Abstract
Importance
Preferred second line medication for diabetes treatment after metformin failure remains uncertain.
Objective
We compared time to acute myocardial infarction [AMI], stroke, or death in a cohort of metformin initiators who added insulin or a sulfonylurea.
Design
Retrospective cohort constructed using national Veterans Health Administration, Medicare, and National Death Index databases.
Participants
Veterans initially treated with metformin from 2001 through 2008 who subsequently added either insulin or sulfonylurea. Each insulin intensifier was propensity score matched by characteristics to five sulfonylurea intensifiers. Patients were followed through September, 2011 for primary analyses or September, 2009 for cause of death analyses.
Main Outcome Measures
Risk of a composite outcome of AMI, stroke hospitalization or all-cause death was compared between therapies using marginal structural Cox proportional hazard models to adjust for baseline and time-varying demographics, medications, cholesterol, hemoglobin A1c, creatinine, blood pressure, body mass index, and co-morbidities.
Results
Among 178,341 metformin monotherapy patients, 2,948 and 39,990 added insulin or sulfonylurea, respectively. Propensity score matching yielded 2,436 metformin+insulin and 12,180 metformin+sulfonylurea patients. At intensification, the median (interquartile range) time on metformin was 14 months (5, 30) and HbA1c was 8.1% (7.2, 9.9). There were 172 versus 634 events for the primary outcome among those who added insulin versus sulfonylureas respectively (42 versus 33 events per 1000 person-years, adjusted hazard ratio [aHR] 1.30, 95% confidence interval [CI] 1.07, 1.58, p=0.009). AMI and stroke rates were statistically similar 41 versus 229 (10.2 and 11.9 per 1000 person years, aHR 0.88,95% CI 0.59, 1.30, p=0.52), while all-cause death rates were137 versus 444, respectively (33.7 and 22.7 per 1000 person-years, aHR 1.44, 95% CI,1.15, 1.79, p=0.001). There were 54 versus 258 secondary outcomes: AMI, stroke hospitalizations or cardiovascular deaths (22.8 vs. 22.5 events per 1000 person years aHR 0.98, 95% CI 0.71, 1.34. p=0.87).
Conclusions
Among patients with diabetes using metformin, the addition of insulin versus sulfonylurea was associated with an increased risk of a composite of nonfatal cardiovascular outcomes and all-cause mortality. These findings require further investigation to understand risks associated with insulin use in these patients.
Keywords: Diabetes mellitus, cardiovascular disease, insulin, treatment intensification, comparative effectiveness
Diabetes mellitus (DM) and its complications represent an enormous healthcare burden and result in nearly 200,000 deaths annually. The American Diabetes Association and the European Association for the Study of Diabetes recommend that for patients with preserved renal function, treatment should begin with metformin and lifestyle changes to achieve a glycosylated hemoglobin (HbA1c) of ≤7%. Often patients will require a second agent to reach this goal, but there is no consensus regarding which medication to choose: insulin, sulfonylureas, thiazolidinediones, GLP-1 receptor agonists and DPP-4 inhibitors.1 Evidence to inform treatment choices after metformin monotherapy remains limited.
Providers start insulin to attain fast and flexible control of blood glucose. In addition, a few trials suggested that early insulin initiation is effective in preserving beta cell function.2–4 Accordingly there has been an increase in early initiation of insulin and its use as add on therapy to metformin.5–7 However, patients often want to delay insulin initiation, due to fears of difficulty with administration, weight gain and hypoglycemia.
We sought to compare time to cardiovascular disease (CVD) or death among patients who intensified their diabetes treatment with addition of insulin versus a sulfonylurea. We hypothesized that intensification with insulin would be associated with a lower risk of CVD or death compared with sulfonylurea, based on the superiority of insulin in achieving glycemic control.8
METHODS
Study Design and Data Sources
We assembled a retrospective cohort of Veterans Health Administration (VHA) patients.9 VHA data identified dispensed prescriptions, including medication, date filled, days supplied, pill number and dosage.10 VHA demographic data and ICD9-CM coded diagnostic and procedure information identified inpatient and outpatient encounters.11 We collected laboratory results from standard clinical sources. Vital signs data included all outpatient height, weight and blood pressure measurements. For enrollees in Medicare or Medicaid, we obtained encounter, prescription (Part D) and self-reported race data (coded as White; Black; Hispanic; American Indian; Asian/Pacific Islander; Other) from the Centers for Medicare and Medicaid Services through VHAs interagency exchange agreement.12, 13 We obtained dates of death from VHA vital status files and cause of death from National Death Index (NDI) data from VHA NDI agreements.14 The institutional review boards of Vanderbilt University and the VHA Tennessee Valley Healthcare System approved this study with waiver of informed consent.
Study Population
The study population comprised veterans aged 18 years or older who received regular VHA care (encounter or a prescription fill at least once every 180 days) for at least 2 years. Incident users of metformin from October, 2001 through September, 2008 with ≥365 days of baseline data preceding their first prescription fill who had not filled any diabetic drug within 180 days were identified. These metformin initiators were eligible for the treatment intensification cohort on the date that they subsequently filled either insulin or a sulfonylurea. We selected patients who were adherent to metformin by excluding patients with no metformin available on the date of their insulin or sulfonylurea prescription or the prior 180 days. Follow-up began 180 days after the intensified prescription to distinguish between patients who continued intensified therapy and those who switched to either insulin or sulfonylurea monotherapy. We excluded patients receiving hospice care or dialysis at intensification.
