Abstract
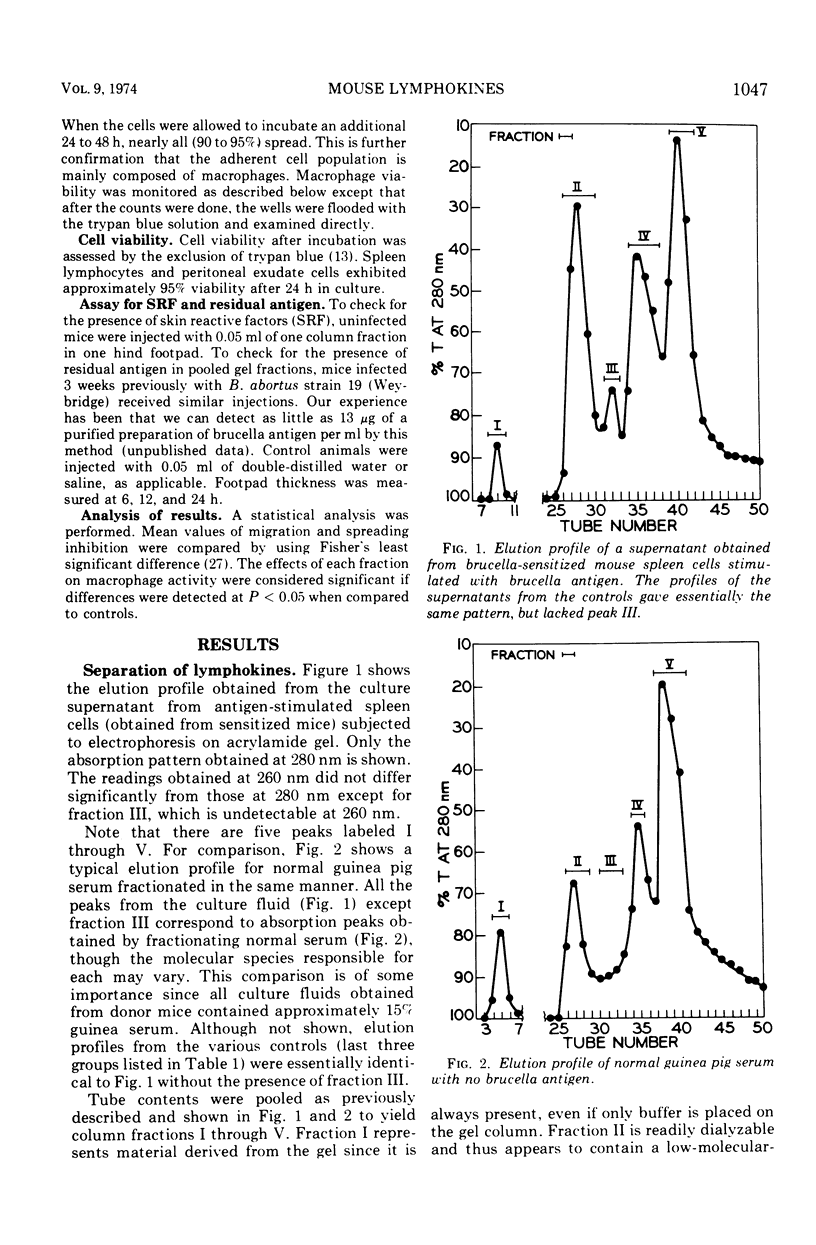
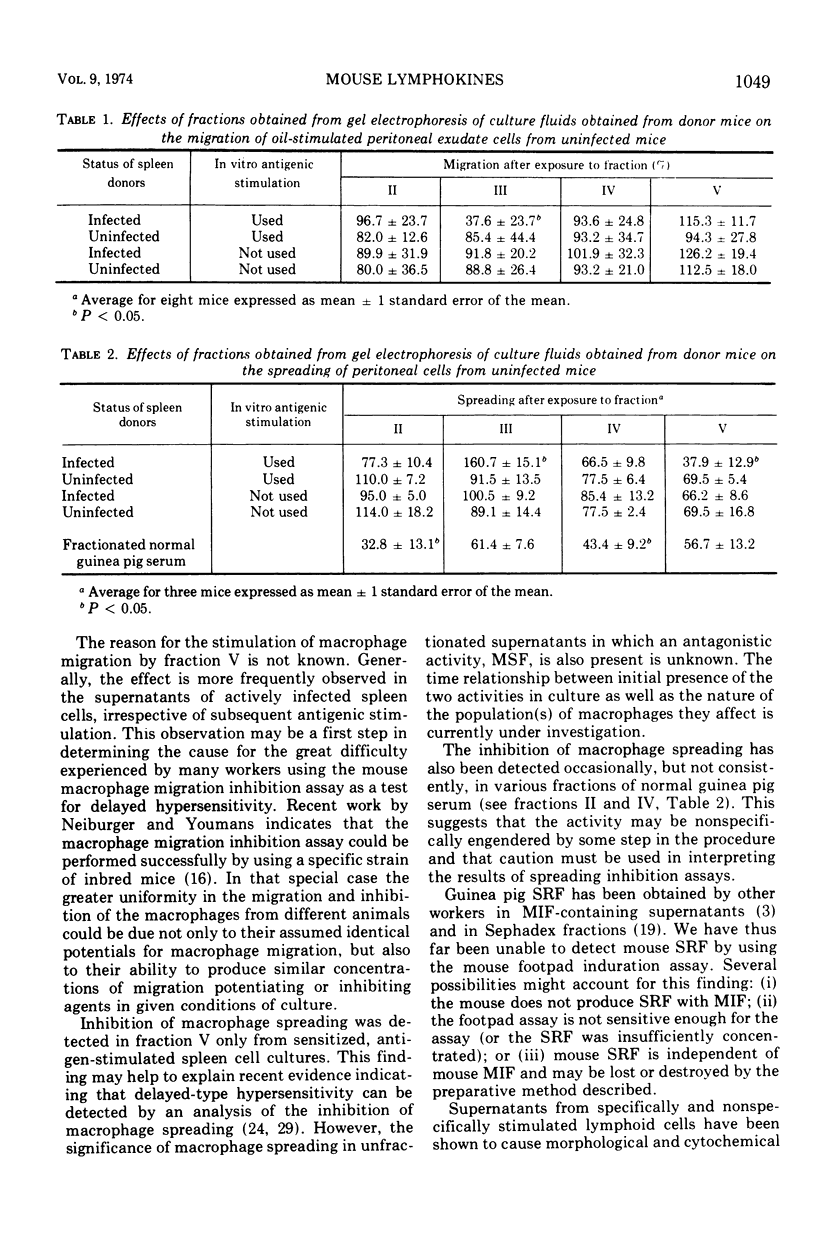
Cultures of brucella-sensitized mouse spleen cells exposed to Brucella abortus antigens in vitro release macrophage migration inhibition factor (MIF) and macrophage spreading factor. Subjecting the supernatants from such cultures to preparative scale electrophoresis in acrylamide gel yields several fractions, one of which contains both MIF and macrophage spreading factor. This material has properties attributable to guinea pig MIF: it is nondialyzable, heat stable, nontoxic to macrophages from heterologous murine donors, and has a greater anodal electrophoretic mobility than guinea pig serum albumin. Another fraction from the gel column inhibits macrophage spreading; its electrophoretic mobility is similar to that of guinea pig gamma globulin. Neither brucella antigen nor skin reactive substances were detectable in any acrylamide gel column fraction when tested by the mouse footpad induration assay technique.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos H. E., Lachmann P. J. The immunological specificity of a macrophage inhibition factor. Immunology. 1970 Feb;18(2):269–278. [PMC free article] [PubMed] [Google Scholar]
- Balow J. E., Rosenthal A. S. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973 Apr 1;137(4):1031–1041. doi: 10.1084/jem.137.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Relation of the migration inhibitory factor (MIF) to delayed-type hypersensitivity reactions. Ann N Y Acad Sci. 1970 Feb 13;169(1):258–265. doi: 10.1111/j.1749-6632.1970.tb55994.x. [DOI] [PubMed] [Google Scholar]
- David J. R., Schlossman S. F. Immunochemical studies on the specificity of cellular hypersensitivity. The in vitro inhibition of peritoneal exudate cell migration by cehmically defined antigens. J Exp Med. 1968 Dec 1;128(6):1451–1459. doi: 10.1084/jem.128.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonde D. C., Page D. A., Matthew M., Wolstencroft R. A. Role of lymphocyte activation products (LAP) in cell-mediated immunity. I. Preparation and partial purification of guinea-pig LAP. Clin Exp Immunol. 1972 Jan;10(1):25–47. [PMC free article] [PubMed] [Google Scholar]
