This research examined the involvement of Rho-associated, coiled-coil protein kinase (ROCK) in human embryonic stem cell- (hESC) and induced pluripotent stem cell (iPSC)-derived retinal pigmented epithelial cell (RPE) culture to attempt to overcome passage limitations for development of therapies for age-related macular degeneration. They report that inhibiting ROCK1/2 with Y-27632 allows extended passage of hESC-derived RPE and iPSC-RPE.
Keywords: Embryonic stem cells, Retinal pigmented epithelium, Cellular therapy, Cell culture, Cell proliferation
Abstract
Human embryonic stem cells (hESCs) offer a potentially unlimited supply of cells for emerging cell-based therapies. Unfortunately, the process of deriving distinct cell types can be time consuming and expensive. In the developed world, age-related macular degeneration (AMD) is the leading cause of blindness in the elderly, with more than 7.2 million people afflicted in the U.S. alone. Both hESC-derived retinal pigmented epithelium (hESC-RPE) and induced pluripotent stem cell-derived RPE (iPSC-RPE) are being developed for AMD therapies by multiple groups, but their potential for expansion in culture is limited. To attempt to overcome this passage limitation, we examined the involvement of Rho-associated, coiled-coil protein kinase (ROCK) in hESC-RPE and iPSC-RPE culture. We report that inhibiting ROCK1/2 with Y-27632 allows extended passage of hESC-RPE and iPSC-RPE. Microarray analysis suggests that ROCK inhibition could be suppressing an epithelial-to-mesenchymal transition through various pathways. These include inhibition of key ligands of the transforming growth factor-β pathway (TGFB1 and GDF6) and Wnt signaling. Two important processes are affected, allowing for an increase in hESC-RPE expansion. First, ROCK inhibition promotes proliferation by inducing multiple components that are involved in cell cycle progression. Second, ROCK inhibition affects many pathways that could be converging to suppress RPE-to-mesenchymal transition. This allows hESC-RPE to remain functional for an extended but finite period in culture.
Introduction
Human embryonic stem cells (hESCs) offer a potentially unlimited supply of cells for emerging cell-based therapies. Unfortunately, the process of deriving distinct cell types can be time consuming and expensive. Furthermore, differentiated cells typically have a finite lifespan and can be passaged only a limited number of times. Expanding the propagation potential of stem cell-derived cells would be extremely useful for transplantation procedures and drug-screening efforts.
Clinical trials are currently under way using hESC-derived retinal pigmented epithelium (hESC-RPE) as a treatment for age-related macular degeneration (AMD) [1, 2]. In the developed world, AMD is the leading cause of blindness in the elderly, with more than 7.2 million people afflicted in the U.S. alone [3, 4]. AMD is a progressive disorder that eventually results in RPE death, photoreceptor death, and profound loss of central vision. It is generally agreed that AMD pathology is primarily linked to the dysfunction and death of the RPE, which acts to support and maintain the photoreceptors. When RPE degenerates, the photoreceptors can no longer function, and they eventually die [5, 6]. There are two clinically recognized forms of the disease: exudative (wet) AMD and atrophic (dry) AMD. The neovascular genesis component of wet AMD can be successfully managed using anti-vascular endothelial growth factor (anti-VEGF) drugs such as ranibizumab, bevacizumab, or aflibercept [7]. However, these drugs do not act to inhibit the underlying RPE degeneration, and patients with wet AMD can progress to more advanced stages of atrophic AMD characterized by macular regions devoid of RPE and photoreceptors [8, 9]. Other than a vitamin cocktail that can only slow the progression of AMD in 25% of patients taking the supplement, there is no effective treatment for dry AMD [10, 11].
Both hESC-RPE and induced pluripotent stem cell-derived RPE (iPSC-RPE) [12] are being developed for AMD therapies by multiple groups, using either cell suspensions or differentiated monolayers on scaffolds [13]. The currently preferred derivation process for RPE follows a spontaneous differentiation protocol involving the removal of fibroblast growth factor 2 in continuously adherent cultures of pluripotent stem cells [1, 14]. This method can take up to 100 days before enrichment of the pigmented population, followed by several months of additional culture to obtain relatively homogeneous, mature RPE ready for transplantation. The hESC-RPE, iPSC-RPE, and fetal RPE (fRPE) are passaged every 30 days and can be maintained only in culture for five to six passages before the cells display abnormal characteristics and are thought to undergo an epithelial-to-mesenchymal transition (EMT) [15–18]. Some reports have claimed an even more limited passage potential for iPSC-RPE, finding that only one passage following enrichment was possible [19]. These late-passage cells lose their pigmented, cobblestone morphology and appear more fibroblastic and often senesce and die. Senescence in cultured primary somatic cells is commonly observed as telomeres shorten and the Hayflick limit is reached [20].
To attempt to overcome this passage limitation, we examined the involvement of Rho-associated, coiled-coil protein kinase (ROCK) in hESC-RPE and iPSC-RPE culture. Two distinct ROCK isoforms, ROCK1 and ROCK2, are activated by RHOA GTPase and initiate signal-transduction cascades to modulate central cell functions, including proliferation, apoptosis, cytoskeletal rearrangements, and migration [21–23]. The RHOA/ROCK pathway has been studied extensively for its downstream effects on stress fiber formation [24, 25] and actin filament stabilization [21, 26]. ROCK inhibition using synthetic compounds is currently being tested in clinical trials for pulmonary hypertension treatments, with positive animal-model results [23, 27, 28]. Current research suggests increasing roles for ROCK inhibitors, including ROCK1/2 inhibitor Y-27632, in regulating various cell processes [23, 29–31]. Previous studies showed that ROCK inhibition increased passage abilities of certain epithelial cell types [32–36] and the clonability of hESCs [37, 38]. We have previously shown the beneficial effects of Y-27632 treatment following initial enrichment of RPE during directed differentiation from hESCs [39].
In this paper, we report that inhibiting ROCK1/2 with Y-27632 allows extended passage of hESC-RPE. Importantly, the resulting hESC-RPE maintains proper gene expression, protein localization, function, and karyotype. We also show evidence that iPSC-RPE extended passage is possible following ROCK1/2 inhibition. The simple culture methods described allow a much greater yield of hESC-RPE and iPSC-RPE and can provide abundant cells for disease modeling, drug screening, and further development of cellular therapies.
Materials and Methods
Cell Culture
Pluripotent Stem Cell Culture
H9 hESCs were obtained from the WiCell Research Institute in Madison, Wisconsin (http://www.wicell.org). Vector-free iPS cell lines DF 4.9, Mycell, and DF 19.11 were obtained from James Thomson and David Gamm (University of Wisconsin, Madison, WI). hESCs and iPS cells were maintained in mTeSR1 medium (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) and grown on Matrigel-coated plates (1:100 dilution; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) at 37°C 5% CO2 under normoxic conditions.
Continuously Adherent Retinal Pigmented Epithelium Differentiation and Enrichment
H9 hESCs and iPS cells were passaged onto Matrigel-coated plates and allowed to overgrow for 8–14 days. mTeSR1 medium was then changed to X-VIVO 10 (Lonza, Basal, Switzerland, http://www.lonza.com), after which the X-VIVO 10 medium was changed every other day. X-VIVO 10 is a xeno-free medium that has been used with cells designed for therapeutic use and increases the efficiency of RPE differentiation [40]. Pigmented cells typically appeared after 4–6 weeks. After 90 days in X-VIVO 10, nonpigmented cells were removed via mechanical dissection and the remaining, mostly pigmented cells were incubated with TrypLE Express (Life Technologies, Grand Island, NY, http://www.lifetech.com) for 5 minutes at 37°C. The resulting hESC-RPE- or iPSC-RPE-enriched cell suspension was passed through a 30 μm strainer and replated on Matrigel-coated plates using X-VIVO 10. Enriched RPE cultures were maintained at 5% CO2 and 37°C in X-VIVO 10. The medium was changed every other day. Every 30 days, the cells were harvested using TrypLE Express and replated at a density of 100,000 cells per cm2. All passage 2 cells used in comparison with passage 13 Y-27632-treated cells were cultured following this method.