Exposures
The exposures of interest were insulin (long acting, premixed, or short/fast acting insulin) and sulfonylurea (glyburide, glipizide or glimeperide) as metformin co-therapies. Follow-up continued through a study outcome or the study end. The study end was September 30, 2011 for all analyses except those that included cause of death as an outcome, for which the study end was September 30, 2009. Patients were censored for loss of follow-up, defined as the 181st day of no contact with any VHA facility (inpatient, outpatient or pharmacy use); non-persistence, defined as 90 days without metformin; or prescription for a third anti-diabetic drug. In our population, allowing 90 days to refill medications approximates 80% adherence.15
Primary Outcome: Cardiovascular disease and all-cause death
The primary composite outcome was acute myocardial infarction (AMI), stroke hospitalization, or all-cause death. We defined AMI by a 410.x ICD9-CM primary discharge diagnosis (positive predictive value [PPV] 90% versus VHA medical record review). Stroke hospitalizations encompassed those with a primary discharge diagnosis for ischemic stroke (433.x1, 434 [excluding 434.x0], or 436), intracerebral hemorrhage (431), and subarachnoid hemorrhage (430), and excluded traumatic brain injury (800–804, and 850– 854) (PPV 81%).16
We determined all-cause death using Vital Status file, which combines information from Medicare, VHA, Social Security and VHA compensation and pension benefits to determine date of death (sensitivity 98.3%, specificity 99.8% relative to NDI).17 When the date of death in the VHA vital status file conflicted with the NDI date of death (<3%), we used the NDI date of death.
Secondary outcomes included CVD events (AMI and stroke combined); all-cause deaths; and a composite of AMI, stroke and cardiovascular death (through September 30, 2009). Cardiovascular deaths were identified from death certificates with an ICD-10 cause of death including I00-I78 (cardiovascular deaths) or R98, R99, R960, R961 (unattended deaths), excluding I30.X (diseases of the pericardium). This definition included the Centers for Disease Control and Prevention broad definition of cardiac death and a validated strategy for identification of sudden cardiac deaths.18
Covariates
Study covariates were collected in the 730 days before intensification and as time varying covariates and included: age, sex, race (white, black, other), fiscal year, indicators of healthcare utilization (hospitalization, months from hospitalization to intensification, nursing home use, number of outpatient visits, Medicare or Medicaid use in past year) physiologic variables (blood pressure, creatinine, HbA1c, low density lipoprotein levels, presence of proteinuria, and body mass index), duration of metformin monotherapy prior to intensifying diabetes regimen (diabetes duration), selected medications, smoking, and presence of co-morbidities (eTable 1). Since race can influence study outcome, it was included in all models.19
For patients missing covariates we conducted multiple imputation using the Markov Chain Monte Carlo method and a non-informative Jeffreys prior.20 All covariates from the primary analysis, survival time, and a censoring indicator were included in twenty imputation models and used to compute the final estimates.
Statistical Analyses
The primary analysis was time to the composite: AMI, stroke or all-cause death in a propensity score-matched cohort. The propensity score modeled the probability of metformin+ insulin use given covariates and Veterans Integrated Service Network (VISN) of care. Because of size differences between the 2 groups, metformin+ insulin observations were propensity score-matched to metformin+ sulfonylurea observations using a 1:5 optimal matching algorithm.21, 22 (eTable 2 and 3, and eFigure1)
Marginal structural Cox proportional hazards models (MSM) were used to compare outcomes for metformin+ insulin versus metformin+ sulfonylurea (referent) while controlling for baseline and time-varying covariates in the matched cohort (eTables 2–4). Since MSM estimates can be unstable in the presence of disproportionately large inverse probability treatment weights (IPTW),23, 24 the primary analysis used stabilized IPTW and truncated weights at 5, the 99th percentile. Thus, the proportional hazards MSM included the main effects of metformin+ insulin versus metformin + sulfonylurea weighting by IPTW. The proportional hazards assumptions were verified through examination of log-log plots. Statistical significance was considered a two sided p value <0.05.
Sensitivity and subgroup analyses
First, in an approach similar to intention to treat analyses, we used the intensification regimen to define drug exposure and ignored subsequent changes (persistent exposure not required [PENR]). Since patients were not censored for non-persistence, this increases follow-up and events. Second, we changed the stabilized IPTWs threshold (untruncated, truncated at 100, and 10). Third, we conducted subgroup analyses stratifying by CVD history, and age (<65 and ≥65 years), to assess effect modification. Among the subgroup with death certificates, we analyzed specific causes of death to identify cardiovascular, cancer, and all other deaths. Finally, we estimated the absolute prevalence difference of a hypothetical unmeasured binary confounder that would be required to yield a statistically non-significant association between exposure and outcome.25 We assumed a confounder-outcome association similar to our observed covariates (hazard ratio= 1.25) and considered a broad range of confounder prevalences in both exposures. Analyses were conducted using R (http://www.r-project.org. modules optmatch26 and RI tools27) and SAS for Windows 9.2. (SAS Institute, Cary, NC modules Proc MI, ProcPHREG for MSM and Proc Lifetest).
