figs. 6a, c, e, g, i and 7a, c, e, g, i). However, upon culture in ADM, plated mesenspheres as well as dissociated cells exhibited lipid vacuole formation within 7 days (not shown) and by 14 days of differentiation, lipid vacuoles were more widespread within cultures recovered from mesenspheres (Fig. 6d, f, h, j) than in monolayer cultures after 28 days of differentiation (Fig. 6b). To quantitatively analyze whether the adipogenic differentiation potential of MSCs was enhanced following 3D suspension culture, Oil Red O stain was extracted from monolayers and compared to stain extracted from plated mesenspheres and dissociated cells (all after 14 days of differentiation). Plated mesenspheres and dissociated cells stained more robustly for Oil Red O than monolayers, with plated spheroids exhibiting a greater than 20-fold increase (p<0.0001) and dissociated cells exhibiting a greater than 40-fold increase (p<0.0001) in lipid vacuole formation compared to monolayers (Fig. 6k). Additionally, dissociated cells stained significantly more positive for lipids than plated mesenspheres (p<0.0001).
Fig. 6.
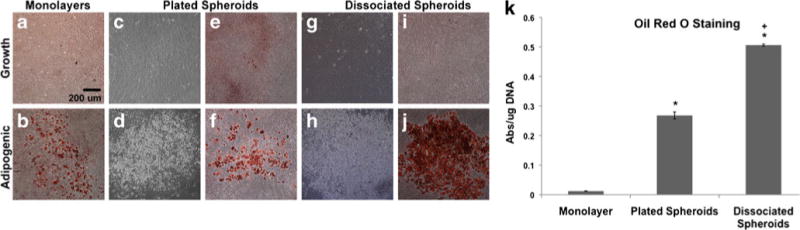
Adipogenic differentiation potential of MSCs following 3D culture. Following 7 days of suspension culture, MSCs were recovered from mesenspheres, plated onto TCPS and maintained under growth or adipogenic culture conditions. The adipogenic differentiation potential of recovered cells was compared to that of conventional monolayer cultures. Brightfield (a, b, e, f, i, j) and phase (c, d, g, h) images of Oil Red O-stained MSCs were obtained following differentiation. a, c, e, g, i Lipid vacuole formation was not evident in any cultures maintained under growth conditions. b, d, f, h, j Lipid vacuoles were more widespread within cultures recovered from mesenspheres following 14 days of differentiation than in monolayer cultures after 28 days of differentiation. Scale bar 200 μm. k Quantitative staining for adipogenesis exhibited significant differences between monolayer cultures and cells recovered from mesenspheres. Cells recovered from mesenspheres stained more robustly for Oil Red O than monolayers at day 14 of differentiation, with plated spheroids exhibiting a greater than 20-fold increase (*p< 0.0001) and dissociated spheroids exhibiting a greater than 40-fold increase (*p<0.0001) in the amount of lipid vacuole formation compared to monolayers. Dissociated mesenspheres also underwent greater adipogenesis (+p<0.0001) than plated spheroids
Fig. 7.
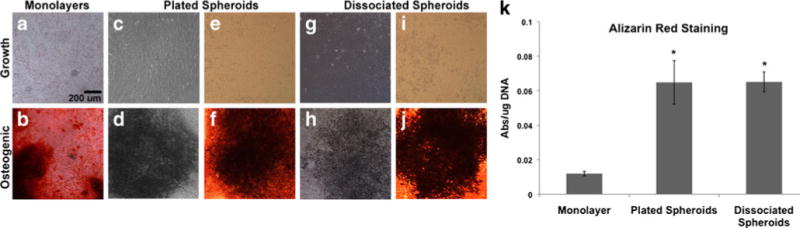
Osteogenic differentiation potential of MSCs following 3D culture. Following 7 days of suspension culture, MSCs were recovered from mesenspheres, plated onto TCPS and maintained under growth or osteogenic culture conditions. The osteogenic differentiation potential of recovered cells was compared to that of conventional monolayer cultures. Brightfield (a, b, e, f, i, j) and phase (c, d, g, h) images of Alizarin Red stained MSCs were obtained following differentiation. a, c, e, g, i Mineralization was not evident in any cultures maintained under growth conditions. b, d, f, h, j Mineralized regions were widely visible throughout all monolayer and recovered MSC osteogenic cultures by 14 days of differentiation. Scale bar 200 μm. k Quantitative staining for calcium deposits exhibited significant differences between monolayer cultures and cells recovered from mesenspheres. Cells recovered from mesenspheres stained more robustly for Alizarin Red than monolayers at day 14 of differentiation, with plated spheroids and dissociated cells exhibiting a greater than five-fold increase (*p<0.0001) in the amount of matrix mineralization compared to monolayers
Plated mesenspheres and dissociated cells cultured in ODM exhibited matrix mineralization within 4 days (not shown) and by 14 days of differentiation, mineralized regions were widely visible throughout cultures (Fig. 7d, f, h, j). To quantitatively analyze whether the osteogenic differentiation potential of MSCs was enhanced following 3D suspension culture, Alizarin Red stain was extracted from plated mesenspheres and dissociated cells and compared to stain extracted from MSC monolayers (Fig. 7b). Similar to the adipogenic differentiation results, a significant increase in the osteogenic potential of cells recovered from 3D cultures was evident, with plated mesenspheres and dissociated cells exhibiting a greater than five-fold increase in matrix mineralization compared to conventional monolayer cultures (Fig. 7k; p<0.0001). Thus, lipid vacuole formation in MSCs recovered from 3D culture was significantly accelerated (7 vs 21 days for lipid vacuole formation) and both osteogenic and adipogenic differentiation potential were significantly enhanced compared to conventional adherent monolayer cultures at similar time points.