Extended Passage Protocol of hESC-RPE and iPSC-RPE
Directly following enrichment, hESC-RPE or iPSC-RPE were plated into four Matrigel-coated wells at 25,000 cells per cm2 in X-VIVO 10. Two wells were treated with Y-27632 (10-μM; Tocris Bioscience, Bristol, U.K., http://www.tocris.com), and the other two received an equal volume of water as a control. One day after confluence (∼4–5 days), one Y-27632-treated well and one control well were passaged and seeded again at 25,000 cells per cm2. Passaging continued in this fashion until cells failed to reach confluence. The two wells not undergoing passaging continued to be supplemented with 10 μM Y-27632 or water for 14 days. On day 30, RNA was harvested and images were taken on the Olympus CKX41 (Olympus, Center Valley, PA, http://www.olympus-global.com). Passage 13 hESC-RPE used in all experiments were derived using this protocol. The extended-passage experiment was performed with seven separate enrichment cultures of H9 hESC-RPE and three enrichments per iPSC-RPE line. Every enrichment was produced from a distinct culture of H9 human embryonic stem cells and was differentiated separately.
MTT Assay
To determine the effect of ROCK inhibition on hESC-RPE proliferation, cells were plated into 96-well plates in X-VIVO 10 and allowed to attach for 2 hours. hESC-RPE is extremely adherent, and we observed that following only 2 hours, most of the cells had attached. The medium was then removed and replaced with fresh X-VIVO 10 with or without 10 μM Y-27632. The media were changed every 2 days for the duration of the experiment with continual Y-27632 supplementation. Cell proliferation was assessed by determining the number of viable cells using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay according to supplier instructions (Life Technologies).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated using the Qiagen RNeasy Plus Extraction Kit (Qiagen, Valencia, CA, http://www.qiagen.com), and cDNA was generated using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, http://www.bio-rad.com). The extent of expression of genes of interest was then evaluated in triplicate using the following TaqMan gene expression assays (Life Technologies): RPE-specific protein 65kDa (RPE65) Hs01071462_m1; bestrophin 1 (BEST1) Hs00188249_ml; retinaldehyde binding protein 1 (RLBP1) Hs00165632_ml; microphthalmia-associated transcription factor (MITF) isoform 2 AJD1S3G; premelanosome protein (PMEL) Hs00173854_m1; tyrosinase-related protein 1 (TYRP1) Hs00167051_m1; tyrosinase (TYR) Hs00165976_ml; paired box 6 (PAX6) Hs01088112_m1; marker of proliferation Ki-67 (MKI67) Hs01032443_m1; zinc finger protein 42 (REX1) Hs01124465_m1; spalt-like transcription factor 4 (SALL4) Hs00360675_m1; microtubule-associated protein 2 (MAP2) Hs00258900_m1; integrin, α 2 (ITGA2) Hs00158127_m1; platelet/endothelial cell adhesion molecule 1 (PECAM1) Hs00169777_m1; S100 calcium binding protein A4 (S100A4) Hs00243202_m1; and housekeepers: eukaryotic translation initiation factor 2B, subunit 2 β (EIF2B2) Hs00204540_m1; ubiquitin-conjugating enzyme E2R 2 (UBE2R2) Hs00215107_m1; and small EDRK-rich factor 2 (SERF2) Hs00428481_m1 (Life Technologies). The relative level of expression for each gene was determined by normalizing to the geometric mean of the housekeeping gene set using CFX Manager (Bio-Rad) and Excel software (Microsoft, Redmond, WA, http://www.microsoft.com).
Immunocytochemistry
Passage 2 hESC-RPE and passage 13 Y-27632-maintained hESC-RPE were seeded onto Matrigel-coated eight-chambered slides at 100,000 cells per cm2. The passage 13 hESC-RPE were treated with 10 μM Y-27632 for 14 days in medium, as described by Maminishkis et al. [41]. Forty-five days after plating, the cells were washed with phosphate-buffered saline (PBS; Life Technologies) and fixed with 4% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 7 minutes at 4°C. The fixed cells were then washed with PBS and blocked with PBS containing 5% bovine serum albumin (BSA) and 0.2% Triton X-100 for 1 hour at 4°C. The cells were then probed with primary antibodies against MITFC5 (1:1,000; Abcam, Cambridge, MA, http://www.abcam.com), orthodenticle homeobox 2 (OTX2; 1:4,000; Millipore, Billerica, MA, http://www.millipore.com), RPE65 (1:100; Abcam), BEST1 (1:100; Abcam), PMEL (1:100; Abcam), tight junction protein ZO-1 (TJP1; 1:100; Life Technologies), or MKI67 (1:1,000; Abcam) in PBS with 5% BSA overnight at 4°C. Following three washes to remove the primary antibodies, they were incubated with appropriate Alexa Fluor conjugated secondary antibody (1:300; Life Technologies) for 1 hour at 4°C. Following the incubation with secondary antibody, cellular DNA was labeled by the addition of Hoechst (2 μg/ml; Life Technologies) to the medium for 5 minutes at room temperature. The labeled cells were then washed with PBS and imaged using epifluorescent microscopy. For both passages, five enrichments of hESC-RPE were probed with this panel of primary antibodies.
Karyotype Analysis
Karyotyping of passage 3 and passage 13 hESC-RPE, from the same enrichment, was performed by Cell Line Genetics (Madison, WI, http://www.clgenetics.com). Cells were analyzed prior to reaching confluence.
Rod Outer Segment Phagocytosis Assay
hESC-RPE and human fRPE (fRPE kindly provided by Lincoln Johnson, Center for the Study of Macular Degeneration, University of California Santa Barbara, and Dean Bok, Jules Stein Eye Institute, University of California Los Angeles) were cultured using the medium and methods of Maminishkis et al. [41]. ARPE19 cells were cultured in Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 and sodium pyruvate (Life Technologies), supplemented with GlutaMAXI (1×; Life Technologies), 10% FBS (Atlas Biologicals, Fort Collins, CO, http://www.atlasbio.com), and 15 mM HEPES (Life Technologies). Human umbilical vein endothelial cells (HUVECs) were grown in endothelial cell growth medium with supplement mix (PromoCell, Heidelberg, Germany, http://www.promocell.com). All cells were plated in quadruplicate at 100,000 cells per cm2 onto 0.1% gelatin-coated wells and cultured for 30 days. hESC-RPE from passage 13 were treated with 10 μM Y-27632 for 14 days.
Rod outer segments (ROSs) were isolated from bovine retinas [42] (Sierra for Medical Science, Whittier, CA, http://www.sierra-medical.com) and fluorescently labeled with the FluoReporter fluorescein isothiocyanate (FITC) protein labeling kit (Life Technologies). The cultured cells were treated with or without αVβ5 function-blocking antibody (62.5 μg/ml; Abcam) or IgG (62.5 μg/ml; Abcam) isotype control for 30 minutes at 37°C 5% CO2. Following the initial antibody incubation, the cells were challenged with 1 × 106 FITC-ROS per well for 5 hours at 37°C 5% CO2 [39, 43] in media supplemented with a fresh aliquot of antibody. After ROS incubation, the wells were washed six times with PBS and then 0.4% trypan blue was added for 20 minutes to quench any fluorescence originating from residual extracellular ROS. Each well was imaged using epifluorescent microscopy, and integrated pixel density of photomicrographs were generated with Image J software (National Institutes of Health, Bethesda, MD) using a rolling pixel radius of 50. The phagocytosis assay was performed with three independent enrichments of hESC-RPE. The fRPE and ARPE19 cells serve as positive controls, and the HUVEC line was used as a negative control. All experiments were normalized to a single ARPE19 ROS experimental data set.
