Abstract
Objective
To examine the relationship between asymmetric dimethylarginine (ADMA) and HIV-associated pulmonary arterial hypertension (PAH).
Design
HIV infection is an independent risk factor for PAH, but the underlying pathogenesis remains unclear. Chronic inflammation resulting in nitric oxide-mediated endothelial dysfunction is a key mechanism underlying other types of PAH. ADMA is an endogenous inhibitor of endothelial nitric oxide synthase. Among uninfected individuals, ADMA is associated with PAH and predicts disease-related mortality.
Methods
We measured ADMA, high sensitivity C-reactive protein, interleukin-6 (IL-6), D-dimer, and pulmonary artery systolic pressure (PASP) using echocardiography in HIV-infected individuals. Right heart catheterization (RHC) was performed in individuals with a PASP at least 30 mmHg. We performed multivariable analysis to identify factors associated with high PASP by echocardiogram and PAH by RHC.
Results
Among 214 HIV-infected individuals, the median age was 50 years, 82% were men, 71% were on antiretroviral therapy, and 4.2% carried a prior diagnosis of PAH. ADMA and IL-6 were associated with increased values of PASP following multivariable adjustment (7.2% per 0.1 μmol/l, P =0.0049 and 3.9% per doubling, P =0.027, respectively). In adjusted analysis among the 85 participants who underwent RHC, ADMA and IL-6 were associated with higher values of mean PAP (14.2% per 0.1 μmol/l, P =0.0014 and 5.8% per doubling, P =0.038, respectively). However, only ADMA was associated with PAH (prevalence ratio =1.74, P =0.029).
Conclusion
Elevated levels of ADMA are independently associated with PAH among HIV-infected individuals. Our findings suggest that chronic HIV-associated inflammation leading to an accumulation of ADMA and subsequent nitric oxide-mediated endothelial dysfunction may represent a novel mechanism for HIV-associated PAH.
Keywords: asymmetric dimethylarginine, endothelial dysfunction, HIV, nitric oxide, pulmonary arterial hypertension
Introduction
First described in 1987, infection with the HIV is now a well recognized independent risk factor for pulmonary arterial hypertension (PAH) [1]. PAH develops in 0.5% of HIV-infected individuals regardless of antiretroviral therapy (ART), a prevalence that is nearly 2500 times greater than that of the general population and may be an underestimation because it does not account for asymptomatic individuals [2,3]. We have previously reported that asymptomatic HIV-infected individuals have a high prevalence of elevated pulmonary artery systolic pressure (PASP) that is independent of other risk factors of PAH [4]. Furthermore, PAH is an independent risk factor for mortality in HIV infection with a median survival of 0.5–3.6 years depending on ART and New York Heart Association class, making it an exceedingly important complication of HIV infection [5–7].
The pathogenesis of HIV-associated PAH, however, remains poorly defined with various HIV proteins (e.g., HIV-1 Nef) and HIV-related inflammation potentially causally implicated [8]. Recent investigation in other forms of PAH including idiopathic, collagen vascular disease, and congenital heart disease supports nitric oxide-mediated endothelial dysfunction of the pulmonary vasculature as an underlying mechanism [9–11]. Nitric oxide is a potent vasodilator that inhibits cellular proliferation, inflammation, and thrombosis, which are all central processes in PAH. Thus, a reduction in nitric oxide bioactivity would disrupt pulmonary vascular homeostasis and lead to chronic vasoconstriction and elevated pulmonary arterial pressures [12]. Elevated levels of perivascular inflammatory cells [13] and inflammatory biomarkers [e.g. interleukin-6 (IL-6)] [14,15] in the aforementioned types of PAH suggest that chronic inflammation may also play a role in HIV-associated PAH.
Asymmetric dimethylarginine (ADMA) competitively inhibits endothelial nitric oxide synthase and, thus, is a mediator of endothelial dysfunction. ADMA accrues via dual mechanisms, namely degradation of methylated proteins, and reduced bioactivity of its metabolizing enzyme, dimethylarginine dimethylaminohydrolase (DDAH); DDAH is a redox sensitive enzyme whose activity declines with oxidative stress [16]. Among uninfected individuals, impaired activity of DDAH and endothelial nitric oxide synthase, and correspondingly increased ADMA levels, are associated with several forms of PAH [9–11,17]. Importantly, higher ADMA levels appear to predict PAH-related mortality [11].
ADMA levels are elevated in the setting of HIV infection [18,19] independent of traditional cardiovascular risk factors, and the degree of elevation reflects the extent of HIV-associated inflammation as indicated by the CD4+ T-cell count and HIV RNA level [20]. Additionally, baseline levels of D-dimer, high sensitivity C-reactive protein (hsCRP), and IL-6 are associated with increased cardiovascular risk among HIV-infected individuals [21]. However, the role of ADMA and HIV-associated inflammation in PAH in the setting of HIV infection has not been described; accordingly, the purpose of our study was to evaluate the association between ADMA, inflammatory biomarkers, and HIV-associated PAH. We hypothesized that higher levels of ADMA and inflammation would be associated with elevated pulmonary arterial pressures among HIV-infected individuals, and therefore, implicate nitric oxide-mediated endothelial dysfunction as a key contributor to the pathogenesis of HIV-associated PAH.