RESULTS
Study Cohort and Patient Characteristics
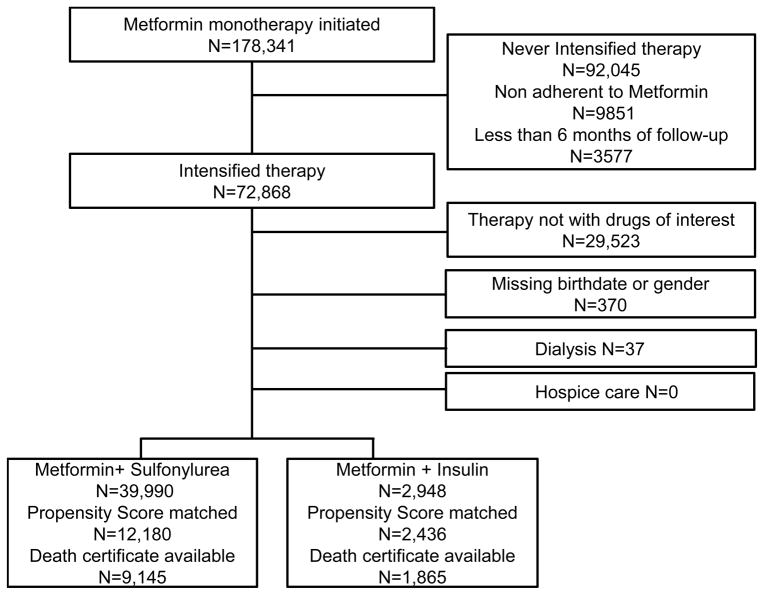
There were 178,341 patients who initiated metformin during 2001–2008. Fifty-two percent (N=92,045) never intensified therapy (median follow-up 50 months [19, 67]); 6% (N=9,851) stopped metformin; and 2% (N=3577) had <6 months of follow-up. Among the remaining 41% (N=72,868) of metformin initiators who started another therapy, 40% (29,523/72,868) were excluded because their regimen excluded metformin or included non-study medications.
Fifty nine percent (43,345/72,868) of metformin patients intensified with one of the two study regimens. We excluded <1% (N=407) of patients with data errors (N=370), hospice (N=0), or dialysis (N=37). The cohort included 2948 (7%) patients who added insulin (47% long acting, 22% both long and short acting, 17% premixed, 11% short acting) and 39,990 (93%) patients who added sulfonylurea (55% glipizide, 43% glyburide, 2% glimepiride). Seventy six percent of matched patients who died had a death certificate available (Figure 1).
Figure 1.
Flow of eligible patients.
Patients were 95% male and 70% white. Patients intensified with insulin after a median of 14 months on metformin versus 18 months for sulfonylurea intensifiers, and had higher median HbA1c, 8.5% versus 7.6%. Insulin intensifiers had a higher prevalence of co-morbidities than sulfonylurea intensifiers. The proportion prescribed metformin+insulin increased over time, with the odds increasing by an average of 17% (14%, 20%) per year, p<0.001. After propensity score matching, we included 14,616 patients: 2436 metformin+ insulin and 12,180 metformin+ sulfonylurea. Baseline characteristics were not statistically different (Table 1).
Table 1.
Characteristics of patients at the time of treatment intensification
| Characteristics | Full Cohort | Propensity matched Cohort | ||||
|---|---|---|---|---|---|---|
|
| ||||||
|
Metformin+ Sulfonylurea N=39,990 |
Metformin+ Insulin N=2948 |
Standardized differences* |
Metformin+ Sulfonylurea N=12180 |
Metformin+ Insulin N=2436 |
Standardized differences* |
|
| Age, median (IQR) | 61 (56, 69) | 60 (54, 67) | −0.13 | 60 (54, 68) | 60 (55, 68) | 0.02 |
| Male N (%) | 38345 (96) | 2787 (95) | −0.07 | 11521 (95) | 2315 (95) | 0.02 |
| Race, N (%) | ||||||
| White | 29458 (74) | 2023 (69) | −0.11 | 8612 (71) | 1726 (71) | 0.00 |
| Black | 5161 (13) | 571 (19) | 0.19 | 2028 (17) | 400 (16) | −0.01 |
| Hispanic/ Other | 1832 (5) | 124 (4) | −0.02 | 512 (4) | 111 (5) | 0.02 |
| Missing | 3539 (9) | 230 (8) | −0.04 | 1028 (8) | 199 (8) | −0.01 |
| Time to intensification†, months median (IQR) | 18 (7, 34) | 14 (5, 30) | −0.13 | 14 (6, 31) | 14 (5, 30) | −0.01 |
| HbA1c, % median (IQR) | 7.6 (7.0, 8.6) | 8.5 (7.0, 10.7) | 0.54 | 8.1 (7.2, 9.9) | 8.1 (6.9,9.9) | −0.07 |
| Missing measurement, N (%) | 5470 (14) | 573 (19) | 0.17 | 2315 (19) | 470 (19) | 0.01 |
| Low Density Lipoprotein mg/dL, median (IQR) | 87 (70, 110) | 87 (67, 113) | −0.02 | 86 (67, 110) | 87 (67, 113) | 0.02 |
| Missing measurement, N (%) | 8492 (21) | 851 (29) | 0.19 | 3408 (28) | 694 (28) | 0.01 |
| Creatinine mg/dL, median (IQR) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.2) | 0.04 | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.2) | 0.00 |
| Glomerular filtration rate ml/min, median (IQR) | 81 (70, 95) | 82 (69, 100) | 0.06 | 82 (70, 98) | 82 (70, 98) | 0.01 |
| Missing measurement, N (%) | 5978 (15) | 555 (19) | 0.11 | 2372 (19) | 468 (19) | −0.01 |
| Proteinuria, N (%) negative | 20909 (52) | 1489 (50) | 6044 (50) | 1214 (50) | ||
| trace through 4+ | 7468 (19) | 615 (21) | 0.01 | 2534 (20) | 503 (20) | 0.00 |
| Missing measurement, N (%) | 11613 (29) | 844 (29) | 0.01 | 3602 (30) | 719 (30) | 0.00 |
| Systolic Blood pressure mm/Hg, median (IQR) | 132 (122, 143) | 131 (120, 142) | −0.08 | 131 (120, 143) | 131 (120, 142) | 0.01 |