Pigment Epithelium-Derived Factor and Vascular Endothelial Growth Factor Enzyme-Linked Immunosorbent Assay
Passage 2 hESC-RPE and passage 13 Y-27632-treated hESC-RPE were grown on Matrigel-coated 0.45-μm HA inserts (0.6 cm2; Millipore) seeded at 100,000 cells per cm2 in medium, as described by Maminishkis et al. [41]. The hESC-RPE passage 13 cells were treated with 10 μM Y-27632 for 14 days. On day 30, media were collected from both the apical and basal chambers following a 72-hour exposure to the cells. The amount of pigment epithelium-derived factor (PEDF; BioProducts MD, LLC, Middletown, MD, http://www.bioproductsmd.com) and vascular endothelial growth factor A (Life Technologies) in the basal and apical compartments were determined by enzyme-linked immunosorbent assay according to the manufacturer’s instructions. All media volumes were kept constant, and the growth area and volume were taken into consideration when calculating the data.
Agilent Whole Human Genome Microarray
hESC-RPE was cultured following the extended passage protocol. At passage 5, control and Y-27632 lysates were collected 2 days after plating, prior to reaching confluence. RNA from four separate biological replicates was isolated using the Qiagen miRNeasy Kit (Qiagen) for each treatment group. Global transcriptome analysis was performed with the Agilent Whole Human Genome 4 × 44K in situ oligonucleotide array platform (G4112F; Agilent Technologies, Santa Clara, CA, http://www.agilent.com) using the reagents and methods of the manufacturer and a two-color experimental design pairing the control and Y-27632 samples. After background and Lowess correction, the 200 replicate probe sets were averaged, and the entire array data set was quantile normalized. Array data are reported as normalized net intensity levels.
Results
ROCK Inhibition Allows Extended Passage of hESC-RPE and iPSC-RPE
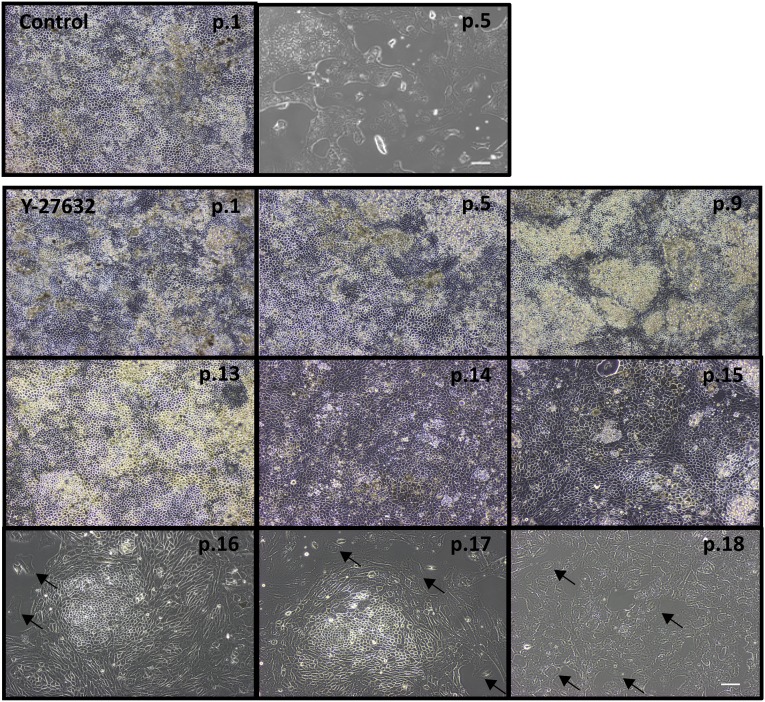
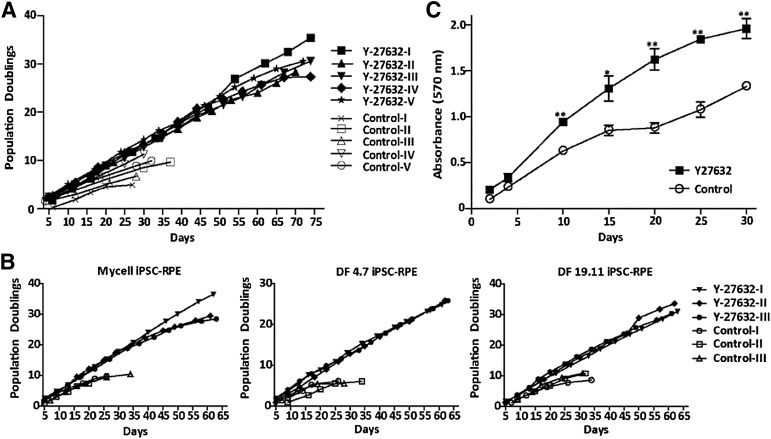
hESC-RPE or iPSC-RPE can typically be passaged only five or six times before undergoing a switch in phenotype suggestive of an EMT and eventually senescing [15–18]. We sought to determine whether inhibition of the Rho kinases ROCK1 and ROCK2 could extend the effective passaging of hESC-RPE and iPSC-RPE. To test the role of ROCK1/2 in this process, the synthetic compound, Y-27632, which is a competitive inhibitor of the ATP binding domain of both ROCK1 and ROCK2 isoforms [23], was added to the culture medium, and cells were continuously passaged, plating at a fourth of the usual seeding density. For each passage, a portion of cells were grown for 30 days to allow them to mature and reach confluence, with removal of the ROCK inhibitor at day 14. Phase contrast images of the resulting cultures are shown in Figure 1. In contrast to untreated hESC-RPE, which underwent an EMT and failed to become confluent following five passages, Y-27632-treated cells were able to assume their typical RPE cobblestone morphology up to passage 14; however, after 15 passages, patches of larger cells displaying a mesenchymal morphology (examples shown by arrows in Fig. 1) were intermixed with areas of cells with epithelial morphology. By passage 18, cells failed to reach confluence following 30 days in culture and never regained the typical RPE morphology. All iPSC-RPE lines acted in a similar fashion to hESC-RPE but were not passaged beyond passage 13 (images not shown). Bright field images of control passage 2 and Y-27632-treated passage 13 hESC-RPE show similar pigmentation patterns (supplemental online Fig. 1). To determine the effect of Y-27632 on the rate of proliferation, cell counts were taken at each passage as hESC-RPE and iPSC-RPE underwent the extended passage protocol. Cells treated with Y-27632 showed a substantial increase in population doublings, allowing an average of 30 doublings compared with only 9 doublings in control cultures for both hESC-RPE and iPSC-RPE (Fig. 2A, 2B). In the presence of the ROCK inhibitor, cell numbers increase exponentially, with an average doubling time of 2.4 days, whereas the control doubling time was significantly longer, with an average of 3.8 days, for hESC-RPE. There was some line-to-line variation in iPSC-RPE; however, the average Y-27632-treated cell doubling time was 2.1 days compared with 3.6 days in control cells. All control cultures could not be propagated beyond five passages, suggesting that they had senesced.
Figure 1.