Methods
Study population
HIV-infected individuals were recruited from the Observational Study of the Consequences of the Protease Inhibitor Era (SCOPE) cohort, which is an ongoing prospective UCSF clinic-based cohort of more than 1500 HIV-infected adults. The sole inclusion criterion was documented HIV infection. There were no exclusion criteria – enrollment of participants was not based on symptoms, known history of or risk factors for PAH, or HIV-related factors. We enrolled 214 consecutive participants into our study. Of these individuals, nine (4.2%) had a known diagnosis of PAH. All participants underwent Doppler transthoracic echocardiography (TTE), and individuals with a PASP at least 30 mmHg were given the opportunity to undergo right heart catheterization (RHC). All study participants gave written informed consent and the UCSF Committee on Human Research approved this study.
Study design
Each participant completed an extensive study intake including review of demographic characteristics, specific risk factors for PAH (see below under Statistical Covariates), traditional cardiovascular risk factors, and HIV disease characteristics. A comprehensive chart review was done to determine duration of HIV infection, nadir CD4+ cell count, and detailed history of ART.
Blood measurements
All study participants provided fasting blood samples. Lipid panel, hsCRP (Dade Behring, Deerfield, Illinois, USA), current CD4+ cell count, and HIV RNA level were measured at the San Francisco General Hospital clinical laboratory. The nadir CD4+ cell count was the lowest documented value before study entry. Blood samples for ADMA were initially centrifuged at 4°C and then stored at −80°C. Plasma levels of ADMA were measured using a previously described modified high-performance liquid chromatography protocol (Oxonon BioAnalysis, Emeryville, California, USA) [22]. The coefficient of variation of ADMA was 4.1%. Additional markers of coagulation (D-dimer) and inflammation (IL-6) were measured in a subset of HIV-infected individuals. D-dimer was measured in EDTA plasma using an immunoturbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, New Jersey, USA). IL-6 was measured using an ELISA assay (R&D Systems HS600B, Minneapolis, Minnesota, USA).
Echocardiography
TTE was performed on all study participants by a trained sonographer (C.D.) using a GE Vivid Seven Imaging System (General Electric, Milwaukee, Wisconsin, USA). Peak tricuspid regurgitant jet velocity as determined by continuous-wave Doppler was used to calculate the pressure gradient between the right atrium and right ventricle utilizing the modified Bernoulli equation [23]. PASP was then estimated by adding that pressure gradient to the mean right atrial pressure [24]. Of note, PASP was presumed to be normal in participants with trace or no tricuspid regurgitation because individuals with clinically significant PAH typically have a detectable regurgitant jet [25]. Diastolic dysfunction and mitral regurgitation were measured per the American Society of Echocardiography guidelines to evaluate for elevated left heart filling pressures as a cause for increased PASP [26].
Right heart catheterization
Participants with a PASP at least 30 mmHg were given the option to undergo RHC. After placing a 7 French sheath into the femoral or internal jugular vein, a pulmonary artery thermodilution catheter (Edwards Biosciences, Irvine, California, USA) was advanced to the pulmonary artery. Comprehensive hemodynamic measurements including mean pulmonary artery pressure (mPAP) and pulmonary capillary wedge pressure (PCWP) were recorded.
Definitions and outcomes
Pulmonary hypertension is classically defined as a resting mPAP at least 25 mmHg. PAH is a subclass of pulmonary hypertension (WHO Group I) in which the PCWP is 15 mmHg or less. We screened participants for PAH noninvasively using a cut-off of PASP at least 30 mmHg as this is a widely accepted screening threshold and is consistent with prior studies of HIV-associated PAH [4]. Participants found to have PAH on RHC underwent routine pulmonary function testing and ventilation-perfusion imaging to rule out chronic obstructive pulmonary disease (WHO Group III) and chronic thromboembolic disease (WHO Group IV), respectively, as additional causes of pulmonary hypertension.
We, therefore, evaluated four outcomes: PASP by TTE assessed as a continuous variable, PASP by TTE assessed as a dichotomous variable at least 30 mmHg, mPAP by RHC assessed as a continuous variable, and PAH by RHC (mPAP ≥25 mmHg with PCWP ≤15 mmHg).
Statistical covariates
Candidate covariates of PASP/mPAP included demographic characteristics (age, gender, and race/ethnicity), specific risk factors for PAH, ADMA, and markers of coagulation (D-dimer) and inflammation (hsCRP and IL-6). Risk factors for PAH included cigarette smoking, intravenous drug use (IVDU), and stimulant use (i.e. cocaine and/or amphetamine/methamphetamine use).