| Diastolic Blood pressure mm/Hg, median (IQR) | 77 (70, 84) | 76 (68, 83) | −0.07 | 76 (68, 83) | 76 (68, 84) | −0.01 |
| Missing measurement, N (%) | 1689 (4) | 187 (6) | 0.10 | 788 (6) | 159 (7) | 0.00 |
| Body Mass Index (kilograms/meter2), median (IQR) | 32.5(28.9,36.7) | 32.4 (28.3, 37.0) | −0.04 | 32.3(28.6,37.0) | 32.6(28.4,37.1) | 0.00 |
| Missing measurement, N (%) | 2098 (5) | 236 (8) | 0.12 | 961 (8) | 191 (8) | 0.00 |
| Baseline Co-morbidities N (%)‡ | ||||||
| Malignancy | 3059 (8) | 273 (9) | 0.06 | 1115 (9) | 223 (9) | 0.00 |
| Liver/ respiratory failure | 1156 (3) | 213 (7) | 0.25 | 668 (5) | 117 (5) | −0.04 |
| HIV | 125 (0.3) | 24 (0.8) | 0.09 | 69 (0.6) | 14 (0.6) | 0.00 |
| Congestive heart failure | 2222 (6) | 306 (10) | 0.21 | 1053 (9) | 209 (9) | 0.00 |
| Cardiovascular disease | 11849 (30) | 1056 (36) | 0.14 | 4125 (34) | 825 (34) | 0.00 |
| Serious mental illness | 11162 (28) | 1028 (35) | 0.15 | 3878 (32) | 768 (32) | −0.01 |
| Smoking | 7719 (19) | 685 (23) | 0.10 | 2581 (21) | 528 (22) | 0.01 |
| Chronic Obstructive Pulmonary Disease/ Asthma | 6114 (15) | 634 (22) | 0.17 | 2378 (20) | 481 (20) | 0.01 |
| Cardiac valve disease | 766 (2) | 84 (3) | 0.07 | 296 (2) | 62 (2) | 0.01 |
| Arrhythmia | 3449 (9) | 338 (11) | 0.10 | 1274 (10) | 255 (10) | 0.00 |
| Parkinson’s | 192 (0.5) | 36 (1) | 0.10 | 107 (0.9) | 21 (0.9) | 0.00 |
| Year N (%) | 0.14 | −0.03 | ||||
| 2002–03 | 1354 (3) | 104 (3) | 474 (4) | 93 (4) | ||
| 2004 | 3047 (8) | 191 (6) | 837 (7) | 171 (7) | ||
| 2005 | 4698 (12) | 282 (10) | 1171 (10) | 250 (10) | ||
| 2006 | 6737 (17) | 450 (15) | 1848 (15) | 379 (16) | ||
| 2007 | 7659 (19) | 451 (15) | 1895 (16) | 401 (16) | ||
| 2008 | 6544 (16) | 546 (19) | 2209 (18) | 428 (18) | ||
| 2009 | 5162 (13) | 475 (16) | 1915 (16) | 369 (15) | ||
| 2010 | 3691 (9) | 353 (12) | 1463 (12) | 275 (11) | ||
| 2011 | 1098 (3) | 96 (3) | 368 (3) | 70 (3) | ||
| Use of Medications N (%) | ||||||
| ACE Inhibitors or ARBs | 28685 (72) | 2072 (70) | −0.03 | 8576 (70) | 1727 (71) | 0.01 |
| Anti hypertensive medications | 28945 (72) | 2147 (73) | 0.01 | 8894 (73) | 1762 (72) | −0.02 |
| Statin and non-statin lipid lowering agents | 32206 (81) | 2210 (75) | −0.14 | 9250 (76) | 1858 (76) | 0.01 |
| Anti-arrhythmics, digoxin and other inotropes | 569 (1) | 78 (3) | 0.10 | 274 (2) | 57 (2) | 0.01 |
| Anticoagulants, platelet inhibitors | 4603 (12) | 482 (16) | 0.15 | 1849 (15) | 363 (15) | −0.01 |
| Nitrates | 3821 (10) | 376 (13) | 0.11 | 1472 (12) | 297 (12) | 0.00 |
| Aspirin | 9441 (24) | 872 (30) | 0.14 | 3411 (28) | 666 (27) | −0.02 |
| Loop Diuretics | 4204 (11) | 545 (18) | 0.25 | 2022 (17) | 395 (16) | −0.01 |
| Antipsychotics | 3254 (8) | 405 (14) | 0.20 | 1436 (12) | 279 (11) | −0.01 |
| Indicators of health care utilization N (%) | ||||||
| Hospitalized in last year | 5692 (14) | 1023 (35) | 0.57 | 3274 (27) | 631 (26) | −0.03 |
| Hospitalized in the 90 days prior to intensification | 2286 (6) | 679 (23) | 0.06 | 1732 (14) | 334 (14) | −0.02 |
| Nursing Home encounter in last year | 38 (0.1) | 11 (0.4) | 0.08 | 24 (0.2) | 4 (0.2) | −0.01 |
| Outpatient Visits in past year | 6 (4, 10) | 7 (4, 12) | 0.15 | 7 (4, 12) | 7 (4, 12) | −0.03 |
| Medicare use in last year | 11349 (28) | 1066 (36) | 0.17 | 4191 (34) | 843 (35) | 0.00 |
| Medicaid use in last year | 1046 (3) | 202 (7) | 0.25 | 590 (5) | 122 (5) | 0.01 |
Standardized differences are the absolute difference in means or percent divided by an evenly weighted pooled standard deviation, or the difference between groups in number of standard deviations. All P values in the unmatched cohort demonstrated statistically significant differences at p<0.001. In the matched cohort all standardized differences were statistically insignificant except HbA1c at p=0.05.