Effect of Rho-associated, coiled-coil protein kinase inhibition on human embryonic stem cell-derived retinal pigmented epithelium (hESC-RPE) passage. Control hESC-RPE and Y-27632-treated hESC-RPE were serially passaged and cultured, as detailed in Materials and Methods. Phase contrast images are shown of control and Y-27632-treated hESC-RPE, with passage number indicated in the upper right corner. All images were collected on day 30. Images are from a single experiment that is representative of nine experiments. Arrows indicate examples of cells with mesenchymal-like phenotype at passages 16, 17, and 18. Seeding density: 25,000 cells per cm2. Scale bar = 100 μm. Abbreviation: p., passage.
Figure 2.
Rho-associated, coiled-coil protein kinase inhibition affects human embryonic stem cell-derived retinal pigmented epithelium (hESC-RPE) proliferation. (A): Population doubling (PD) is plotted versus time in cultures with and without addition of Y-27632. Each point represents a passage, n = 5. PD = log2(number of cells counted at time of passage divided by the number of cells plated). (B): PD of three iPSC-RPE lines throughout the extended passage protocol. n = 3 per line. (C): Passage 4 hESC-RPE grown in the presence or absence of Y-27632, and cell number was quantified by measuring MTT reduction. Error bars represent ±SEM. ∗, p ≤ .05, ∗∗, p ≤ .01 compared with control for the same time point. n = 3 (same enrichment). Abbreviation: iPSC-RPE, induced pluripotent stem cell-derived retinal pigmented epithelial cell.
In addition to monitoring cell expansion at the time of each passage, over numerous passages, cell proliferation was measured more directly within a single passage. Similar effects of Y-27632 on hESC-RPE growth rate were observed when the number of living cells within a single passage was monitored as a function of time using an MTT assay (Fig. 2C). When passage 4 hESC-RPE were grown in the continual presence or absence of Y-27632, a significant increase in the number of cells was detected by 10 days in the Y-27632-treated cells and persisted to at least day 30. This experiment shows that ROCK inhibition speeds up the rate of proliferation of hESC-RPE. Both control and Y-27632-treated passage 4 cells retained RPE morphology at day 30; however, the characteristics of these particular cells at higher passages were not examined. We are currently testing other compounds that are known to affect proliferation on various different passages of hESC-RPE and fRPE.
Gene Expression During Extended Passage of hESC-RPE
In an effort to assess the effects of Y-27632 on gene expression, we determined the relative amounts of a selected set of RPE and non-RPE marker transcripts. As shown in Figure 3, control hESC-RPE showed a decrease in the abundance of RPE RNAs (RPE65, BEST1 RLBP1, and MITF) as a function of passage, with significant differences being observed at passage 5 (Fig. 3). Interestingly, levels of pigment-related mRNAs PMEL, TYRP1, and TYR remained constant in untreated hESC-RPE. PAX6, a neural retina and immature RPE marker, increased over passage but not significantly. In contrast, in Y-27632-treated hESC-RPE, all seven RPE marker RNA levels remained relatively stable over the course of 13 passages, and PAX6 mRNA levels did not increase. We believe that the large error bars for several control passage 3 and passage 5 transcripts is due to the mixed population of cells arising within the well as the RPE begins to undergo EMT.
Figure 3.
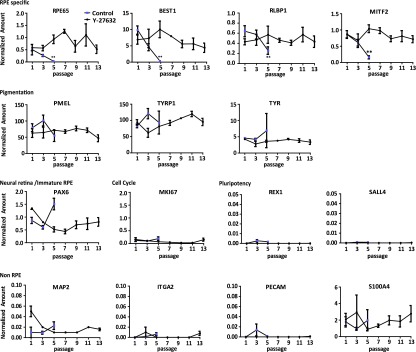
Gene expression in extended-passage human embryonic stem cell-derived (hESC-derived) RPE. RPE-specific, pigmentation, neural retina/immature-RPE, cell cycle, pluripotent, and non-RPE gene expression was analyzed as a function of passage at 30 days after plating. All data were normalized to geometric mean of three housekeeper mRNAs. Positive control cell values for non-RPE genes: H9 hESC, REX1 (4.09 ± 0.09), SALL4 (10.93 ± 0.45); neuroblastoma cell line SH-SY5Y, MAP2 (0.78 ± 0.29); smooth muscle cells, ITGA2 (2.02 ± 0.24); human umbilical vein endothelial cells, PECAM (15.7 ± 0.53); Hs27, S100A4 (20.13 ± 1.09). Error bars represent ±SEM. ∗, p ≤ .05, ∗∗, p ≤ .01 compared with passage one within the same treatment group. n = 3. Abbreviation: RPE, retinal pigmented epithelial cell.
In addition, although Y-27632 treatment preserves the mitotic potential of hESC-RPE, there is no evidence for increased expression of MKI67, a marker of mitosis, in confluent 30-day-old cultures of Y-27632-treated cells relative to that seen with untreated cells. This would imply that although cells proliferate more rapidly in the presence of Y-27632 (Fig. 2), the effects of Y-27632 are not lasting (Fig. 3). After removal of ROCK inhibition, cells reach confluence and exit the cell cycle.
We also examined markers for pluripotency and potential contaminating or transdifferentiated cell types. The level of the pluripotent mRNAs REX1 and SALL4 remained negligible with extended passage, as did the neuronal marker MAP2, the smooth muscle marker ITGA2, the endothelial marker PECAM, and the fibroblastic marker S100A4. (Positive control cell values for non-RPE gene markers are described in the legend for Fig. 3).
Protein Expression and Localization During Extended Passage of hESC-RPE
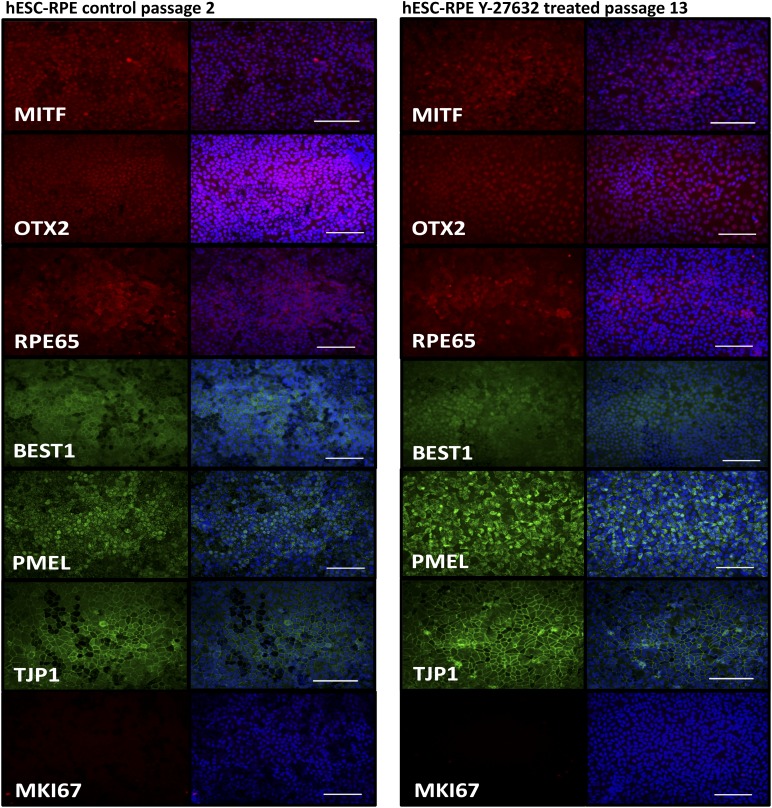
To assess whether there were any gross differences in protein localization between differentiated cultures of Y-27632-treated, extended-passage cells versus minimally passaged control cells, 45-day cultures of each condition were evaluated using immunocytochemistry. Cells were probed with antibodies directed against the RPE marker proteins MITF, OTX2, RPE65, BEST1, PMEL, and ZO-1 (TJP1) as well as an antibody against the mitosis-associated antigen MK167 (Fig. 4). In all cases, the pattern of protein localization was indistinguishable between control cells at passage 2 and Y-27632-treated cells at passage 13. The mature RPE markers BEST1 and RPE65 were both localized to the cytoplasm, with additional localization of BEST1 to the plasma membrane being evident in passage 2. The pigmentation-associated protein PMEL was found to be colocalized with distinct melanosomes and the tight junction protein ZO-1 (TJP1) to cell borders. Consistent with the lack of evidence for the presence of MKI67 mRNA in 30-day control passage 2 or Y-27632-treated passage 13 cells, no MIK67 immunoreactivity was detected.
