Abstract
Background
A rapid and early monoclonal (M) protein response during initial therapy in patients with multiple myeloma has been identified as a predictor of superior long-term outcome in some, but not all studies.
Methods
To determine if the parameter of M protein reduction was of value in the relapsed and/or refractory setting, we performed a retrospective landmark analysis at the end of cycles 2 and 4 of a phase III study which randomized such patients to receive bortezomib alone, or pegylated liposomal doxorubicin (PLD) with bortezomib. Results: Compared with a <25% reduction in M protein at the landmark time point, patients with a 50-<75% reduction after cycle 2 had a significantly lower hazard ratio for time to progression [hazard ratio (HR)=0.41; 95% confidence interval [CI] (0.26, 0.64); P<.001], as did those with a ≥75% reduction [HR=0.26; 95% CI (0.15, 0.45); P<.001]. In all of these groups, PLD + bortezomib provided superior outcomes to bortezomib alone, and did so without an increase in the risk of adverse events overall and a predictable toxicity profile.
Conclusions
These analyses support the possibility that a robust early M protein response is a good prognostic factor for long-term outcome of myeloma patients with relapsed and/or refractory disease receiving bortezomib or PLD + bortezomib.
Keywords: relapsed/refractory multiple myeloma, pegylated liposomal doxorubicin, bortezomib, monoclonal (M) protein, response rapidity
Introduction
Approximately 20,180 new cases of multiple myeloma will be diagnosed in the United States during 2010, and approximately 10,650 people will die of the disease during the same period1. In part due to the availability of new therapies for multiple myeloma, 5-year survival rates and median survival have increased substantially over the past several decades. The latter, for example, has improved from approximately 2-3 years from the time of diagnosis in 1985 through 1998, to approximately 5-7 years for patients diagnosed in 20052, 3. Although multiple myeloma remains incurable, novel, rationally designed regimens such as pegylated liposomal doxorubicin (PLD; DOXIL®) and the proteasome inhibitor bortezomib (VELCADE®) appear to be improving outcomes in the relapsed and/or refractory setting as well. Indeed, in a phase III randomized trial comparing PLD + bortezomib with bortezomib alone in this patient population, PLD + bortezomib improved time to progression (TTP) from 6.5 months with bortezomib alone, to 9.3 months in patients treated with the combination4. Moreover, therapy with PLD + bortezomib improved the complete + very good partial response rate, the response duration, the progression-free survival, and the overall survival compared to single agent bortezomib.
Prognostic factors, such as advanced age, low serum albumin, greater number of prior therapies, and increased β2-microglobulin (β2M) levels have been recognized to unfavorably affect treatment outcomes in both front-line and relapsed settings and impact subsequent treatment choices5. Other factors may also influence the choice of salvage therapy in relapsed/refractory multiple myeloma, including various disease characteristics such as the monoclonal (M) protein burden. Several studies have suggested that there is a positive relationship between rapidity of response, as measured by the reduction in M protein, to outcomes in patients with newly diagnosed multiple myeloma. For example, Ross et al. noted that those patients who achieved at least a partial response after one cycle of infusional vincristine and doxorubicin with oral dexamethasone (VAD) had a superior event-free survival compared to those with lesser quality responses6. Similarly, Schaar et al. reported that an at least 30% reduction in the M-protein, and especially a 40% reduction after one cycle of melphalan and prednisone (MP), predicted for superior survival7. Thus, an early response to induction therapy in multiple myeloma may benefit long-term outcome measures, and may result in an improvement in symptom burden. To investigate the possibility that a relationship between rapid response and outcomes is also present in the relapsed and/or refractory setting, we retrospectively evaluated the impact of an early reduction in the M protein in the phase III trial of PLD + bortezomib versus bortezomib alone in patients with relapsed and/or refractory multiple myeloma.