Time to treatment intensification represents the median number of months on metformin monotherapy. It is an approximation of the duration of diabetes since patients were free of all hypoglycemic medications for 180 days prior to starting metformin.
Definitions of comorbidities available in eTable 1
The most common reasons for censoring were therapy change (58.7% metformin+ insulin versus 61.7% metformin+ sulfonylurea), leaving VHA (1.3% versus 2.5%) or reaching study end (32.9% versus 30.6%). The median number of years before censoring or the outcome was 1.15 (0.5, 2.4) among metformin+ insulin patients and 1.15 (0.5, 2.2) among metformin+ sulfonylurea patients. At one year median HbA1c declined to 7% (6.3, 8.0) among metformin+ insulin users and 6.9% (6.4, 7.7) among metformin+ sulfonylurea users. Patient characteristics at one year were not statistically different (eTable 5).
Absolute and Relative Hazards of Cardiovascular Events and Deaths
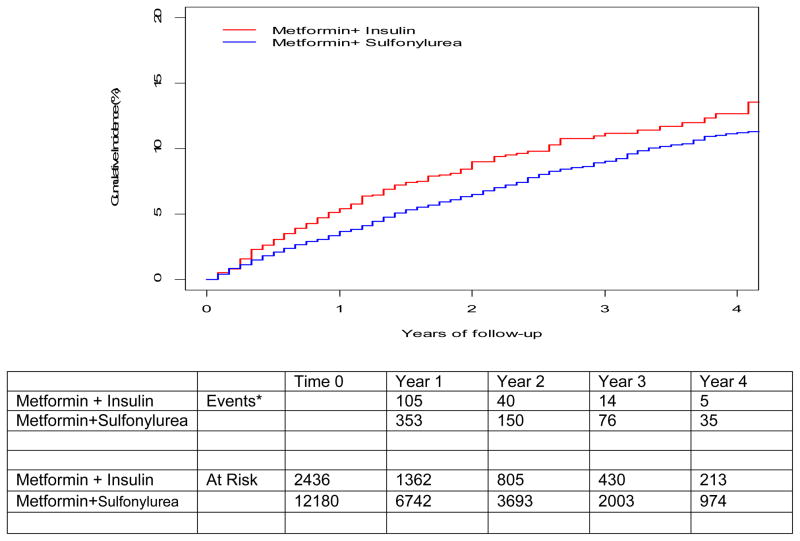
There were 172 versus 634 events for the primary outcome events among those who added insulin versus sulfonylureas respectively (42.7 versus 32.8 events per 1000 person-years, adjusted Hazard ratio [aHR] 1.30, 95% confidence intervals [CI] 1.07, 1.58 p=0.009). (Table 2, Figure 2A). CVD (AMI and stroke) events were 41 and 229, among those who added insulin or sulfonylurea, respectively (10.2 and 11.9 per 1000 person-years, aHR 0.88 95% CI 0.59, 1.30, p=0.52). All-cause deaths were 137 versus 444, respectively (33.7 and 22.7 per 1000 person-years aHR1.44, 95% CI 1.15, 1.79, p=0.001).
Table 2.
Rates and adjusted hazard ratios (95% confidence interval [CI]) for risk of cardiovascular disease or death (primary composite outcome) and cardiovascular events or deaths (secondary outcomes) among those who intensify with metformin + insulin versus metformin + sulfonylurea among propensity score matched cohort
| Persistent Exposure required* | Metformin+ Sulfonylurea | Metformin+ Insulin |
|---|---|---|
| Sample Size (N) | 12,180 | 2,436 |
| Person Years | 19,315 | 4,025 |
| Composite Cardiovascular events or all-cause death (N events) | 634 | 172 |
| Unadjusted Rate/1000 person-years (95% CI) | 32.8 (30.4, 35.4) | 42.7 (36.9, 49.4) |
| Adjusted Hazard Ratio† (95% CI) | Reference | 1.30 (1.07, 1.58) |
| AMI and Stroke hospitalizations events (N events) | 229 | 41 |
| Unadjusted Rate/1000 person-years (95% CI) | 11.9 (10.4, 13.5) | 10.2 (7.5, 13.8) |
| Adjusted Hazard Ratio † (95% CI) | Reference | 0.88 (0.59, 1.30) |
| All-cause death (N events) ‡ | 444 | 137 |
| Person Years | 19,596 | 4,071 |
| Unadjusted Rate/1000 person-years | 22.7 (20.7, 24.8) | 33.7 (28.5, 39.6) |
| Adjusted Hazard Ratio † (95% CI) | Reference | 1.44 (1.15, 1.79) |
| Composite Cardiovascular events or cardiovascular death (N Events) § | 258 | 54 |
| Sample size (N) | 9,145 | 1,865 |
| Person Years | 11,473 | 2,364 |
| Unadjusted Rate/1000 person-years (95% CI) | 22.5 (19.9, 25.4) | 22.8 (17.5, 29.7) |
| Adjusted Hazard Ratio † (95% CI) | Reference | 0.98 (0.71, 1.34) |
Primary analysis requires persistence on metformin; patients are censored after 90 days without metformin.
Adjusted hazard is derived from Cox proportional hazards marginal structural model for time to outcome truncating weights at 5. Refer to eTable 2 for the inverse probability treatment weights and etables 3 and 4 for the models used to derive Inverse probability treatment weights.
For the outcome of all-cause death, patients were followed until death as an outcome and AMI/Stroke events were ignored. In the composite outcome we consider the time to the first event (AMI, stroke, or death), which reduces the number of deaths in the composite outcome.
Death certificates with cause of death were available through September 30, 2009 and only patients with a date of intensification before September 30, 2009 were included in analyses.