Discussion
Isolating cells from native 3D tissues and culturing them on 2D substrates can alter normal cell physiological behaviors such as proliferation and differentiation and conventional stem cell monolayer cultures are limited by the propensity of cells to senesce and/or spontaneously differentiate with time (Bonab et al. 2006; Bork et al. 2010; Stolzing et al. 2006; Wagner et al. 2008). Such loss of MSC potential following ex vivo expansion is a major hurdle for the therapeutic use of these clinically-relevant cells. The results of this study demonstrate the successful development of a 3D, substrate-free culture system for MSCs that may not only enable the maintenance of multipotent MSCs but also may enhance differentiation towards particular phenotypes.
A forced-aggregation method for MSC spheroid formation yielded largely homogeneous populations of mesenspheres of controlled sizes, which could be maintained for extended periods of time in suspension via rotary orbital shaking. Up to at least 7 days of suspension culture, the homogeneity of spheroid populations was maintained, as evidenced by the retention of relatively narrow distributions in mesensphere cross-sectional areas at this time (Fig. 2). Of particular interest, regardless of initial cell seeding density and resultant spheroid size upon formation, small (300) and large (1,000) cell spheroids were comparable in size by day 7 of suspension culture. Surprisingly, intermediate (600) cell spheroids exhibited a reduction in size after 7 days of culture. Although it is beyond the scope of the current study, differences in cell shape and nuclear organization within spheroids (Figs. 4 and 5) of different sizes and under different culture conditions may lend support to the theory that cells within 3D environments alter their cytoskeletal arrangement in response to cell–cell contact and imposed forces as a result of varied stimuli. Importantly, the absence of necrotic core formation at all time points examined (Fig. 4) suggests that the decrease in 600- and 1,000-cell spheroid size was not due to significant cell death but rather could be due to cell compaction as a result of oxygen and nutrient diffusion into larger spheroids (Huang et al. 2010), as well as ECM remodeling and cadherin-, integrin- and fibronectin-mediated cell–cell interactions (Dikovsky et al. 2008; Ferrante et al. 2006; Lin et al. 2006; Robinson et al. 2004).
The introduction of dynamic culture conditions by rotary orbital shaking could account for preserved cell viability in the interior of spheroids despite more limited diffusion and proliferating cells were detected throughout spheroids up to at least 7 days of culture using a BrdU assay. Notably, while the percentage of cells actively cycling within spheroids was small (<5%), limited proliferation (3–5%) has also been noted in MSC monolayers and spheroids formed and maintained using other methodologies, suggesting that cell growth in spheroids is relatively comparable to that in conventional monolayer cultures (Frith et al. 2010). Additionally, in the reported studies, cells recovered from mesenspheres exhibited similar proliferative capacity compared to conventional monolayers (Supplemental Figure 2), confirming the retention of MSC proliferative capacity following 3D culture. Furthermore, the absence of signs of cell death may indicate successful circumvention of anoikis mechanisms (Frisch and Screaton 2001) using a forced-aggregation, substrate-free culture system and is further substantiated by the inability of mouse MSCs to spontaneously form spheroids when inoculated as a single cell suspension in rotary suspension culture (data not shown) (Carpenedo et al. 2010). Thus, high-density cell–cell contact between MSCs seems essential not only to the formation of multi-cellular aggregates but also to the promotion of cell viability in the absence of exogenous matrices or adherent substrates.