Methods
Study treatment and endpoints
Full details of the primary study design, protocol, and treatment have been previously described4. Briefly, patients naïve to bortezomib with measurable disease who had progressed after ≥1 line of therapy, or were refractory to initial treatment, were randomized after stratification by β2M levels and response to initial therapy. The intent-to-treat (ITT) population received PLD at 30 mg/m2 as a 1-hour or longer intravenous (IV) infusion on Day 4 and bortezomib at 1.3 mg/m2 on Days 1, 4, 8, and 11 of every 21-day cycle (n = 324), or bortezomib alone at the same dose and schedule (n = 322). Treatment continued until disease progression or unacceptable toxicity occurred, or for 8 cycles, though patients who were still responding after 8 cycles could continue, provided treatment was tolerated. The primary efficacy endpoint was the TTP, and secondary endpoints included response rate and safety. Assessments of the latter included adverse event reports, changes in clinical laboratory findings, and cardiac function tests (multiple gated acquisition scan/echocardiogram/electrocardiogram).
Post-hoc landmark analyses of M protein (early response)
The percentage decrease from baseline in M protein was evaluated post-hoc using landmark analyses at the end of cycles 2 and 4. Patients were categorized into 4 groups by reduction in M protein at the landmark time points. Patients included in the landmark analyses must have had: 1) baseline M protein ≥1.0; 2) end of cycle 2 (or cycle 4) M protein data; 3) dose intensity >0 for cycle 2 (or cycle 4); and 4) not progressed or been censored by study day used for M protein data at end of cycle 2 (or cycle 4). Using a Cox proportional hazard model, TTP was evaluated by treatment and by the 3 groups with ≥25% M protein reduction compared to the group with <25% M protein reduction.
Results
A total of 199 patients receiving PLD + bortezomib and 205 patients receiving bortezomib alone were included in the landmark analysis at the end of cycle 2, while 116 patients receiving PLD + bortezomib and 129 patients receiving bortezomib alone were included in the landmark analysis at the end of cycle 4. Because our interest was focused on the early responders, the results below focus on the landmark analyses at the end of cycle 2.
Efficacy
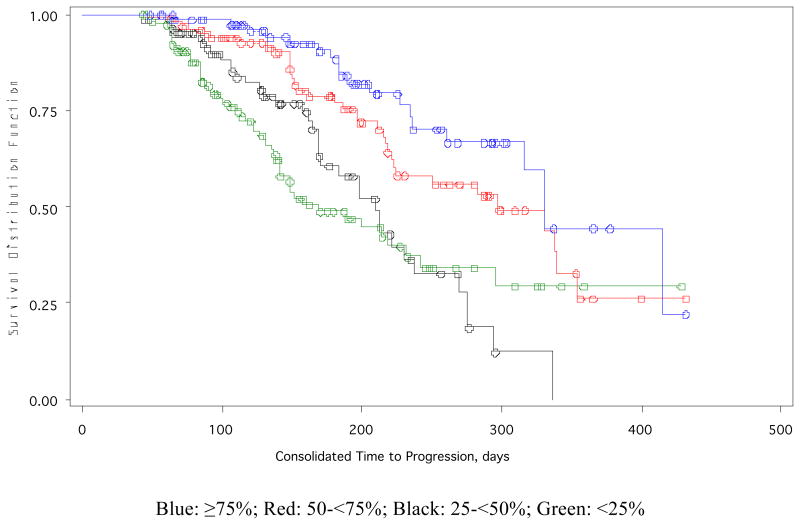
Regardless of the treatment assignment, a decrease in the risk of TTP was observed in patients with a greater reduction in M protein (Table 1). At the cycle 2 landmark analysis, the risk reduction was significant for the 50-<75% M protein reduction group (approximately a 60% risk reduction) and for the ≥75% M protein reduction group (approximately a 75% risk reduction). At the cycle 4 landmark analysis, the risk reduction was significant for the ≥75% M protein reduction group (approximately a 60% risk reduction). PLD + bortezomib continued to be significantly superior to bortezomib alone at cycle 2 and cycle 4, even after adjusting for the % decrease in M protein. TTP was longer as M protein decreased at the end of cycle 2 and improved for PLD + bortezomib vs bortezomib (Table 2; Figure 1). Similar results were observed at the end of the cycle 4 landmark analysis (not shown). At the end of cycle 2, larger reductions in M protein resulted in a longer duration of response as well as in increasing percentages of patients having a complete response or partial response (CR + PR). Patients in the ≥75% M protein reduction group had the highest CR + PR rates of 97.9% and 95.0% in the PLD + bortezomib and bortezomib alone treatment groups, respectively.
