Summary
Serotonin is an essential neuromodulator, but the precise circuit connectivity that regulates serotonergic neurons has not been well defined. Using rabies virus tracing strategies Weissbourd et al., and Dorocic et al., in this issue of Neuron and Ogawa et al., in Cell Reports provide a comprehensive map of the inputs to serotonergic neurons; highlighting the complexity and diversity of potential upstream cellular regulators.
Serotonin, a neurotransmitter largely produced by neurons in the raphe nuclei, is involved with a diversity of behaviors such as mood, arousal, food intake, decision-making, reward, and aggression (Lucki, 1998; Nakamura, 2013). Despite serotonin's involvement in many essential behaviors, the precise circuit connectivity that regulates serotonin-producing neurons remains poorly understood. Neuroanatomical structures such as the prefrontal cortex and lateral habenulasend axonal projections to the dorsal raphe(Aghajanian and Wang, 1977), and can regulate raphe neuronal activity(Challis et al., 2014; Warden et al., 2012). However, the dorsal raphe is extremely heterogeneous, and contains discrete cells expressing genes associated not only with serotonin production, but also for other neurotransmitters such as glutamate (Hioki et al., 2010) and GABA(Bang and Commons, 2012). Given the diversity of genetically and electro physiologically(Kirby et al., 2003; Vandermaelen and Aghajanian, 1983) defined cell types within the raphe, it is likely that upstream neuronal cell populations selectively interface with serotoninergic (and other) cell types, however with classical neuroanatomical tracing techniques this has been difficult to establish. In papers this month by Weissbourd et al. and Dorocic et al. in Neuron, and Ogawa et al. in Cell Reports, this hurdle was overcome by utilizing rabies virus tracing strategies, in combination with cell type specificcre-driver mouse lines, to identify the precise brain-wide location of input neurons that interface with raphe cell populations. All three studies used modified GFP-encoding rabies virus pseudotyped with an avian envelop protein, which only transduces neurons that express a cognate receptor (TVA receptor), to target the initial entry of rabies viral particles to genetically defined raphe cell types(Wickersham et al., 2007) such as serotonergic neurons. By also expressing the rabies virus envelop glycoprotein, which promotes transsynaptic spread (Wickersham et al., 2007) input neurons that synapse onto the raphe starter cell populations are also labeled (Fig. 1). While this general strategy was utilized in all three studies, there are many interesting distinctions that set the three papers apart from each other.
Fig. 1. Rabies viral tracing strategies to target inputs onto raphe neurons.
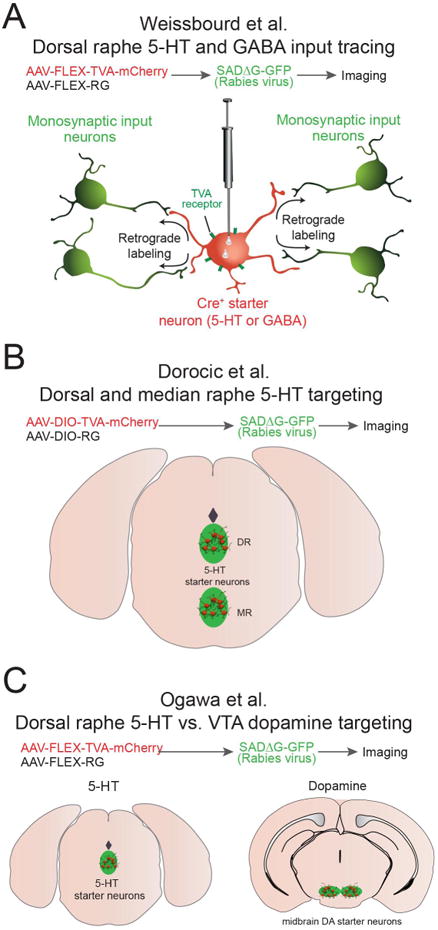
A. In this study, Weissbourd et al. targeted both serotonin and GABA neurons within the dorsal raphe nucleus. B. Dorocic et al., targeted serotonin neurons in both dorsal and medial raphe. C. Ogawa et al., compared inputs from serotoninergic and midbrain dopaminergic neurons.