Mesenspheres subjected to differentiation protocols in suspension culture exhibited commitment to the adipogenic and osteogenic lineages, as evidenced by Nile Red staining of lipid vacuoles and von Kossa staining of calcium mineral deposits, respectively (Figs. 4 and 5). In addition, mesenspheres could be maintained under growth conditions for a prolonged period of time (up to 16 days of culture, the longest time point examined) in an undifferentiated state (i.e. without spontaneous differentiation of cells), as evidenced by the lack of abundant lipid vacuole formation or extracellular mineral deposition in growth spheroids (Figs. 4 and 5). However, while beyond the scope of the current study, these results motivate future serial passaging studies to determine multipotent MSC expansion and extended maintenance and differentiation potential in scaffold-free 3D culture systems (i.e. to more specifically assess the ability of this culture platform to circumvent passage limitations associated with MSC senescence in long-term adherent cultures).
Of particular interest in this study was whether MSCs cultured as 3D spheroids in suspension exhibited compromised differentiation potential compared to traditional adherent monolayer cultures. To this end, mesenspheres were either directly plated onto TCPS or dissociated into a single-cell suspension and plated onto TCPS following 7 days of suspension culture under growth conditions. MSC monolayers were concurrently plated at the same density as dissociated cell cultures (to eliminate potential cell density effects on differentiation) and all three culture sets were maintained in growth medium prior to being subjected to adipogenic and osteogenic differentiation protocols. The preservation of typical MSC morphology and proliferation potential was confirmed in plated and dissociated cells via visual microscopy inspection. Furthermore, to directly compare the differentiation potential of cells following 2D and 3D culture, quantitative Oil Red O and Alizarin Red staining was performed on all cell cultures after 14 days of differentiation and the quantities of extracted stains were normalized to the DNA content of the cultures (Figs. 6 and 7). MSCs exhibited significantly enhanced differentiation potential following 3D culture, as evidenced by increased Oil Red O and Alizarin Red staining. Additionally, the appearance of lipid vacuoles under adipogenic culture conditions was noted earlier in cells recovered from 3D suspension than in MSC monolayers (7 vs 21 days). These results suggest that 3D culture of mesenchymal stem cells may yield more uniformly differentiated populations than traditional monolayer culture techniques and are of particular note since the efficiency of differentiation (i.e. the percentage of cells within a population that commit to an intended phenotype) is a common limitation to conventional directed differentiation protocols for MSCs and many types of stem cells in general.
Taken together, the results of this study demonstrate the successful development of a 3D scaffold-free culture system for MSCs that maintains and enhances the in vitro multipotent differentiation of these cells. The use of such a culture system may circumvent several limitations associated with current methodologies for in vitro stem cell expansion and differentiation. Additionally, the development of 3D culture methods for stem and progenitor cells enables precise control over a number of parameters critical to tissue morphogenic events (such as aggregate size, resultant cell density and the hydrodynamic microenvironment) and can therefore provide a convenient in vitro model for the study of complex cell–cell and cell–matrix interactions, as well as trophic factor production and ECM deposition by cells, in the absence of exogenous substrates. Such systems more closely resemble in vivo tissue microenvironments and enable the study of cell behaviors without introducing many of the artifacts attributable to adherent cell culture practices. Thus, these results provide for directed, precise spatiotemporal control over cell behavior and may directly facilitate the fabrication of 3D cellular and acellular constructs from multipotent stem cells for tissue repair and regeneration.
Supplementary Material
Acknowledgments
The authors thank Ms. Martha Lesniewski for help with cell culture and spheroid size analyses, Ms. Sha’Aqua Asberry for assistance with histology sample preparation and Ms. Melissa Kinney for help with image processing. Dr. Baraniak is supported by a Postdoctoral Fellowship from the American Heart Association and this work was supported in part by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00441-011-1215-5) contains supplementary material, which is available to authorized users.