Statistical analysis
We built smoothing splines using generalized additive models to assess the association between ADMA and PASP/mPAP. Multivariable linear regression models adjusting for all aforementioned candidate covariates and with robust standard errors were constructed to examine the relationship of each candidate covariate with PASP in all study participants, and with mPAP in the subset that underwent RHC. Due to their skewed distribution, PASP and mPAP were both log-transformed in all linear regression analyses; results were back-transformed to estimate percentage differences in both variables. We also used a multivariable Poisson regression model with a robust variance estimator to calculate a prevalence ratio, a risk ratio that estimates the relative risk for factors associated with PASP at least 30 mmHg and mPAP at least 25 mmHg. Given that the markers of coagulation and inflammation were only available in a subset of individuals, we used multiple imputation with the Markov chain Monte Carlo method to impute missing data, with five imputations yielding approximately 95% relative efficiency.
We performed Bayesian model averaging as an alternative model building approach; predictors with posterior probabilities less than 35% were excluded from the model. These two approaches yielded very similar models. Bayesian model averaging was done using the BMA package for the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were conducted using the SAS system, version 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Participant characteristics
ADMA levels were measured in 214 HIV-infected individuals, and nine (4.2%) of these individuals carried a prior diagnosis of PAH and were on PAH-specific therapy (Table 1). Overall, 82% were men, the median [interquartile range (IQR)] age was 50 years (43–56), 36% used cigarettes, 8% engaged in IVDU, and 18% used stimulants. For HIV-related characteristics, the median duration of HIV infection was 15 years (IQR 8–20), the median current CD4+ cell count was 586 cells/μl (IQR 370–761), 71% had undetectable HIV RNA levels, and 71% were on ART.
Table 1.
Baseline characteristics of HIV-infected participants.
| Parameter | All participants (N =214) | RHC (N =85) |
|---|---|---|
| Age (years) | 50 (43–56) | 52 (46–57) |
| Female | 38 (18%) | 16 (19%) |
| Race | ||
| White | 113 (53%) | 43 (51%) |
| African–American | 68 (32%) | 27 (32%) |
| Latino | 20 (9%) | 10 (12%) |
| Other | 13 (6%) | 5 (6%) |
| Coronary artery disease | 17 (8%) | 6 (7%) |
| Diabetes mellitus | 23 (11%) | 13 (15%) |
| Hypertension | 95 (44%) | 44 (52%) |
| Hyperlipidemia | 62 (29%) | 28 (33%) |
| hsCRP (mg/l) | 2.0 (0.9–4.3) | 2.5 (1.1–5.0) |
| BMI (kg/m2) | 26 (24–31) | 26 (24–32) |
| Cigarette smoking (ever) | 139 (65%) | 58 (68%) |
| Cigarette smoking (current) | 76 (36%) | 39 (46%) |
| IVDU (ever) | 79 (37%) | 43 (51%) |
| IVDU (current) | 18 (8%) | 13 (15%) |
| Stimulant use (ever) | 127 (60%) | 55 (65%) |
| Stimulant use (current) | 39 (18%) | 21 (25%) |
| Hepatitis C | 53 (25%) | 27 (32%) |
| Duration of HIV infection (years) | 15 (8–20) | 16 (11–21) |
| ART use (ever) | 167 (78%) | 76 (89%) |
| ART use (current) | 152 (71%) | 71 (84%) |
| ART duration (years)a | 6.6 (3.1–10.3) | 6.0 (2.8–9.6) |
| Current CD4+ cell count (cells/μl) | 586 (370–761) | 576 (347–761) |
| Nadir CD4+ cell count (cells/μl) | 208 (59–335) | 133 (40–256) |
| HIV RNA (viral load) | ||
| <75 (copies/ml) | 152 (71%) | 65 (76%) |
| 75–1999 (copies/ml) | 23 (11%) | 9 (11%) |
| 2000–9999 (copies/ml) | 14 (7%) | 3 (4%) |
| >10 000 (copies/ml) | 25 (12%) | 8 (9%) |
| History of PAH | 9 (4.2%) | 9 (10.6%) |
| PAH-specific therapy | 9 (4.2%) | 9 (10.6%) |
Data are presented as median (IQR) or numbers (percentage). ART, antiretroviral therapy; IQR, interquartile range; IVDU, intravenous drug use; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PAH, pulmonary arterial hypertension; PI, protease inhibitor; RHC, right heart catheterization.
Duration of ART use among ever users.