Figure 4.
Protein expression and localization in extended-passage human embryonic stem cell-derived retinal pigmented epithelium (hESC-RPE). Control passage 2 (left panel) and Y-27632-treated passage 13 (right panel) cells were stained for RPE markers and a mitotic marker after reaching confluence at day 45. Scale bars = 100 μm. Images shown are representatives of four experiments for each passage. Primary antibody stain is shown in the left column, and the merged image with Hoechst (blue signal) is depicted in the right column.
Normal chromosomal arrangement was observed following karyotype analysis of control passage 3 and Y-27632-treated passage 13 cells (supplemental online Fig. 2).
Functional Analysis of Extended-Passage hESC-RPE
The diurnal phagocytosis of photoreceptor outer segments, the apical secretion of PEDF, and basal VEGF secretion are critical RPE functions [44]. To examine these functions in extended-passage hESC-RPE treated with the ROCK inhibitor, phagocytosis assays and ELISAs were performed. For the phagocytosis assays, hESC-RPE passage 2 and hESC-RPE Y-27632-treated passage 13 cells, as well as negative and positive control cells, were challenged with FITC-labeled bovine ROSs, and the extent ROS uptake was measured using quantitative immunofluorescence microscopy. Passage 13 Y-27632-treated hESC-RPE showed levels of phagocytosis similar to those seen in passage 2 control cells (Fig. 5A). To test whether phagocytosis occurred through the receptor-dependent mechanism used by RPE in vivo, cells were incubated with an integrin αVβ5 function-blocking antibody prior to challenging with FITC-labeled ROSs. This integrin has been shown to be important for RPE phagocytosis [45]. There was a significant decrease in the amount of ROS internalization compared with IgG control-treated cells (p > .05) for fRPE and hESC-RPE passage 2 and hESC-RPE passage 13 cells after αVβ5 activity was blocked. This shows that phagocytosis requires the same integrin receptor after extended passage and that passage 13 hESC-RPE retain normal phagocytosis activity.
Figure 5.
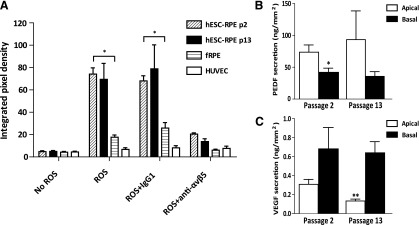
Function of extended-passage hESC-RPE. (A): RPE phagocytosis of bovine photoreceptor outer segments, as determined by pixel density analysis of photomicrographs, is shown. fRPE and HUVEC serve as positive and negative controls, respectively. All experiments are normalized to a single ARPE19 ROS experiment data set. Isotype-matched IgG was added as a negative control for the αVβ5 function-blocking antibody. (B, C): Enzyme-linked immunosorbent assay analysis of PEDF and VEGF. Polarized hESC-RPE showed a higher level of apical PEDF and basal VEGF secretion, consistent between passages. Error bars represent ±SEM. ∗, p ≤ .05; ∗∗, p ≤ .01. Section A compared with samples treated with anti-αvβ5. Sections B and C compared with secretion from opposite cell side. n = 3 for all experiments. Abbreviations: fRPE, fetal retinal pigmented epithelial cell; hESC-RPE, human embryonic stem cell-derived retinal pigmented epithelial cell; HUVEC, human umbilical vein endothelial cell; PEDF, pigment epithelium-derived factor; ROS, rod outer segment; VEGF, vascular endothelial growth factor.
For the determination of apical and basal growth factor secretion, media were collected from the inner and outer chambers of transwell hESC-RPE cultures, and the amount of the PEDF and VEGF in the two media compartments was quantified. There was no significant difference for either apical or basal PEDF release between passage 2 and passage 13 hESC-RPE (Fig. 5B). The amount of basal VEGF secretion also remained unchanged following extended passage in the presence of Y-27632; however, there was a twofold decrease in amount the apical VEGF in the passage 13 cultures compared with passage 2, but this did not reach significance (Fig. 5C).