Figure 2.
Figure 2 Panel A: Cumulative incidence of cardiovascular disease or death among propensity score matched cohort of metformin+ sulfonylurea initiators versus metformin + insulin initiators. All follow-up is through September 30, 2011
* Events are the composite of cardiovascular disease (Acute myocardial infarction, stroke) or all cause death that occurred in the 12 months between each time point.
Figure 2 Panel B: Cumulative incidence of fatal and non-fatal cardiovascular events (Acute myocardial infarction, stroke or cardiovascular deaths) among propensity score matched cohort of metformin+ sulfonylurea initiators versus metformin + insulin initiators. All follow-up is through September 30, 2009
* Events are the composite of fatal and non-fatal cardiovascular events (Acute myocardial infarction, stroke or cardiovascular deaths) that occurred in the 12 months between each time point
For the secondary outcome, fatal and nonfatal cardiovascular events, there were 54 versus 258 events (22.8 vs. 22.5 per 1000 person-years aHR 0.98, 95% CI 0.71, 1.34, p=0.87) (Table 2, Figure 2B).
Sensitivity and Subgroup Analyses
In sensitivity analysis in which persistent exposure was not required (PENR), there were 394 events for the primary outcome among insulin intensifiers (7456 person-years) and 1553 events among sulfonylurea intensifiers (37,237 person-years) yielding 52.8 (48.0, 58.2) and 41.7 (39.7, 43.8) events per 1000 person-years, respectively (aHR 1.29, 95% CI 1.15, 1.46, p<0.0001). Results of the stabilized non-truncated weights in the PENR analysis yielded comparable results (aHR1.30, 95% CI 1.15, 1.46, p<0.0001). MSM analyses which varied the threshold of the maximal stabilized weight, yielded consistent results (e Figure 2). No interaction between exposure and CVD history was detected (p=0.78). Subgroup analyses stratifying by CVD or age were consistent with the primary analysis, but confidence intervals were wide (eFigure 3). In separate analyses that evaluated cause of death, the aHRs for metformin+ insulin versus metformin+ sulfonylureas were increased for all groups, but statistically significant only for cancer death (Table 3). Assuming an association comparable to our measured covariates (i.e. HR 1.25), an unmeasured binary confounder would need to be 30% higher among metformin+ insulin users compared with metformin + sulfonylurea users to yield non-significant results in the main findings and 70% higher to yield statistical non-significance in the outcome of all-cause mortality. (eTables 6,7)
Table 3.
Comparison of specific-causes of death* among propensity score-matched cohort
| Persistent Exposure required† | Metformin+ Sulfonylurea N=9,145 |
Metformin+ Insulin N=1,865 |
|---|---|---|
| Person Years | 11,622 | 2,392 |
| Cardiovascular Death | 91 | 24 |
| Unadjusted Rate/1000 person-years (95% CI) | 7.8 (6.4, 9.6) | 10.0 (6.8, 14.9) |
| Adjusted Hazard Ratio ‡ (95% CI) | Reference | 1.21 (0.74, 2.00) |
| Cancer Death | 82 | 35 |
| Unadjusted Rate/1000 person-years (95% CI) | 7.1 (5.7, 8.7) | 14.6 (10.5, 20.3) |
| Adjusted Hazard Ratio ‡ (95% CI) | Reference | 1.85 (1.21, 2.84) |
| All Other Deaths | 123 | 41 |
| Unadjusted Rate/1000 person-years (95% CI) | 10.6 (8.9, 12.6) | 17.1 (12.7, 23.2) |
| Adjusted Hazard Ratio ‡ (95% CI) | Reference | 1.36 (0.90, 2.04) |
Death certificates with cause of death were available through September 30, 2009 and only patients with a date of intensification before September 30, 2009 were included in analyses.
Primary analysis requires persistence on metformin; patients are censored after 90 days without metformin. Cardiovascular outcomes such as AMI or stroke are ignored.
Adjusted hazard is derived from Cox proportional hazards marginal structural model for time to outcome truncating weights at 5. Refer to eTable 2 for the inverse probability treatment weights and eTables 3 and 4 for the propensity score models and the model used to derive Inverse probability treatment weights
COMMENT
Among patients with diabetes using metformin, the addition of insulin compared with sulfonylurea was associated with an increased hazard of a composite of nonfatal cardiovascular outcomes and all-cause mortality. There is general consensus that metformin is first line diabetes treatment; however, uncertainty remains regarding additional therapy after inadequate control with metformin. Among the options, intensification with either insulin or sulfonylurea is considered a high efficacy strategy with reasonable costs.1
Although sulfonylurea use predominated as add-on therapy, we observed increasing use of insulin intensification over the study years. Reasons may include growing prevalence of obesity and insulin resistance, emphasis on metrics such as glycemic targets,28, 29 increasing comfort with newer analog insulins, and benefit in microvascular outcome prevention.30
Two large randomized trials demonstrated that regimens including greater insulin use and tighter control did not reduce cardiovascular events compared with standard care. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, patients were randomized to intensive control (target HbA1c <6%) or standard care. About 77% of patients in the intensive-group received insulin compared with 55% in the standard group.31 ACCORD was stopped when interim analyses found higher all-cause deaths in the intensive versus standard group (5.0% vs. 4.0%; hazard ratio, 1.22; 95% CI, 1.01, 1.46). Most excess mortality, was due to cardiovascular deaths (2.63% vs. 1.83% over a mean 3.5 observation years, p= 0.02). Whether insulin itself or other effects of intense treatment like hypoglycemia32, 33 contributed to the increased mortality remains unknown.