Relationship between asymmetric dimethylarginine, pulmonary artery systolic pressure, and pulmonary arterial hypertension
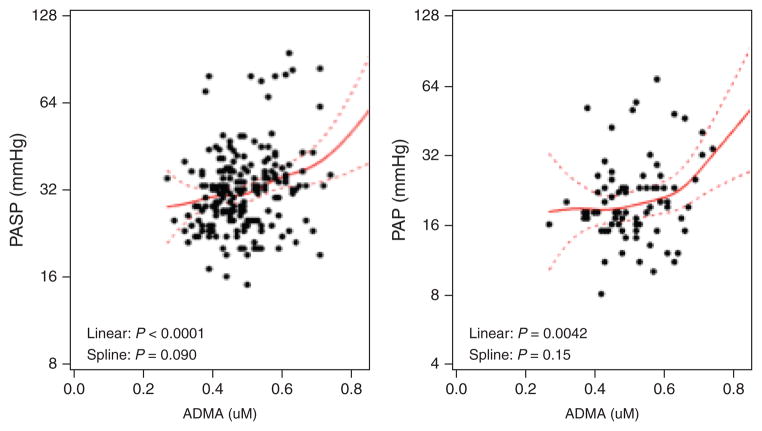
Among all participants, the median ADMA level was 0.48 μmol/l (IQR 0.42–0.55). The median ADMA levels among the 86 participants with a PASP less than 30 mmHg and 128 participants with a PASP at least 30 mmHg were 0.46 μmol/l (IQR 0.41–0.50) and 0.49 μmol/l (IQR 0.43–0.57), respectively. In unadjusted analysis, increased levels of ADMA were associated both with elevated values of PASP (9.5% per 0.1 μmol/l, P=0.0009, Supplemental Digital Content, Table 1S, http://links.lww.com/QAD/A442), and increased risk of having PASP at least 30 mmHg (prevalence ratio =1.19, P=0.043, Table 2). Following multivariable adjustment, the association between ADMA and PASP (assessed as a continuous variable) remained significant (6.4% per 0.1 μmol/l, P=0.0086), whereas the relationship between ADMA and PASP at least 30 mmHg weakened (prevalence ratio 1.13, P=0.20). The positive relationship between ADMA and PASP appeared to be strongest at higher ADMA levels (Fig. 1).
Table 2.
Factors associated with pulmonary artery systolic pressure at least 30 mmHg in HIV-infected participants.
| Parameter | Unadjusted Prevalence ratio (95% CI), P value | Fully adjusted Prevalence ratio (95% CI), P value |
|---|---|---|
| ADMA (per 0.1 μmol/l increase) | 1.19 (1.01, 1.42), P =0.043 | 1.13 (0.94, 1.35), P =0.20 |
| Age (per decade) | 1.11 (0.92, 1.33), P =0.28 | 1.13 (0.92, 1.38), P =0.25 |
| Female vs. male | 1.36 (0.90, 2.1), P =0.15 | 1.10 (0.68, 1.78), P =0.69 |
| African–American vs. white | 1.40 (0.96, 2.1), P =0.080 | 1.24 (0.80, 1.91), P =0.33 |
| Other/Latino vs. white | 1.24 (0.75, 2.0), P =0.40 | 1.23 (0.73, 2.1), P =0.44 |
| IVDU (current vs. never) | 1.85 (1.09, 3.1), P =0.022 | 1.48 (0.73, 3.0), P =0.28 |
| IVDU (past vs. never) | 1.33 (0.91, 1.96), P =0.15 | 1.31 (0.81, 2.1), P =0.27 |
| Cigarette smoking (current vs. never) | 1.39 (0.92, 2.1), P =0.11 | 1.06 (0.65, 1.73), P =0.80 |
| Cigarette smoking (past vs. never) | 1.04 (0.66, 1.64), P =0.87 | 0.99 (0.61, 1.62), P =0.98 |
| Stimulant use (current vs. never) | 1.36 (0.86, 2.1), P =0.19 | 0.97 (0.53, 1.80), P =0.93 |
| Stimulant use (past vs. never) | 0.99 (0.66, 1.47), P =0.95 | 0.77 (0.48, 1.24), P =0.28 |
| hsCRP (per doubling) | 1.05 (0.95, 1.15), P =0.32 | 1.01 (0.91, 1.12), P =0.91 |
| D-dimer (per doubling) | 1.14 (1.00, 1.30), P =0.044 | 1.04 (0.89, 1.22), P =0.60 |
| IL-6 (per doubling) | 1.19 (1.05, 1.34), P =0.0057 | 1.10 (0.95, 1.29), P =0.20 |
Analysis includes all 214 HIV-infected participants; prevalence of outcome was 59.8% (128 of 214 participants). Multiple imputation was used to handle missing covariates: CRP (N =181), D-dimer (N =170), and IL-6 (N =164). ADMA, asymmetric dimethylarginine; CI, confidence interval; IVDU, intravenous drug use; PASP, pulmonary artery systolic pressure; PR, prevalence ratio.
Fig. 1. Association of asymmetric dimethylarginine with pulmonary artery systolic pressure and mean pulmonary artery pressure in HIV infection.
Pulmonary artery systolic pressure (PASP) analysis (left-side scatterplot) comprises all 214 HIV-infected participants; mean pulmonary artery pressure (mPAP) analysis (right-side scatterplot) only includes subset of 85 HIV-infected participants with right heart catheterization (RHC). Solid lines denote predicted level of PASP and mPAP (with dotted 95% confidence intervals) calculated from unadjusted generalized additive models. Linear and spline P values indicate whether the linear and nonlinear curved associations, respectively, are statistically significant. ADMA, asymmetric dimethylarginine.