ROCK Inhibition Affects Transcript Levels Within Many Cell Pathways
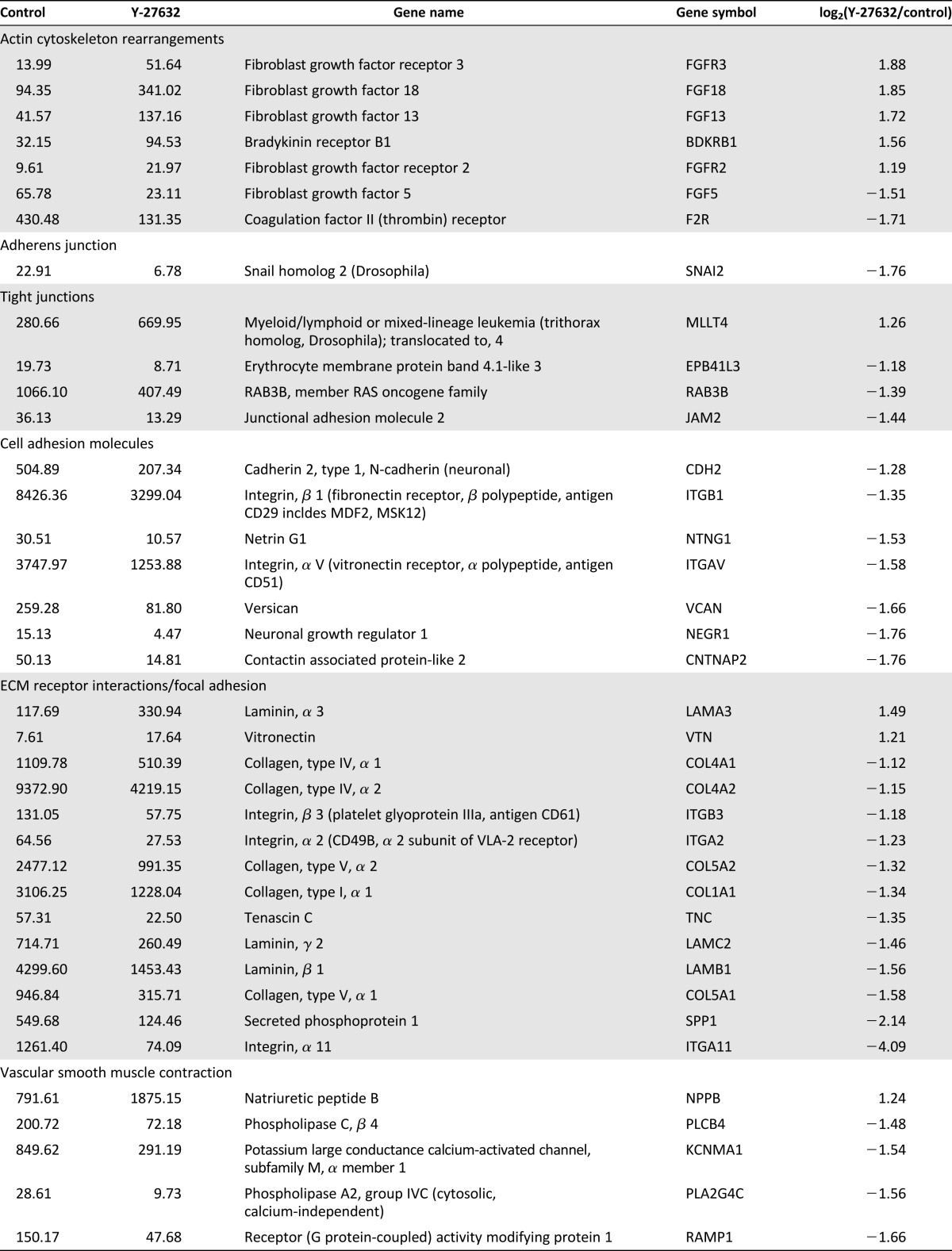
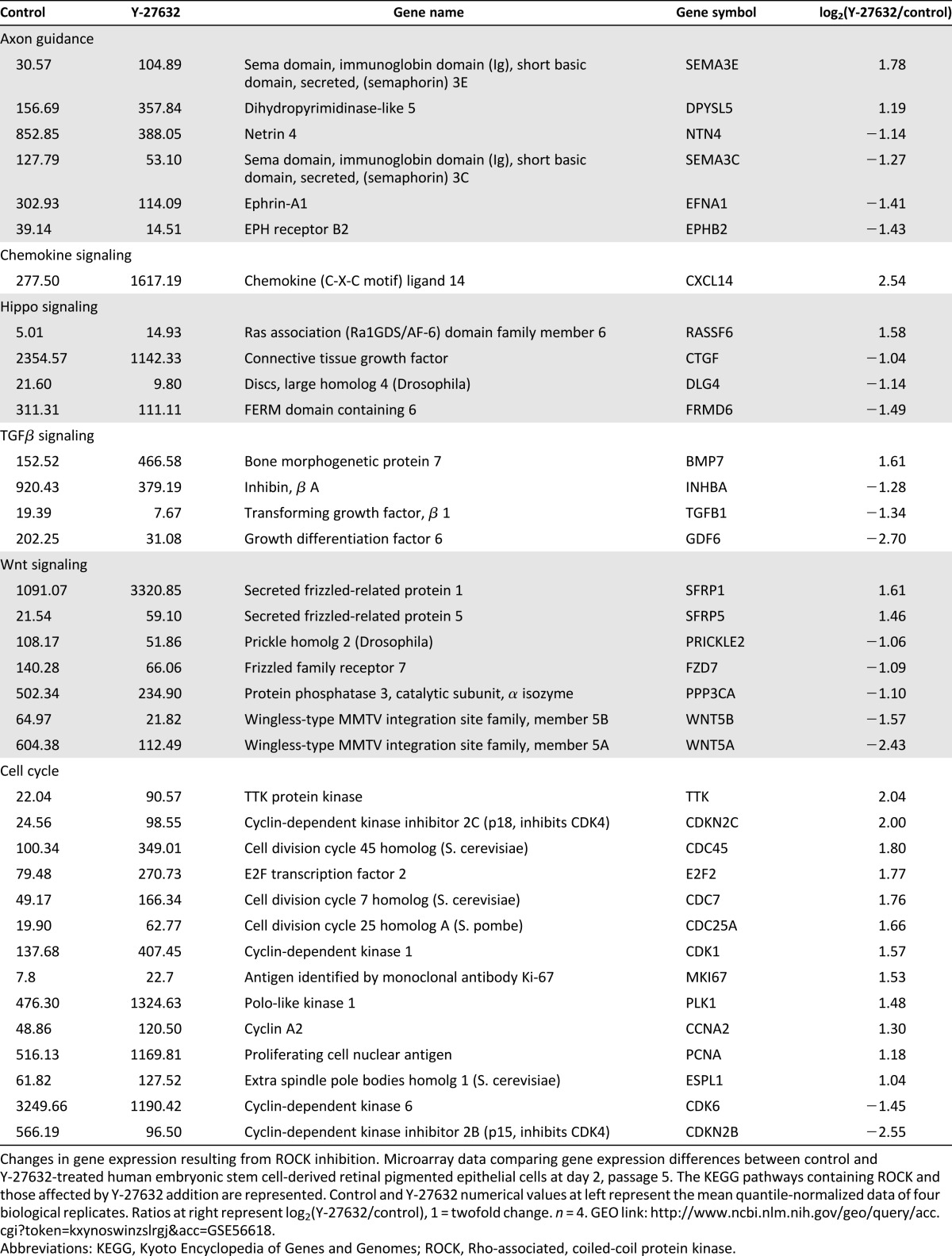
ROCK is known to be involved in numerous pathways within the cell, but its effect on gene transcription has not been fully examined. To gain more insight into how ROCK inhibition allows for extended passage of hESC-RPE, we assessed the global transcription patterns using cDNA microarray analysis in passage 5 control and Y-27632-treated cells 2 days after plating. Microarray analysis revealed that around 12,200 probes had above-background signals. From those, just more than 700 transcripts had at least a 1.5-fold change in each pairwise comparison and an average fold change of two or greater for all experiments. Overall, 369 genes decreased after treatment and 356 increased.
Our microarray data reiterate the published literature showing that ROCK plays a role in several different pathways that all could contribute to the ability of hESC-RPE to undergo extended passage (Table 1) [22, 26, 46, 47]. Table 1 summarizes the microarray data using a pathway-centric organization based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways containing ROCK. These pathways include: actin cytoskeleton rearrangements, adherens junctions, tight junctions, cell adhesion molecules, extracellular matrix (ECM) receptor interactions/focal adhesions, vascular smooth muscle contraction, axon guidance, chemokine guidance, HIPPO signaling, transforming growth factor-β (TGF-β) signaling, Wnt signaling, and the cell cycle. This analysis reveals decreased expression of genes encoding proteins involved in tight junctions, cell adhesion molecules, ECM-receptor interactions, and focal adhesion at day 2 prior to cell confluence, following ROCK inhibition. In addition, examination of the Hippo, TGF-β, and Wnt signaling pathways showed lower levels of transcripts encoding key ligands as a result of ROCK inhibition. No changes in SMADs were detected; however, they are activated through phosphorylation. Consequently, more work is needed to uncover whether ROCK inhibition affects SMAD function. In addition, transcripts tied to progression through the cell cycle increased substantially after Y-27632 treatment. These data were also analyzed using the gene ontology enrichment analysis software DAVID [48, 49]. This analysis revealed several significant gene groups, many of which overlap the KEGG pathway analysis (supplemental online Fig. 3).
Table 1.
Microarray: KEGG pathways (passage 5, day 2; control vs. Y-27632)
Discussion
RPE was first noted in differentiated hESC cultures in 2001 [50] and then expanded and characterized in a landmark paper by Klimanskaya et al. in 2004 [51]. Since then, groups have been attempting to optimize the process for derivation of RPE from hESCs or iPS cells [14, 51]. The main obstacles of culturing pluripotent cell-derived RPE have always been long derivation time and limited potential for expansion once differentiated. Recently, progress has been made in developing more rapid methods for differentiation of hESCs into RPE [39, 52–54]. Buchholz et al. [39], for example, described a method to direct differentiation using a series of growth factors known to play a role in RPE differentiation in vivo; however, once RPE is obtained, they still have a limited functional life span.
We report, in this paper, a novel passaging strategy that relies on inhibition of ROCK and that results in an increased rate of proliferation and extended passage of both hESC-RPE and iPSC-RPE, with retention of function. This phenomenon occurs even when the cells are plated at a fourth of the seeding density that is typically used. Thus, one can use less starting material and passage cells longer, leading to an exponential increase in the number of cells produced from a single culture of hESCs or iPS cells.