Table 1. Hazard Ratios* by TTP for M Protein Reduction Groups at the End of Cycle 2 and Cycle 4 Landmarks.
| Cycle 2 | Cycle 4 | |||
|---|---|---|---|---|
| HR (95% CI) | P | P | ||
| B vs. PLD + B | 1.68 (1.19, 2.37) | .003 | 2.38 (1.47, 3.84) | <.001 |
|
| ||||
| M Protein reduction groups | ||||
|
| ||||
| 25-<50% vs. <25% | 0.84 (0.55, 1.30) | .438 | 0.82 (0.38, 1.75) | .602 |
| 50-<75% vs. <25% | 0.41 (0.26, 0.64) | <.001 | 0.68 (0.35, 1.30) | .238 |
| ≥75% vs. <25% | 0.26 (0.15, 0.45) | <.001 | 0.40 (0.22, 0.73) | .003 |
Abbreviations: B, bortezomib; CI, confidence interval; HR, hazard ratio; M protein, monoclonal protein; PLD, pegylated liposomal doxorubicin; TTP, time to progression
Proportional hazard model.
Table 2. Median TTP by M Protein Reduction Groups at the End of Cycle 2 Landmark.
| PLD + B N=199 |
B N=205 |
|
|---|---|---|
| <25%, n (%) | 58 (29.1) | 63 (30.7) |
|
| ||
| Median TTP, days (95% CI) | 214 (141, N/E) | 151 (116, 242) |
| Duration of response, days (95% CI) | N/E | N/E |
| CR+PR, n/evaluable (%) | 1/58 (1.7%) | 3/63 (4.8%) |
|
| ||
| 25-<50%, n (%) | 40 (20.1) | 48 (23.4) |
|
| ||
| Median TTP, days (95% CI) | 221 (198, 275) | 183 (164, 230) |
| Duration of response, days (95% CI) | 165 (123, 212) | 172 (120, 274) |
| CR+PR, n/evaluable (%) | 11/40 (27.5%) | 17/48 (35.4%) |
|
| ||
| 50-<75%, n (%) | 54 (27.1) | 54 (26.3) |
|
| ||
| Median TTP, days (95% CI) | 338 (331, N/E) | 217 (197, N/E) |
| Duration of response, days (95% CI) | 311 (297, N/E) | 180 (169, N/E) |
| CR+PR, n/evaluable (%) | 48/54 (88.9%) | 46/54 (85.2%) |
|
| ||
| ≥75%, n (%) | 47 (23.6) | 40 (19.5) |
|
| ||
| Median TTP, days (95% CI) | 415 (330, N/E) | 261 (227, N/E) |
| Duration of response, days (95% CI) | 394 (309, 394) | 237 (206, N/E) |
| CR+PR, n/evaluable (%) | 46/47 (97.9%) | 38/40 (95.0%) |
Abbreviations: B, bortezomib; CI, confidence interval; CR+PR, complete response or partial response; M protein, monoclonal protein; N/E, not evaluable; PLD, pegylated liposomal doxorubicin; TTP, time to progression
Figure 1.
Kaplan-Meier estimates for time to progression by M protein reduction groups at the end of Cycle 2 Landmark.
Safety
The safety profiles of the 2 treatment groups, PLD + bortezomib and bortezomib alone, were comparable at the cycle 2 landmark (Table 3). Of the 404 patients evaluated at the cycle 2 landmark, 17% of patients receiving PLD + bortezomib experienced a ≥Grade 3 adverse event, compared with 13% of patients receiving bortezomib alone. As expected, patients receiving PLD + bortezomib experienced more hand-foot syndrome compared with patients receiving bortezomib alone, and also more mucositis/stomatitis, but less peripheral neuropathy both overall, and at the Grade 3 and 4 levels of severity. Overall safety profiles in this analysis were similar to the overall safety profiles for the previously reported phase III study4.
