Abstract
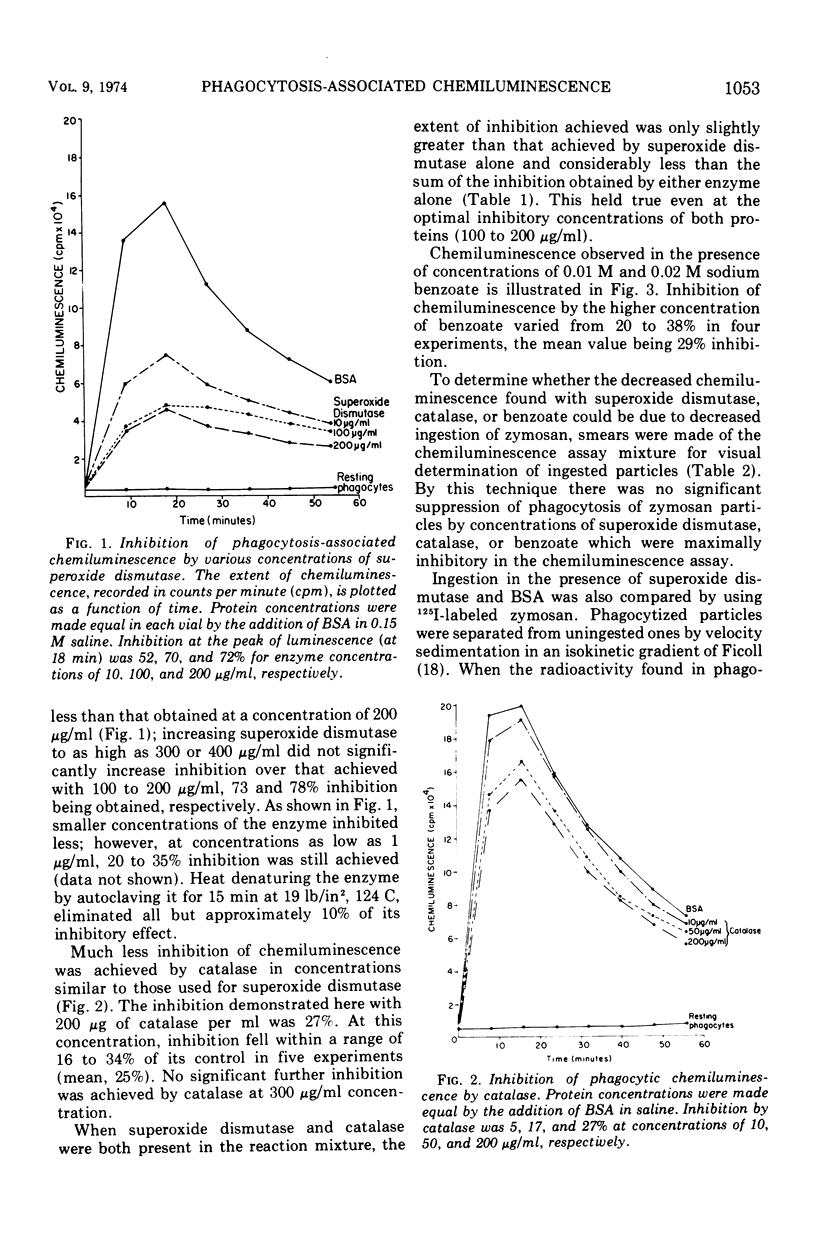
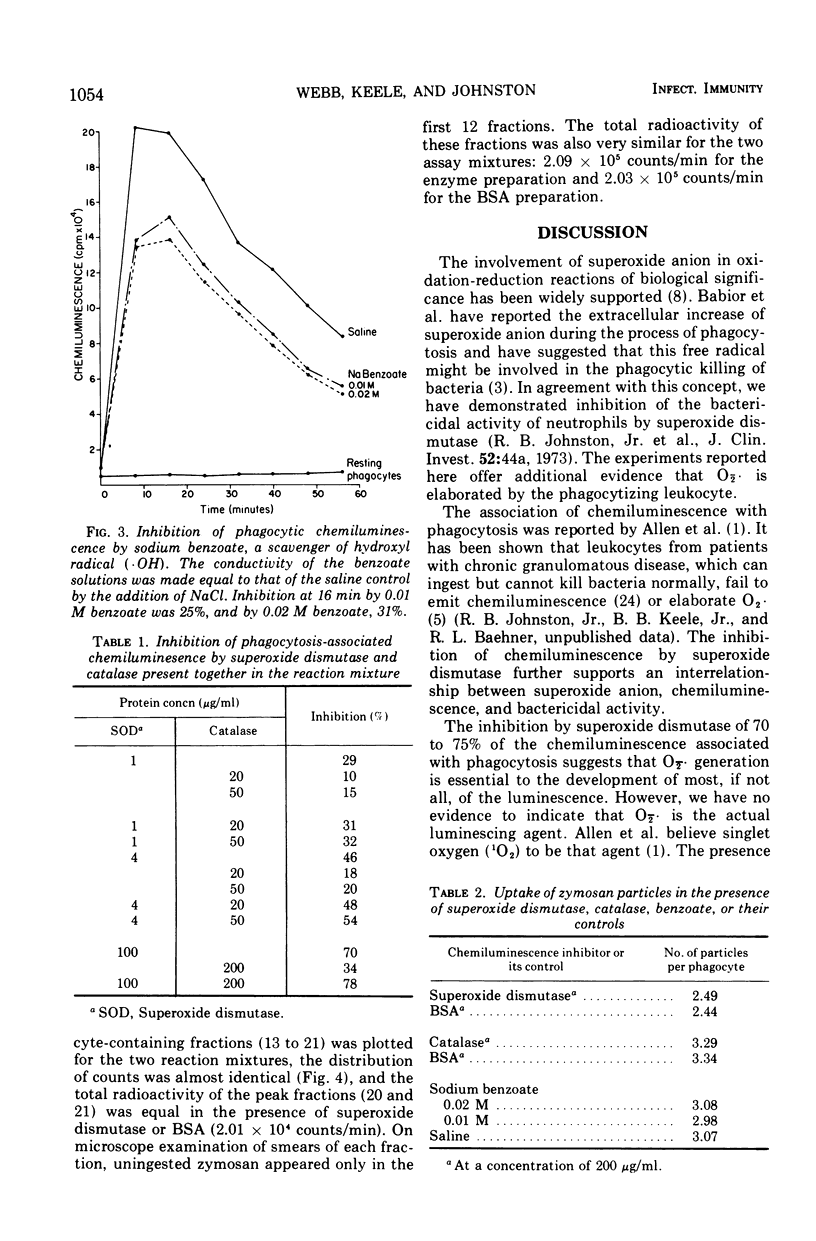
During the process of phagocytosis, human leukocytes emit a burst of luminescence which can be measured in a liquid scintillation spectrometer. The enzyme superoxide dismutase, which removes superoxide anions (O2̄·), inhibited this chemiluminescence by 70% at a concentration of 100 μg/ml. The enzyme did not inhibit phagocytosis. These results support other studies indicating that O2̄· is elaborated by phagocytizing leukocytes. They also indicate that O2̄· plays a major role in phagocytosis-associated chemiluminescence, though not necessarily as the luminescing agent. Catalase and benzoate inhibited the chemiluminescence of phagocytosis to a slight extent, suggesting that hydrogen peroxide and hydroxyl radical, respectively, might also be involved in this phenomenon. The relationship between the mediators of chemiluminescence and those responsible for phagocytic bactericidal activity remains to be defined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agro' A. F., Giovagnoli C., De Sole P., Calabrese L., Rotilio G., Mondovi' B. Erythrocuprein and singlet oxygen. FEBS Lett. 1972 Mar 15;21(2):183–185. doi: 10.1016/0014-5793(72)80132-7. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Arneson R. M. Substrate-induced chemiluminescence of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1970 Feb;136(2):352–360. doi: 10.1016/0003-9861(70)90205-5. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- GREENLEE L., FRIDOVICH I., HANDLER P. Chemiluminescence induced by operation of iron-flavoproteins. Biochemistry. 1962 Sep;1:779–783. doi: 10.1021/bi00911a008. [DOI] [PubMed] [Google Scholar]
- Goda K., Chu J., Kimura T., Schaap A. P. Cytochrome c enhancement of singlet molecular oxygen production by the NADPH-dependent adrenodoxin reductase-adrenodoxin system: the role of singlet oxygen in damaging adrenal mitochondrial membranes. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1300–1306. doi: 10.1016/0006-291x(73)90642-6. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- Khan A. U. Singlet molecular oxygen from superoxide anion and sensitized fluorescence of organic molecules. Science. 1970 Apr 24;168(3930):476–477. doi: 10.1126/science.168.3930.476. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Luberoff D. E. A new method for separating lymphocytes and granulocytes from human peripheral blood using programmed gradient sedimentation in an isokinetic gradient. Immunology. 1973 Jan;24(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C., PILLEMER L. The properdin system and immunity. V. The bactericidal activity of the properdin system. J Exp Med. 1956 May 1;103(5):553–575. doi: 10.1084/jem.103.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]