Extended-passage hESC-RPE maintained appropriate morphology and gene expression of key RPE markers without an increase in pluripotent, fibroblastic, or endothelial transcripts. Immunocytochemistry of passage 13 hESC-RPE exhibited proper localization of RPE transcription factors, proteins involved in pigmentation, tight junctions, and proteins important for RPE-specific functions. Importantly, from a therapeutic-application perspective, no aberrant proliferation was detected at day 30. Functional analysis demonstrated that passage 13 hESC-RPE were normal with respect to ROS phagocytosis and polarized secretion of PEDF and VEGF. In addition, no gross genomic abnormalities were detected following ROCK inhibition and repeated passage.
How does ROCK inhibition lead to extended passage? Microarray analysis suggests that ROCK inhibition could be suppressing an EMT through various pathways. These include inhibition of key ligands of the TGF-β pathway (TGFB1 and GDF6) [55] and Wnt signaling (WNT5A and WNT5B) [56, 57], along with decreasing levels of collagens 1A1 4A2 [58, 59] and SNAI2, known biomarkers of EMT [60]. RPE are thought to undergo EMT after repeated passages in culture, a main reason why hESC-RPE have a finite ability to expand, limiting their production for use in transplantation [17, 61]. TGF-β signaling has been shown to be implicated in activating EMT in RPE [17, 55, 62–64]. Furthermore, several investigations have predicted that the RHO/ROCK pathway is involved in regulating EMT in multiple cell types [65–68], possibly through TGF-β signaling [69]. There has also been evidence linking Wnt activation and β-catenin accumulation to the increased expression of EMT-related genes [56, 70]. Inhibition of ROCK, through addition of Y-27632 or other synthetic compounds, has already been shown in some cases to reverse this transition [71, 72]. Our results are consistent with the idea that decreased expression of TGF-β and Wnt signaling transcripts led to the suppression of EMT. In addition, novel, undocumented pathways could be contributing to the maintenance and increased expansion rate of hESC-RPE that led to the persistence of RPE identity.
Two important processes are affected, allowing for an increase in hESC-RPE expansion. First, ROCK inhibition promotes proliferation by inducing multiple components that are involved in cell cycle progression. This allows RPE to quickly reform tight junctions, which are critical to RPE health [73]. Second, ROCK inhibition affects many pathways that could be converging to suppress RPE-to-mesenchymal transition. This allows hESC-RPE to remain functional for an extended but finite period in culture.
The prevalence of AMD in adults older than 40 years of age in the U.S. alone was calculated to be 7.2 million people; that number is projected to increase 50% by 2020 [4]. Assuming that a 3 × 5 mm patch of a hESC-RPE monolayer on a scaffold might be developed as a dry AMD therapy, it is estimated that 300,000 hESC-RPE will be required for each patient transplant [13, 74]. Based on this number, 3.3 trillion cells would be required to treat all patients affected in 2020. Following the extended passage protocol, a single well of a six-well plate will generate enough cells in 55 days to treat all of those estimated to be affected by the disease in 2020. This does not include the additional cells that would be required for release assays, quality control, and cell loss, but the protocol can easily be scaled up. Compared with the current method of derivation, the extended passage protocol using ROCK inhibition can exponentially increase the yield of mature hESC-RPE to 49 billion from 9 million within the first 60 days in culture—a 5,000-fold increase—in addition to using one-quarter of the starting cell number.
From a cell-engineering perspective, it is clear that these discoveries have the potential to greatly facilitate the production of functional hESC-RPE and iPSC-RPE for both therapeutic and research applications. It is even possible that these findings may be transferable to other epithelial cells derived from pluripotent stem cells. Currently, minimally passaged hESC-RPE are being used in clinical trials. Transitioning to the use of more highly passaged cells deserves further experimentation to characterize efficacy and potential tumorigenicity in animal studies, including a full transcriptome analysis of day 30 Y-27632-treated passage 13 hESC-RPE to ensure complete restoration of the gene profile to a passage 2-like state. In addition, defining the mechanisms of increased proliferation and suppressed EMT and devising methods to manipulate these processes could be extremely beneficial in developing therapeutics for other RPE dysfunction diseases such as geographic atrophy and proliferative vitreal retinopathy, a complication of retinal detachment characterized by RPE-to-mesenchymal transition that can lead to loss of vision [64, 75].
Conclusion
Inhibition of ROCK activity by the synthetic compound Y-27632 allows extended passage of hESC-RPE and iPSC-RPE. Extended passage, in part, seems to be achieved by increased proliferation and through the prevention of gene expression that promotes and contributes to the mesenchymal phenotype. We have shown that inhibiting ROCK allows hESC-RPE and iPSC-RPE to be seeded at one-quarter of the normal density and grow for up to 13 passages. The extended-passage hESC-RPE was comparable to untreated early passage hESC-RPE by all parameters tested. This simple technique, in combination with the published rapid differentiation protocols, could lead to a faster and more efficient way of producing hESC-RPE and iPSC-RPE for clinical trials, basic disease research, and drug screening.
Supplementary Material
Acknowledgments
This work was supported by the Garland Initiative for Vision, Grant DR1-0144 from the California Institute for Regenerative Medicine, Fight for Sight, The Foundation Fighting Blindness Wynn-Gund Translational Research Acceleration Program, the University of California Santa Barbara Institute for Collaborative Biotechnologies through Grant W911NF-09-0001 from the U.S. Army Research Office, the Bright Focus Foundation (M2011064, M.J.R.) and the California Institute for Regenerative Medicine (LA1-02086 [P.J.C.], DR1-01444, CL1-00521, TG2-01151 (D.O.C.), and Major Facilities Grant FA1-00616. R.H.C. is a fellow of the California Institute for Regenerative Medicine. The content of the information does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. We thank Carolyn Radeke for technical support of the microarray experiments and Lincoln Johnson, Dean Bok, James Thomson, and David Gamm for their generous gifts of fetal and induced pluripotent stem cells.
Author Contributions
R.H.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; D.E.B.: conception and design, data analysis and interpretation, manuscript writing; M.J.R.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; W.J.T.: collection and/or assembly of data; Q.H.: collection and/or assembly of data, data analysis and interpretation; P.J.C.: financial support, manuscript writing; D.O.C.: conception and design, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
D.O.C. is an uncompensated patent holder and has uncompensated ownership interest in Regenerative Patch Technologies.