- Heise E. R., Han S., Weiser R. S. In vitro studies on the mechanism of macrophage migration inhibition in tuberculin sensitivity. J Immunol. 1968 Nov;101(5):1004–1015. [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klun C. L., Youmans G. P. The effect of lymphocyte supernatant fluids on the intracellular growth of virulent tubercle bacilli. J Reticuloendothel Soc. 1973 Mar;13(3):263–274. [PubMed] [Google Scholar]
- Klun C. L., Youmans G. P. The induction by Listeria monocytogenes and plant mitogens of lymphocyte supernatant fluids which inhibit the growth of Mycobacterium tuberculosis within macrophages in vitro. J Reticuloendothel Soc. 1973 Mar;13(3):275–285. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath I., Poulter L. W., Turk J. L. Effect of lymphocyte mediators on macrophages in vitro. A correlation of morphological and cytochemical changes. Clin Exp Immunol. 1973 Mar;13(3):455–466. [PMC free article] [PubMed] [Google Scholar]
- Neiburger R. G., Youmans G. P. Inhibition of migration of mouse macrophages by tuberculin-sensitive mouse lymphocytes and by mouse migration inhibitory factor. Infect Immun. 1973 Feb;7(2):190–195. doi: 10.1128/iai.7.2.190-195.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou P. S., Henley W. L., Glade P. R. Production and characterization of migration inhibitory factor(s) (MIF) of established lymphoid and non-lymphoid cell lines. J Immunol. 1972 Feb;108(2):494–504. [PubMed] [Google Scholar]
- Pick E., Brostoff J., Krejci J., Turk J. L. Interaction between "sensitized lymphocytes" and antigen in vitro. II. Mitogen-induced release of skin reactive and macrophage migration inhibitory factors. Cell Immunol. 1970 May;1(1):92–109. doi: 10.1016/0008-8749(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Pick E. Cyclic AMP affects macrophage migration. Nat New Biol. 1972 Aug 9;238(84):176–177. doi: 10.1038/newbio238176a0. [DOI] [PubMed] [Google Scholar]