The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial randomized 12,537 patients with CVD risk factors and pre-diabetes or diabetes to insulin glargine or standard care. Metformin (28%) and sulfonylurea (29%) use were similar in both groups; but insulin reached only 11% in the standard group by study end. After a median of 6 years, there was no difference in cardiovascular death, myocardial infarction or stroke between groups, 2.94 vs. 2.85 per 100 person-years, respectively (HR 1.02; 95% CI, 0.94, 1.11).34 There was also no difference in cancer or cancer death. However, patients in the insulin group had more weight gain and hypoglycemic events.
Several observational studies have also reported no cardiovascular benefit of insulin relative to non-insulin comparators and some suggested worse outcomes. A Canadian study35 reported increased all-cause mortality among insulin users compared to non-users in a dose response manner. Similarly, a study of primary care patients in the UK General Practice Research database36 determined that metformin+ insulin was associated with higher all-cause mortality, cardiovascular and cancer compared to metformin monotherapy. However, these studies did not address confounding by disease severity adequately. The first did not control for HbA1c, and the second compared more intensive therapies such as insulin (alone or in combination) with metformin monotherapy.
Our finding of a modestly increased risk of a composite of cardiovascular events and death in metformin users who add insulin compared to sulfonylurea is consistent with the available clinical trial and observational data. None of these studies found an advantage of insulin compared to oral agents on cardiovascular risk and several reported increased cardiovascular risk and/or weight gain and hypoglycemic episodes, which could result in poorer outcomes. Although insulin remains a reasonable option for patients who have very high glucose or who desire flexible and fast glucose reduction, most patients prefer to delay insulin initiation.37 Our study suggests that intensification of metformin with insulin among those who could add a sulfonylurea (HbA1c less than ~10%), offers no advantage on risk of cardiovascular events, and is associated with some risk.
Our findings must be interpreted in light of limitations. Although we applied an extensive set of strategies to address confounding by indication including rigorous selection criteria, propensity score matching and marginal structural models, residual confounding from difficult to measure factors, such as patient frailty or diabetes severity, remains possible. Nevertheless our sensitivity analyses estimated that a large confounding effect would be needed for an unmeasured confounder to explain our observations. Using similar methods in a VHA diabetes cohort, we previously demonstrated drug effects on lipids, HbA1c and body mass index that were concordant with clinical trials and meta-analyses9, 38–40. Our results are consistent with UKPDS trial results which demonstrated a reduction of cardiovascular events with metformin but not insulin or sulfonylurea.
There are several other limitations to be noted. We utilized refill data as a proxy for medication taking. Nevertheless, prescription fills are a good proxy for medication use. Veterans may not receive all their care or medications in VHA facilities;12, 13 resulting in missing events or medications, which we partially addressed through supplementation with Medicare and Medicaid information. Because we required patients to persist on their medications, censoring was high. In addition, patients who added insulin comprised only 7% of intensifiers. This resulted in a relatively small sample size and limited the precision of some estimates. The statistical significance of our primary outcome was driven by all-cause mortality, and a clinically significant cardiovascular benefit could not be excluded. Our primary analyses considered a matched population. Some patients who were prescribed metformin + insulin did not match metformin + sulfonylurea users. Excluded metformin + insulin users (N=512) had a median HbA1c of 11.8% (8.9%, 13.7%) and 67% of these patients were hospitalized in the 90 days prior to insulin initiation (eTable 8). Results can only be generalized to metformin patients who were eligible to add either medication. Finally, our patients reflect a typical veteran population, with most patients being white and male.
Conclusion
Among patients with diabetes using metformin, the addition of insulin compared with sulfonylurea was associated with an increased risk of a composite of nonfatal cardiovascular outcomes and all-cause mortality. These findings require further investigation to understand risks associated with insulin use in these patients and call into question recommendations that insulin is equivalent to sulfonylureas for patients who may be able to use an oral agent.
Acknowledgments
Role of the Sponsors: The Agency for Healthcare Research and Quality and the Veterans Affairs Information Resource Center reviewed the manuscript prior to submission, but had no other involvement with the study. The other funders of the project had no involvement in the study design or conduct; data collection, management, analysis, and interpretation; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Funding Source
This project was funded by the Agency for Healthcare Research and Quality, US Department of Health and Human Services, Contract No. HHSA2902010000161, as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE 2) program. This work was supported in part by VA CSRD investigator initiated grant I01CX000570-01 (Roumie) and the Center for Diabetes Translation Research P30DK092986 (Elasy, Roumie). Dr. Hung (2-031-09S) was supported by VA Career Development Award. Support for Veterans Affairs/Centers for Medicare & Medicaid Services data provided by the Department of Veterans Affairs, Veterans Affairs Health Services Research and Development Service, Veterans Affairs Information Resource Center (project numbers SDR 02-237 and 98-004).
Footnotes
Disclaimer
The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the Department of Veterans Affairs.
Disclosures
None
Author Contributions
Design (Roumie, Elasy, Grijalva, Griffin)
Conduct/data collection (Roumie, Liu, Greevy, Murff, Griffin)
Analysis (Liu, Greevy)
Drafting manuscript (Roumie)
Critical revision of manuscript (Roumie, Hung, Liu, Greevy, Grijalva, Murff, Elasy, Griffin)
Drs. Roumie and Greevy had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012 Jun;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008 May 24;371(9626):1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 3.Harrison LB, Adams-Huet B, Raskin P, Lingvay I. beta-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care. 2012 Jul;35(7):1406–1412. doi: 10.2337/dc11-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarsson M, Sundkvist G, Lager I, et al. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab. 2008 May;10(5):421–429. doi: 10.1111/j.1463-1326.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed November 25, 2013.];Adults with Diabetes by Diabetes Medication Status, United States, 1997–2011. Available at: http://www.cdc.gov/diabetes/statistics/meduse/fig1.htm.