Of the 128 participants with a PASP at least 30 mmHg, 85 participants elected to undergo RHC. These participants had similar demographics, HIV-related characteristics, and rates of cardiovascular and PAH risk factors as those who deferred RHC (data not shown). Among the subset undergoing RHC, 20 participants had a mPAP at least 25 mmHg; 17 of these 20 had a PCWP 15 or less and, therefore, met hemodynamic criteria for PAH (Supplemental Digital Content, Table 2S, http://links.lww.com/QAD/A442). All 17 participants with PAH were on ART and 53% carried a known diagnosis of PAH and were on PAH-specific therapy prior to study entry; the remaining 47% were asymptomatic. The median ADMA level among the 65 participants with a mPAP less than 25 mmHg was 0.48 μmol/l (IQR 0.43–0.56). Among the 17 participants with PAH, the median ADMA was 0.56 μmol/l (IQR 0.45–0.66). In unadjusted analysis, elevated levels of ADMA were associated with both higher values of mPAP (13.4% per 0.1 μmol/l, P=0.0057, Supplemental Digital Content, Table 3Shttp://links.lww.com/QAD/A442) and PAH (prevalence ratio =1.55, P=0.029, Table 3). Notably, these associations remained significant after multivariable adjustment (13% per 0.1 μmol/l, P=0.0022 and prevalence ratio =1.70, P=0.041, respectively). The positive relationship between ADMA and mPAP was also strongest at higher ADMA levels (Fig. 1). Notably, none of the other candidate covariates demonstrated an independent association with PAH.
Table 3.
Factors associated with pulmonary arterial hypertension in HIV-infected participants.
| Parameter | Unadjusted Prevalence ratio (95% CI), P value | Fully adjusted Prevalence ratio (95% CI), P value |
|---|---|---|
| ADMA (per 0.1 μmol/l increase) | 1.55 (1.05, 2.3), P =0.029 | 1.70 (1.02, 2.8), P =0.041 |
| Age (per decade) | 0.72 (0.43, 1.23), P =0.23 | 0.59 (0.27, 1.28), P =0.18 |
| Female vs. male | 1.33 (0.43, 4.1), P =0.62 | 0.80 (0.16, 4.0), P =0.79 |
| African–American vs. white | 1.06 (0.38, 3.0), P =0.91 | 1.05 (0.26, 4.3), P =0.95 |
| Other/Latino vs. white | 0.64 (0.14, 2.9), P =0.56 | 0.85 (0.15, 4.8), P =0.86 |
| IVDU (current vs. never) | 1.62 (0.40, 6.5), P =0.50 | 0.64 (0.083, 5.0), P =0.67 |
| IVDU (past vs. never) | 1.87 (0.65, 5.4), P =0.25 | 2.2 (0.51, 9.5), P =0.29 |
| Cigarette smoking (current vs. never) | 1.90 (0.61, 6.0), P =0.27 | 1.20 (0.30, 4.7), P =0.80 |
| Cigarette smoking (past vs. never) | 0.71 (0.13, 3.9), P =0.69 | 0.47 (0.076, 3.0), P =0.42 |
| Stimulant use (current vs. never) | 1.43 (0.41, 4.9), P =0.57 | 0.95 (0.12, 7.5), P =0.96 |
| Stimulant use (past vs. never) | 1.24 (0.39, 3.9), P =0.72 | 0.81 (0.15, 4.3), P =0.80 |
| hsCRP (per doubling) | 1.12 (0.85, 1.48), P =0.42 | 1.15 (0.81, 1.64), P =0.44 |
| D-dimer (per doubling) | 1.09 (0.72, 1.65), P =0.69 | 0.91 (0.51, 1.61), P =0.73 |
| IL-6 (per doubling) | 1.29 (0.97, 1.73), P =0.081 | 1.30 (0.84, 2.0), P =0.24 |
Analysis only includes subset of 85 HIV-infected participants who underwent right heart catheterization; prevalence of outcome is 20% (17 of 85 participants). Pulmonary arterial hypertension (PAH) was defined as mean pulmonary arterial pressure at least 25 mmHg and pulmonary capillary wedge pressure 15 mmHg or less. Multiple imputation was used to handle missing covariates: CRP (N =68), D-dimer (N =71), and IL-6 (N =68). ADMA, asymmetric dimethylarginine; CI, confidence interval; IVDU, intravenous drug use; PR, prevalence ratio.
Relationship between coagulation and inflammatory markers, pulmonary artery systolic pressure, and pulmonary arterial hypertension
Additional markers of coagulation and inflammation (D-dimer, hsCRP, and IL-6) were available in the majority of our overall cohort (greater than 75% of individuals). These participants had similar demographics, HIV-related characteristics, and rates of cardiovascular and PAH risk factors as those who did not have inflammatory markers available. In the fully adjusted analysis, elevated levels of IL-6 were significantly associated with both higher values of PASP by TTE (3.9% per doubling, P=0.027, Supplemental Digital Content, Table 1Shttp://links.lww.com/QAD/A442) and higher values of mPAP by RHC (5.8% per doubling, P=0.038, Supplemental Digital Content, Table 3Shttp://links.lww.com/QAD/A442), though both of these associations were weaker than the corresponding ones of ADMA reported above. Additionally, the association between IL-6 and PAH failed to reach statistical significance (P=0.24, Table 3); as reported earlier, only ADMA remained significantly associated with PAH in the fully adjusted model. Neither D-dimer nor hsCRP demonstrated significant associations with PASP, mPAP, or PAH after multivariable adjustment.