- Pick E., Turk J. L. The biological activities of soluble lymphocyte products. Clin Exp Immunol. 1972 Jan;10(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- Remold H. G., David J. R. Further studies on migration inhibitory factor (MIF): evidence for its glycoprotein nature. J Immunol. 1971 Oct;107(4):1090–1098. [PubMed] [Google Scholar]
- Remold H. G., David R. A., David J. R. Characterization of migration inhibitory factor (MIF) from guinea pig lymphocytes stimulated with concanavalin A. J Immunol. 1972 Sep;109(3):578–586. [PubMed] [Google Scholar]
- Remold H. G., Katz A. B., Haber E., David J. R. Studies on migration inhibitory factor (MIF): recovery of MIF activity after purification by gel filtration and disc electrophoresis. Cell Immunol. 1970 May;1(1):133–145. doi: 10.1016/0008-8749(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Sandok P. L., Hinsdill R. D., Albrecht R. M. Migration inhibition of mouse macrophages by Brucella antigens. Infect Immun. 1971 Oct;4(4):516–518. doi: 10.1128/iai.4.4.516-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubergen D. G., Feldman J. D., Pollock E. M., Lerner R. A. Production of macrophage migration inhibition factor by continuous cell lines. J Exp Med. 1972 Feb 1;135(2):255–266. doi: 10.1084/jem.135.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselic B., Dekaris D., Hrsak K. M. In vitro studies of delayed-type hypersensitivity. The time course of the primary immunological reaction in individual rats determined by macrophage spreading inhibition. Immunology. 1973 Feb;24(2):375–384. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Characterization of the Experimental System. Infect Immun. 1970 Jun;1(6):587–594. doi: 10.1128/iai.1.6.587-594.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L., Ribi E. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Relationship Between Inhibitory Activity of Lung Cells and Resistance to Airborne Challenge with Mycobacterium tuberculosis H37Rv. Infect Immun. 1970 Jun;1(6):595–599. doi: 10.1128/iai.1.6.595-599.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Janeway C. A., Jr, Paul W. E. Activity of migration inhibitory factor in the absence of antigen. J Immunol. 1972 Aug;109(2):201–206. [PubMed] [Google Scholar]