- 6.Cohen FJ, Neslusan CA, Conklin JE, Song X. Recent antihyperglycemic prescribing trends for US privately insured patients with type 2 diabetes. Diabetes Care. 2003 Jun;26(6):1847–1851. doi: 10.2337/diacare.26.6.1847. [DOI] [PubMed] [Google Scholar]
- 7.Holden SE, Poole CD, Morgan CL, Currie CJ. Evaluation of the incremental cost to the National Health Service of prescribing analogue insulin. BMJ Open. 2011 Jan 1;1(2):e000258. doi: 10.1136/bmjopen-2011-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 9.Hung AM, Roumie CL, Greevy RA, et al. Comparative effectiveness of incident oral antidiabetic drugs on kidney function. Kidney Int. 2012 Apr;81(7):698–706. doi: 10.1038/ki.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold N, Hines D, Stroupe K. Comparison of VA Outpatient Prescriptions in the DSS Datasets and the PBM Database. Hines, IL: Edward Hines, Jr. VA Hospital; Jan 15, 2006. [Google Scholar]
- 11.International Classification of Diseases, Ninth Revision, Clinical Modification. Washington, DC: Public Health Service, US Dept of Health and Human Services; 1988. [Google Scholar]
- 12.Humensky J, Carretta H, de Groot K, Brown MM, Tarlov E, Hynes D. Service Utilization of Veterans Dually Eligible for VA and Medicare Fee-For-Service: 1999–2004. Medicare & Medicaid Research Review. 2012;2(3) doi: 10.5600/mmrr.002.03.a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes DM, Koelling K, Stroupe K, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care. 2007 Mar;45(3):214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy JF, Valenstein M, Kim HM, Ilgen M, Zivin K, Blow FC. Suicide mortality among patients receiving care in the veterans health administration health system. Am J Epidemiol. 2009 Apr 15;169(8):1033–1038. doi: 10.1093/aje/kwp010. [DOI] [PubMed] [Google Scholar]
- 15.Greevy RA, Jr, Huizinga MM, Roumie CL, et al. Comparisons of persistence and durability among three oral antidiabetic therapies using electronic prescription-fill data: the impact of adherence requirements and stockpiling. Clin Pharmacol Ther. 2011 Dec;90(6):813–819. doi: 10.1038/clpt.2011.228. [DOI] [PubMed] [Google Scholar]
- 16.Niesner K, Murff HJ, Griffin MR, et al. Validation of VA administrative data algorithms for identifying cardiovascular disease hospitalization. Epidemiology. 2013 Mar;24(2):334–335. doi: 10.1097/EDE.0b013e3182821e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001 Dec;58(12):1161–1167. doi: 10.1001/archpsyc.58.12.1161. [DOI] [PubMed] [Google Scholar]
- 19.Office of Minority Health. Heart Disease and African Americans. US department of Health and Human Services; [Accessed February 19, 2014.]. Available at: http://minorityhealth.hhs.gov/templates/content.aspx?ID=3018. [Google Scholar]
- 20.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0) SAS reference documents version 3.0. 2011 [Google Scholar]
- 21.Parsons L. Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. :214–226. [Google Scholar]
- 22.D’Agostino R, Rubin D. Estimating and using Propensity Scores with partially missing data. Journal of the American Statistical Association. 2000 Sep;95(451):749–759. 2000. [Google Scholar]
- 23.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000 Sep;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000 Sep;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005 Jan;16(1):17–24. doi: 10.1097/01.ede.0000147164.11879.b5. [DOI] [PubMed] [Google Scholar]
- 26.Version: R package version 0.9–1. 2010. optmatch: functions for optimal matching [computer program] [Google Scholar]
- 27.Version: R package version 0.1–11. 2010. RItools: Randomization Inference Tools [computer program] [Google Scholar]
- 28.Selby JV, Uratsu CS, Fireman B, et al. Treatment intensification and risk factor control: toward more clinically relevant quality measures. Med Care. 2009 Apr;47(4):395–402. doi: 10.1097/mlr.0b013e31818d775c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerr EA, Gerzoff RB, Krein SL, et al. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med. 2004 Aug 17;141(4):272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 30.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 31.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonds DE, Miller ME, Dudl J, et al. Severe hypoglycemia symptoms, antecedent behaviors, immediate consequences and association with glycemia medication usage: Secondary analysis of the ACCORD clinical trial data. BMC Endocr Disord. 2012;12:5. doi: 10.1186/1472-6823-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012 Jul 26;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 35.Gamble JM, Simpson SH, Eurich DT, Majumdar SR, Johnson JA. Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab. 2010 Jan;12(1):47–53. doi: 10.1111/j.1463-1326.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 36.Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013 Feb;98(2):668–677. doi: 10.1210/jc.2012-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes. 2010 Apr;4 (Suppl 1):S11–18. doi: 10.1016/S1751-9918(10)60004-6. [DOI] [PubMed] [Google Scholar]
- 38.Roumie CL, Huizinga MM, Liu X, et al. The effect of incident antidiabetic regimens on lipid profiles in veterans with type 2 diabetes: a retrospective cohort. Pharmacoepidemiol Drug Saf. Nov 12; doi: 10.1002/pds.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012 Nov 6;157(9):601–610. doi: 10.7326/0003-4819-157-9-201211060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roumie CL, Liu X, Choma NN, et al. Initiation of sulfonylureas versus metformin is associated with higher blood pressure at one year. Pharmacoepidemiol Drug Saf. 2012 May;21(5):515–523. doi: 10.1002/pds.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]