Discussion
Despite recent advances in the epidemiology of HIV-associated PAH and insights into the pathogenetic role of viral proteins such as HIV-1 Nef, the underlying mechanism by which HIV infection causes pulmonary hypertension remains poorly understood. Our study demonstrates that, among HIV-infected individuals, elevated levels of ADMA are independently associated with PAH and higher levels of IL-6 are independently associated with increased mean pulmonary arterial pressures, findings that have not been previously reported. These observations are similar to prior work in other subtypes of PAH – namely, collagen vascular disease, congenital heart disease, and idiopathic PAH [9–11,14,15]. Taken together, our findings strongly suggest that HIV-associated PAH is driven by chronic inflammation resulting in an accumulation of ADMA and subsequent nitric oxide-mediated endothelial dysfunction.
PAH is a devastating complication of HIV infection that carries a poorer median survival and has a several thousand times higher prevalence than other forms of PAH [2,3,5–7]. The World Health Organization/UNAIDS 2009 Global Report estimates that 820 000 individuals in Europe are living with HIV. Using the reported prevalence of HIV-associated PAH of 0.5%, approximately 4100 Europeans would be expected to have HIV-associated PAH. However, recently published cohorts in Europe have been much smaller (N=19–82) with median survival periods of less than 4 years and rates of sudden cardiac death of 5–6% [2,3,6,7]. Although the reasons why more HIV-infected individuals are not presenting with clinically manifest PAH are unclear at this time, earlier detection of HIV-associated PAH remains critical given its poor prognostic implications. A recent study in idiopathic PAH demonstrated that ADMA (median level of 0.55 compared with 0.56 μmol/l in our study) was an independent predictor of mortality [11]. This observation coupled with our finding that elevated levels of ADMA are independently associated with PAH in HIV-infected individuals suggests that ADMA is a potential screening biomarker for HIV-associated PAH and may help identify individuals at higher risk for worse clinical outcomes.
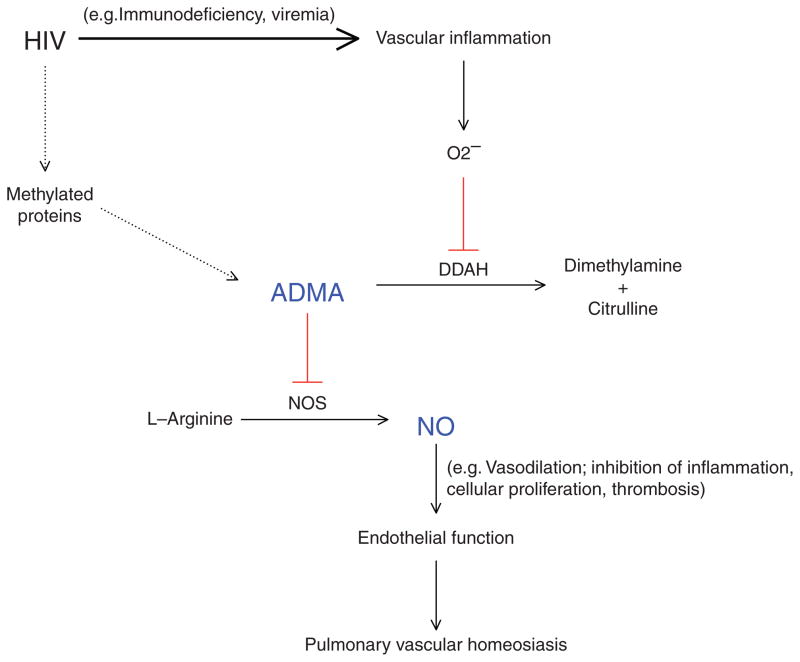
The impact of HIV disease characteristics and ART on HIV-associated PAH remains controversial. Whereas certain studies report CD4+ cell count [3,7] and ART [27] as prognostic factors, recent prospective studies in European HIV cohorts did not show a significant impact of ART on the development of PAH or survival [2,6]. Furthermore, the overall prevalence of HIV-associated PAH in the ARTera remains largely similar to that of pre-ART cohorts [2,3,5]. ADMA represents a potential mechanistic factor that links HIV infection to HIV-associated PAH. Previous studies have demonstrated that ADMA is independently elevated in the setting of HIV infection [18,19] and reduced by ART [28]. Our group recently reported that HIV-specific markers of chronic inflammation (low CD4+ cell count and high viral load) were independently associated with increased levels of ADMA [20]. We also found that even individuals with ART-suppressed HIV infection had higher levels of ADMA compared with uninfected controls, which may partly explain why ART has not significantly impacted the epidemiology of HIV-associated PAH. The principal mechanism by which ADMA accumulates in HIV infection appears to be through chronic HIV-associated inflammation leading to oxidative stress and subsequent impairment of DDAH. Prior studies have shown that certain markers of coagulation and inflammation (e.g. D-dimer, hsCRP, and IL-6) are elevated in the setting of HIV infection [29] and are independently associated with increased cardiovascular events [21], though HIV-associated PAH was not specifically evaluated in these studies. In our current study, ADMA was the only independent predictor of HIV-associated PAH, though IL-6 independently predicted elevated pulmonary arterial pressures on both TTE and RHC. Collectively, these findings, in addition to previously published reports of reduced DDAH activity and higher ADMA and IL-6 levels in other forms of PAH [9–11,14,15,17], provide strong evidence that HIV-associated chronic inflammation and the ADMA-DDAH-nitric oxide axis play a central role in HIV-associated PAH (Fig. 2).