Contributor Information
Priya R. Baraniak, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, USA
Todd C. McDevitt, Email: todd.mcdevitt@bme.gatech.edu, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, USA, The Parker H. Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA, 313 Ferst Drive, Suite 2102, Atlanta, GA 30332, USA
References
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9(1):54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9(1):31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3(5):e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11(8):922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- Marrero B, Messina JL, Heller R. Generation of a tumor spheroid in a microgravity environment as a 3D model of melanoma. In Vitro Cell Dev Biol Anim. 2009;45(9):523–534. doi: 10.1007/s11626-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SM, Zhao Z, Arooz T, Zhao D, Zhang S, Du T, Wasser M, van Noort D, Yu H. Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials. 2010;31(6):1180–1190. doi: 10.1016/j.biomaterials.2009.10.049. [DOI] [PubMed] [Google Scholar]
- Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–151. doi: 10.1007/978-1-59745-443-8_8. [DOI] [PubMed] [Google Scholar]
- Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103(5):389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- Shukla S, Nair R, Rolle MW, Braun KR, Chan CK, Johnson PY, Wight TN, McDevitt TC. Synthesis and organization of hyaluronan and versican by embryonic stem cells undergoing embryoid body differentiation. J Histochem Cytochem. 2010;58(4):345–358. doi: 10.1369/jhc.2009.954826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30(13):2507–2515. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XZ, Kataoka K, Medina R, Yamamoto K, Than SS, Miyazaki M, Huh NH. A novel three-dimensional culture system for isolation and clonal propagation of neural stem cells using a thermo-reversible gelation polymer. Tissue Eng Part C Methods. 2009;15(4):615–623. doi: 10.1089/ten.TEC.2008.0516. [DOI] [PubMed] [Google Scholar]
- Wan F, Zhang S, Xie R, Gao B, Campos B, Herold-Mende C, Lei T. The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol. 2010;20(5):877–889. doi: 10.1111/j.1750-3639.2010.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius H, Kokaia Z. Isolation and generation of neurosphere cultures from embryonic and adult mouse brain. Methods Mol Biol. 2010;633:241–252. doi: 10.1007/978-1-59745-019-5_18. [DOI] [PubMed] [Google Scholar]
- Garzoni LR, Rossi MI, de Barros AP, Guarani V, Keramidas M, Balottin LB, Adesse D, Takiya CM, Manso PP, Otazu IB, Meirelles Mde N, Borojevic R. Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp Cell Res. 2009;315(19):3406–3418. doi: 10.1016/j.yexcr.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zund G, Hoerstrup SP. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol. 2010;148(1):46–55. doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Djonov V, Ittner LM, Fluri D, Born W, Hoerstrup SP, Fussenegger M. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12(8):2151–2160. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- Langenbach F, Naujoks C, Kersten-Thiele PV, Berr K, Depprich RA, Kubler NR, Kogler G, Handschel J. Osteogenic differentiation influences stem cell migration out of scaffold-free microspheres. Tissue Eng Part A. 2010;16(2):759–766. doi: 10.1089/ten.TEA.2009.0131. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cell. 2007;25(9):2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cell Tissue Organ. 2002;170(2–3):73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Bancroft JD. Theory and practice of histological techniques. 5. Churchill Livingstone; Edinburgh: 2002. [Google Scholar]
- Rim JS, Mynatt RL, Gawronska-Kozak B. Mesenchymal stem cells from the outer ear: a novel adult stem cell model system for the study of adipogenesis. FASEB J. 2005;19(9):1205–1207. doi: 10.1096/fj.04-3204fje. [DOI] [PubMed] [Google Scholar]
- Huang X, Wang J, Xie H, Zhang Y, Wang W, Yu W, Liu Y, Ma X. Microcapsules Embedded with Three-Dimensional Fibrous Scaffolds for Cell Culture and Tissue Engineering. Tissue Eng Part C Methods. 2010 doi: 10.1089/ten.TEC.2009.0545. (in press) [DOI] [PubMed] [Google Scholar]
- Dikovsky D, Bianco-Peled H, Seliktar D. Defining the role of matrix compliance and proteolysis in three-dimensional cell spreading and remodeling. Biophys J. 2008;94(7):2914–2925. doi: 10.1529/biophysj.107.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Rainaldi G, Indovina P, Indovina PL, Santini MT. Increased cell compaction can augment the resistance of HT-29 human colon adenocarcinoma spheroids to ionizing radiation. Int J Oncol. 2006;28(1):111–118. [PubMed] [Google Scholar]
- Lin RZ, Chou LF, Chien CC, Chang HY. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res. 2006;324(3):411–422. doi: 10.1007/s00441-005-0148-2. [DOI] [PubMed] [Google Scholar]
- Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15(3):973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. (Translated from eng) Tissue Eng Part C Methods. 2010;16(4):735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. (Translated from eng) Curr Opin Cell Biol. 2001;13(5):555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Carpenedo RL, Seaman SA, McDevitt TC. Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation. J Biomed Mater Res Part A. 2010;94(2):466–475. doi: 10.1002/jbm.a.32710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