References
- 1.Croze RH, Clegg DO. Differentiation of pluripotent stem cells into retinal pigmented epithelium. In: Casaroli-Marano RP, Zarbin MA, eds. Developments in Opthalmology. Vol. 53. Cell-Based Therapy for Retinal Degenerative Disease. Basel, Germany: Karger, 2014:81–96 In: Casaroli-Marano RP, Zarbin MA, editors. [Google Scholar]
- 2.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- 6.Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 7.Gehrs KM, Anderson DH, Johnson LV, et al. Age-related macular degeneration—emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120:3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris FL, III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowes Rickman C, Farsiu S, Toth CA, et al. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54:ORSF68–80. doi: 10.1167/iovs.13-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 13.Bharti K, Rao M, Hull SC, et al. Developing cellular therapies for retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2014;55:1191–1202. doi: 10.1167/iovs.13-13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227:457–466. doi: 10.1002/jcp.22814. [DOI] [PubMed] [Google Scholar]
- 15.Burke JM. Epithelial phenotype and the RPE: Is the answer blowing in the Wnt? Prog Retin Eye Res. 2008;27:579–595. doi: 10.1016/j.preteyeres.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405. [PubMed] [Google Scholar]
- 17.Lee SC, Kwon OW, Seong GJ, et al. Epitheliomesenchymal transdifferentiation of cultured RPE cells. Ophthalmic Res. 2001;33:80–86. doi: 10.1159/000055648. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Phillips MJ, Kuai D, et al. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Invest Ophthalmol Vis Sci. 2013;54:6767–6778. doi: 10.1167/iovs.13-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Q, Lu SJ, Klimanskaya I, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 20.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 21.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 22.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 23.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung T, Chen XQ, Manser E, et al. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Street CA, Bryan BA. Rho kinase proteins—pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011;31:3645–3657. [PMC free article] [PubMed] [Google Scholar]
- 27.Doggrell SA. Rho-kinase inhibitors show promise in pulmonary hypertension. Expert Opin Investig Drugs. 2005;14:1157–1159. doi: 10.1517/13543784.14.9.1157. [DOI] [PubMed] [Google Scholar]
- 28.Pankey EA, Byun RJ, Smith WB, II, et al. The Rho kinase inhibitor azaindole-1 has long-acting vasodilator activity in the pulmonary vascular bed of the intact chest rat. Can J Physiol Pharmacol. 2012;90:825–835. doi: 10.1139/y2012-061. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): Potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res. 2012;95:60–67. doi: 10.1016/j.exer.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horani A, Nath A, Wasserman MG, et al. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyashita H, Yokoo S, Yoshida S, et al. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Translational Medicine. 2013;2:758–765. doi: 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Bogaard EH, Rodijk-Olthuis D, Jansen PA, et al. Rho kinase inhibitor Y-27632 prolongs the life span of adult human keratinocytes, enhances skin equivalent development, and facilitates lentiviral transduction. Tissue Eng Part A. 2012;18:1827–1836. doi: 10.1089/ten.tea.2011.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman S, Liu X, Meyers C, et al. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Ohgushi M, Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011;21:274–282. doi: 10.1016/j.tcb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Buchholz DE, Pennington BO, Croze RH, et al. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Translational Medicine. 2013;2:384–393. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: Improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- 43.Rowland TJ, Blaschke AJ, Buchholz DE, et al. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J Tissue Eng Regen Med. 2013;7:642–653. doi: 10.1002/term.1458. [DOI] [PubMed] [Google Scholar]
- 44.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 45.Lin H, Clegg DO. Integrin alphavbeta5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39:1703–1712. [PubMed] [Google Scholar]
- 46.Duong-Quy S, Bei Y, Liu Z, et al. Role of Rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol Ther. 2013;137:352–364. doi: 10.1016/j.pharmthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 49.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 51.Klimanskaya I, Hipp J, Rezai KA, et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 52.Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Zahabi A, Shahbazi E, Ahmadieh H, et al. A new efficient protocol for directed differentiation of retinal pigmented epithelial cells from normal and retinal disease induced pluripotent stem cells. Stem Cells Dev. 2012;21:2262–2272. doi: 10.1089/scd.2011.0599. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Carido M, Meinhardt A, et al. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS One. 2013;8:e54552. doi: 10.1371/journal.pone.0054552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SC, Kim SH, Koh HJ, et al. TGF-betas synthesized by RPE cells have autocrine activity on mesenchymal transformation and cell proliferation. Yonsei Med J. 2001;42:271–277. doi: 10.3349/ymj.2001.42.3.271. [DOI] [PubMed] [Google Scholar]
- 56.Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicol Rev. 2010;26:245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao L, Wang M, Yang S, et al. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. Biomed Res Int. 2013;2013:987064. doi: 10.1155/2013/987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Billings PC, Whitbeck JC, Adams CS, et al. The transforming growth factor-β-inducible matrix protein βig-h3 interacts with fibronectin. J Biol Chem. 2002;277:28003–28009. doi: 10.1074/jbc.M106837200. [DOI] [PubMed] [Google Scholar]
- 59.Kim JE, Park RW, Choi JY, et al. Molecular properties of wild-type and mutant betaIG-H3 proteins. Invest Ophthalmol Vis Sci. 2002;43:656–661. [PubMed] [Google Scholar]
- 60.Scanlon CS, Van Tubergen EA, Inglehart RC, et al. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamiya S, Liu L, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest Ophthalmol Vis Sci. 2010;51:2755–2763. doi: 10.1167/iovs.09-4725. [DOI] [PubMed] [Google Scholar]
- 62.Stocks SZ, Taylor SM, Shiels IA. Transforming growth factor-beta1 induces alpha-smooth muscle actin expression and fibronectin synthesis in cultured human retinal pigment epithelial cells. Clin Experiment Ophthalmol. 2001;29:33–37. doi: 10.1046/j.1442-9071.2001.00368.x. [DOI] [PubMed] [Google Scholar]
- 63.Gamulescu MA, Chen Y, He S, et al. Transforming growth factor beta2-induced myofibroblastic differentiation of human retinal pigment epithelial cells: Regulation by extracellular matrix proteins and hepatocyte growth factor. Exp Eye Res. 2006;83:212–222. doi: 10.1016/j.exer.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Ko M, Joo CK. Rho plays a key role in TGF-beta1-induced cytoskeletal rearrangement in human retinal pigment epithelium. J Cell Physiol. 2008;216:520–526. doi: 10.1002/jcp.21424. [DOI] [PubMed] [Google Scholar]
- 65.Hu YB, Li X, Liang GN, et al. Roles of Rho/Rock signaling pathway in silica-induced epithelial-mesenchymal transition in human bronchial epithelial cells. Biomed Environ Sci. 2013;26:571–576. doi: 10.3967/0895-3988.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Wei J, Li Z, Ma C, et al. Rho kinase pathway is likely responsible for the profibrotic actions of aldosterone in renal epithelial cells via inducing epithelial-mesenchymal transition and extracellular matrix excretion. Cell Biol Int. 2013;37:725–730. doi: 10.1002/cbin.10082. [DOI] [PubMed] [Google Scholar]
- 67.Clay MR, Halloran MC. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development. 2013;140:3198–3209. doi: 10.1242/dev.095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Liu X, Liu Y, et al. Epithelial-mesenchymal transition of rat peritoneal mesothelial cells via Rhoa/Rock pathway. In Vitro Cell Dev Biol Anim. 2011;47:165–172. doi: 10.1007/s11626-010-9369-0. [DOI] [PubMed] [Google Scholar]
- 69.Cho HJ, Yoo J. Rho activation is required for transforming growth factor-beta-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol Int. 2007;31:1225–1230. doi: 10.1016/j.cellbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Azar KM, Xiao L, Ma J. Baseline obesity status modifies effectiveness of adapted diabetes prevention program lifestyle interventions for weight management in primary care. Biomed Res Int. 2013;2013:191209. doi: 10.1155/2013/191209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu L, Gao Q, Ni L, et al. Fasudil inhibits epithelial-myofibroblast transdifferentiation of human renal tubular epithelial HK-2 cells induced by high glucose. Chem Pharm Bull (Tokyo) 2013;61:688–694. doi: 10.1248/cpb.c13-00066. [DOI] [PubMed] [Google Scholar]
- 72.Das S, Becker BN, Hoffmann FM, et al. Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol. 2009;10:94. doi: 10.1186/1471-2121-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- 74.Tribukait A, Rosenhall U, Osterdahl B. Morphological characteristics of the human macula sacculi. Audiol Neurootol. 2005;10:90–96. doi: 10.1159/000083364. [DOI] [PubMed] [Google Scholar]
- 75.Chen HC, Zhu YT, Chen SY, et al. Wnt signaling induces epithelial-mesenchymal transition with proliferation in ARPE-19 cells upon loss of contact inhibition. Lab Invest. 2012;92:676–687. doi: 10.1038/labinvest.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.