Fig. 2. Asymmetric dimethylarginine-dimethylarginine dimethylaminohydrolase-nitric oxide axis in pathogenesis of HIV-associated pulmonary arterial hypertension.
Solid line = primary pathway; dashed line = secondary pathway; Red = inhibits. ADMA, asymmetric dimethylarginine; DDAH, dimethylarginine dimethylaminohydrolase; NO, nitric oxide; NOS, nitric oxide synthase; O2−, oxidative stress; PAH, pulmonary arterial hypertension.
ADMA may also represent a possible therapeutic target given its likely role in the pathogenesis of HIV-associated PAH. A recent study in rats showed that treatment of endothelial cells with a combined phosphodiesterase 3 and 4 inhibitor promoted endothelial regeneration and slowed development of PAH through increased DDAH expression/activity, reduced ADMA levels, and elevated nitric oxide levels [30]. Thus, differential modulation of the ADMA-DDAH-nitric oxide axis may be a potential avenue for molecular therapy to selectively target PAH in the future. In addition, IL-6 inhibitor therapy may also be a possible therapeutic opportunity in HIV-associated PAH.
Though contemporary registries of PAH without HIV report a higher risk of PAH in women (female to male ratios of 1.6–3.9) [31,32], most studies of HIV-associated PAH (N=35–82) demonstrate either no gender association [3,7] or an increased risk among men [2]. A smaller study of 19 Swiss patients with HIV-associated PAH reported a higher proportion of women (63%), but like our study, this cohort had a small number of patients overall [6]. In our study, we did not observe a significant relationship between gender and HIV-associated PAH, though the small number of individuals with PAH (N=17) – of whom only four (24%) were women – likely limited our ability to evaluate the association between gender and HIV-associated PAH. Nonetheless, our findings (76% of the HIV-associated PAH cohort were men) were consistent with those of the previously described studies, suggesting a trend toward an increased predilection of HIV-associated PAH in men rather than women
Our study has important limitations. First, our study’s cross-sectional design inherently limits the ability to establish causality. However, the consistency between our findings in an HIV population and the findings in other cross-sectional studies of other forms of PAH (e.g. collagen vascular disease, congenital heart disease, idiopathic) strongly support the biological pathway of elevated levels of ADMA causing nitric oxide-mediated endothelial dysfunction as a key mechanism of PAH [9–11,17]. Second, Doppler TTE is known to be an inaccurate measure of PASP; specifically in HIV-infected individuals, our group recently reported that Doppler TTE, in comparison with the gold-standard RHC, inaccurately estimated PASP in nearly 20% of cases and failed to capture approximately 33% of cases of PAH [33]. Although we performed RHC on patients with a PASP at least 30 mmHg, these findings suggest that we may have missed cases of PAH in patients with a PASP less than 30 mmHg and further highlight the critical need for better screening of HIV-associated PAH. Third, we did not directly measure endothelial function (e.g. brachial artery flow-mediated dilation). However, studies in both the general population and high cardiovascular risk groups have shown that ADMA is inversely related to flow-mediated dilation [34,35]. Finally, among the RHC subset, the median difference in ADMA that was detected between HIV-infected patients with and without PAH was modest (0.08 μmol/l) compared with that between other subtypes of PAH and healthy controls (0.17–0.19 μmol/l). This discrepancy was likely explained by the fact that HIV infection itself raises ADMA levels [18–20]. Notably, the median ADMA level we report in HIV-associated PAH (0.56 μmol/l) was similar to that reported in congenital heart disease-related PAH (0.55 μmol/l) [10] and idiopathic PAH (0.51 μmol/l) [11].
In conclusion, increased levels of ADMA are independently associated with HIV-associated PAH. Additionally, higher levels of IL-6 are independently associated with elevated mean pulmonary arterial pressures among HIV-infected individuals. These novel findings strongly support our hypothesis that chronic HIV-associated inflammation leading to dysregulation of the ADMA-DDAH-nitric oxide axis is an important mechanism of HIV-associated PAH. Therefore, ADMA and IL-6 may have utility as biomarkers for earlier recognition of HIV-associated PAH as well as potential therapeutic targets for disease modifying therapy in the PAH clinical arena. However, future studies will be needed to establish highly sensitive thresholds for capturing HIV-associated PAH and to determine whether elevated levels of ADMA and IL-6 are predictive of disease progression and mortality in HIV-associated PAH.
Supplementary Material
Acknowledgments
R.V.P. was responsible for the conception, design, interpretation of the data, drafting and revising the article, and final approval of the article submitted. R.S. and E.M.N. were responsible for collection and statistical analysis of the data. A.L.’s laboratory was responsible for measuring plasma ADMA levels. S.H. and V.M. conducted informed consent with all of the study participants and constructed the clinical database. J.S.M. performed all of the right heart catheterizations. J.N.M., S.G.D., P.G., and P.Y.H. contributed to the analysis and interpretation of data, revising the article, and approving the final paper.