The first study by Weissbourd et al., (2014) used rabies viral tracing, in situ hybridization, and brain slice electrophysiology to examine both long range and local inputs onto both dorsal raphe serotoninergic and GABAergic neurons (Fig. 1A). In addition to providing a comprehensive dataset of brain-wide inputs to these distinct dorsal raphe cell types this study also utilized two different forms of the TVA receptor in order to optimize their anatomical tracing at both the distal and local level. The first form was the traditional TVA receptor (TCB), which is highly efficient for long-range tracing, but can significantly increase background labeling at the initial site of viral transduction. The second form was a mutated TVA receptor (TC66T), which decrease stranssynaptic spread of the rabies virus, but also lowers non-specific viral labeling at the injection site, which was integral for quantifying local raphe connectivity to demonstrate both serotonin-to-serotonin and GABA-to-serotonin neuronal connectivity. Previous studies have indicated that the glutamatergic projection from the prefrontal cortex to the dorsal raphe can modulate both stress and depression-like behaviors (Amat et al., 2005; Warden et al., 2012). Using optogenetic stimulation of defined prefrontal inputs to the dorsal raphe Weissbourd et al., also show that DR serotonergic neurons, not GABAergic neurons, are preferentially innervated by prefrontal cortex glutamatergic neurons. Conversely, a larger proportion of DR GABAergic neurons receive input from central amygdala GABAergic neurons. In summary, Weissbourd et al. identified both long range and local inputs onto dorsal raphe serotoninergic and GABAergic neurons that likely coordinate raphe function, serotonin release, and behavior.
The study by Dorocic et al. (2014) examined the connectivity between afferents to serotonin neurons within both the DR and median raphe (MR) (Fig. 1B). In contrast with previous findings that utilized classical tracing strategies(Nakamura, 2013; Varga et al., 2001), Dorocic et al., show that DR serotonin neurons receive direct input from brain regions such as the PFC and lateral habenula (LHb). Additionally, they found that the lateral hypothalamus (LH) sends a strong projection to serotonin neurons in the DR. By utilizing immunohistochemistry in conjunction with rabies virus tracing, the authors also demonstrated that this neuronal projection originates from LH neurons that produce important neuropeptides such as melanin-concentrating hormone (MCH) vasopressin, and orexin. Furthermore, a functional projection from dopamine D1-receptor expressing neurons in the ventral striatum that preferentially innervates DR serotoninergic neurons was also identified.
In the study by Ogawa et al. (2104) presynaptic inputs onto serotonergic neurons from both the medial raphe and DR are quantified, and highlight the similarities and differences to input neurons that projection to another important monoaminergic cell group; midbrain dopaminergic neurons (Watabe-Uchida et al., 2012; Fig. 1C). Interestingly, the authors found that ventral tegmental area (VTA) dopamine neurons and DR serotonergic neurons receive input from strikingly similar presynaptic structures. Ogawa et al. also describe a ventral striatal input to the dorsal raphe, however it is noted that the ventral striatal neuronal population that innervated the DR is significantly less compared to the population of ventral striatal neurons that innervate the VTA. Additionally, DR serotonergic neurons synapse directly onto VTA dopaminergic neurons further suggesting coordinated and tightly regulated circuit-level activity between these two neuromodulatory cell groups.
While these three studies elegantly provide long-range and local circuit maps for the precise inputs to serotonin neurons, there are a few considerations related to approach used in all three studies. First, there may be subtle differences in which types of synapses (i.e. excitatory, inhibitory, neuromodulatory) rabies virus can effectively utilize to propagate, which in turn could lead to biased labeling of input neuron populations. For example Wall et al., (2013) recently reported that midbrain dopaminergic neurons are likely under-labeled when tracing inputs to direct and indirect pathway striatal neurons using a similar approach. Second, while quantitative whole brain cell counting data is useful, demonstrating functional connectivity between genetically defined pre- and postsynaptic neurons can greatly aide in determining whether a given input circuit is physiologically relevant. Lastly, the transynaptic labeling strategies used in these three studies rely upon the high degree of colocalization in expression of crerecombinase and markers for cell type specific function (i.e. Sert, Gad, and others). While many cre-diver lines are exquisitely specific for distinct cell types, it is likely that none of these mouse lines are entirely specific due to changing dynamics of gene expression over the developmental lifespan, as well as potential changes in the gene expression landscape due to neural activity and plasticity. Taken together, these studies begin to provide a cyclopedic map of the neuronal inputs to the serotonin system, thus offering an important circuit blueprint to put into context previous and future physiological and behavioral studies of the raphe nuclei.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain research. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Commons KG. Forebrain GABAergic projections from the dorsal raphe nucleus identified by using GAD67-GFP knock-in mice. The Journal of comparative neurology. 2012;520:4157–4167. doi: 10.1002/cne.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Beck SG, Berton O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Frontiers in behavioral neuroscience. 2014;8:43. doi: 10.3389/fnbeh.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorocic IP, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlén M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014 doi: 10.1016/j.neuron.2014.07.002. this issue. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. The Journal of comparative neurology. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biological psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K. The role of the dorsal raphe nucleus in reward-seeking behavior. Frontiers in integrative neuroscience. 2013;7:60. doi: 10.3389/fnint.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Uchida N, Watabe-Uchida M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Reports. 2014 doi: 10.1016/j.celrep.2014.06.042. doi? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain research. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pawath striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L. Presynaptic Partners of Dorsal Raphe Serotonergic and GABAergic Neurons. Neuron. 2014 doi: 10.1016/j.neuron.2014.06.024. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