The study received grant support from R01HL095130 (P.Y.H.), R01HL095126 (P.Y.H.), SCOPE*.
SCOPE cohort supported by NIAID (RO1 AI087145, K24AI069994), UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), and CFAR Network of Integrated Systems (R24 AI067039).
Footnotes
Conflicts of interest
P.Y.H. has received honoraria from Gilead and Pfizer. The remaining authors declare no conflicts of interest.
This work was presented in part at the Conference on Retroviruses and Opportunistic Infections, 5–8 March 2012, Seattle, WA, USA.
References
- 1.Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol. 1987;18:1293–1296. doi: 10.1016/s0046-8177(87)80417-3. [DOI] [PubMed] [Google Scholar]
- 2.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 3.Degano B, Guillaume M, Savale L, Montani D, Jais X, Yaici A, et al. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 4.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–833. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22:S35–S40. doi: 10.1097/01.aids.0000327514.60879.47. [DOI] [PubMed] [Google Scholar]
- 6.Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med. 1997;155:990–995. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 7.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 8.Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC. Pathogenesis of HIV-associated pulmonary hypertension. Proc Am Thorac Soc. 2011;8:308–312. doi: 10.1513/pats.201006-046WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitroulas T, Giannakoulas G, Sfetsios T, Karvounis H, Dimitroula H, Koliakos G, et al. Asymmetrical dimethylarginine in systemic sclerosis-related pulmonary arterial hypertension. Rheumatology. 2008;47:1682–1685. doi: 10.1093/rheumatology/ken346. [DOI] [PubMed] [Google Scholar]
- 10.Gorenflo M, Zheng C, Werle E, Fiehn W, Ulmer HE. Plasma levels of asymmetrical dimethyl-l-arginine in patients with congenital heart disease and pulmonary hypertension. J Cardiovasc Pharmacol. 2001;37:489–492. doi: 10.1097/00005344-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Kielstein JT, Bode-Boger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–1418. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 12.Ganz P, Vita JA. Testing endothelial vasomotor function. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 13.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 15.Gourh P, Arnett FC, Assassi S, Tan FK, Huang M, Diekman L, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther. 2009;11:R147. doi: 10.1186/ar2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke JP, Ghebremariam YT. DDAH says NO to ADMA. Arterioscler Thromb Vasc Biol. 2011;31:1462–1464. doi: 10.1161/ATVBAHA.111.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J. 2005;19:1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 18.Jang JJ, Berkheimer SB, Merchant M, Krishnaswami A. Asymmetric dimethylarginine and coronary artery calcium scores are increased in patients infected with human immunodeficiency virus. Atherosclerosis. 2011;217:514–517. doi: 10.1016/j.atherosclerosis.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Kurz K, Teerlink T, Sarcletti M, Weiss G, Zangerle R, Fuchs D. Plasma concentrations of the cardiovascular risk factor asymmetric dimethylarginine (ADMA) are increased in patients with HIV-1 infection and correlate with immune activation markers. Pharmacol Res. 2009;60:508–514. doi: 10.1016/j.phrs.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Parikh RV, Scherzer R, Grunfeld C, Nitta EM, Leone A, Martin JN, et al. Elevated levels of asymmetric dimethylarginine are associated with lower CD4+ count and higher viral load in HIV-infected individuals. Atherosclerosis. 2013;229:246–252. doi: 10.1016/j.atherosclerosis.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, et al. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 23.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 24.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 25.Borgeson DD, Seward JB, Miller FA, Jr, Oh JK, Tajik AJ. Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr. 1996;9:832–837. doi: 10.1016/s0894-7317(96)90475-7. [DOI] [PubMed] [Google Scholar]
- 26.Gibson DG, Francis DP. Clinical assessment of left ventricular diastolic function. Heart. 2003;89:231–238. doi: 10.1136/heart.89.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuber JP, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with anti-retroviral therapy. Clin Infect Dis. 2004;38:1178–1185. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 28.Baker JV, Neuhaus J, Duprez D, Freiberg M, Bernardino JI, Badley AD, et al. HIV replication, inflammation, and the effect of starting antiretroviral therapy on plasma asymmetric dimethylarginine, a novel marker of endothelial dysfunction. J Acquir Immune Defic Syndr. 2012;60:128–134. doi: 10.1097/QAI.0b013e318252f99f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pullamsetti SS, Savai R, Schaefer MB, Wilhelm J, Ghofrani HA, Weissmann N, et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases/clinical perspective. Circulation. 2011;123:1194–1204. doi: 10.1161/CIRCULATIONAHA.110.941484. [DOI] [PubMed] [Google Scholar]
- 31.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 32.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 33.Selby VN, Scherzer R, Barnett CF, MacGregor JS, Morelli J, Donovan C, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26:1967–1969. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction – its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 35.Juonala M, Viikari JSA, Alfthan G, Marniemi J, Kahonen M, Taittonen L, et al. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in Young Finns study. Circulation. 2007;116:1367–1373. doi: 10.1161/CIRCULATIONAHA.107.690016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.