Table 3. Adverse Events of Interest at the End of Cycle 2 Landmark.
| n (%) | PLD + B (n=199) |
B (n=205) |
||
|---|---|---|---|---|
| All | ≥Grade 3 | All | ≥Grade 3 | |
| Peripheral neuropathy | 77 (38.7) | 9 (4.5) | 93 (45.4) | 21 (10.2) |
| Febrile neutropenia | 5 (2.5) | 5 (2.5) | 3 (1.5) | 3 (1.5) |
| Bleeding/hemorrhage | 23 (11.6) | 5 (2.5) | 15 (7.3) | 2 (1.0) |
| Mucositis/stomatitis | 43 (21.6) | 4 (2.0) | 9 (4.4) | 1 (0.5) |
| Hand-foot syndrome | 39 (19.6) | 11 (5.5) | 0 | 0 |
| Thromboembolic events | 1 (0.5) | 0 | 2 (1.0) | 1 (0.5) |
| Alopecia | 1 (0.5) | 0 | 1 (0.5) | 0 |
Abbreviations: B, bortezomib; PLD, pegylated liposomal doxorubicin
Discussion
Analyses of modern therapeutic approaches to multiple myeloma with regimens incorporating novel agents have generally supported the hypothesis that response quality predicts long-term outcomes, with deeper responses being associated with longer TTP and overall survival2. However, there is some controversy about whether the early response rapidity is similarly predictive. Schaar and colleagues conducted a study that prospectively evaluated the relationship between survival and the rate of M protein decrement during the first cycles of therapy in 262 patients with newly diagnosed multiple myeloma that were included in a phase III trial (HOVON-16)7. In this study, the median M protein decrease after the first cycle of MP was 21% for IgG and 27% for IgA, and declined to <5% after 4 cycles. A survival advantage was seen for patients who had an M protein decrease of at least 30% after the first MP cycle, which became significant when an M protein decrease of 40% or more was reached. They also found that established prognostic parameters, including Salmon & Durie stage, serum creatinine, and hemoglobin, remained significant, leading to the conclusion that early response to MP treatment predicted for survival in multiple myeloma. However, in 2 retrospective studies by Shimizu et al., also in the frontline setting, the post-treatment nadir in M protein level was prognostic for survival outcome, but the percentage fall in M protein was not prognostic8, 9. Moreover, van Rhee et al. have reported that a rapid reduction in myeloma burden, as measured by a top-tertile reduction in the serum-free light chain levels before cycle 2, predicted for an inferior progression-free and 2-year overall survival after their induction therapy and tandem transplantation approach10.
In that these previous studies have focused predominantly on patients with newly-diagnosed disease, we sought to determine the possible impact of rapid early disease response in the relapsed and/or refractory setting. In a follow-up to the SUMMIT and CREST clinical trials, patients with relapsed and/or refractory multiple myeloma who had progressive or stable disease after single-agent bortezomib therapy were eligible to receive dexamethasone as add-on therapy. Patients with an improved response to the combination experienced a median reduction of 36.7% in M protein level, compared to a median reduction of 15.4% in responders on bortezomib alone11. Our analyses showed that, compared to the group of patients with a less than 25% reduction, a 50% or greater, and especially a 75% or greater reduction in M protein levels at cycle 2 resulted in a significant risk reduction for time to progression. Indeed, for both the PLD + bortezomib and bortezomib alone cohorts, patients with a ≥75% M protein reduction had a TTP that was nearly double that of the group with a <25% reduction. In addition, over 90% of patients with a ≥75% M protein reduction had CR + PR, compared with less than 5% in the <25% reduction group. For all of the response categories studied, PLD + bortezomib retained its superiority to bortezomib alone in the primary study endpoint, TTP. Moreover, these benefits were not associated with a significantly higher risk for patients to develop Grade 3 or higher adverse events, and were associated with a predictable toxicity profile.
Limitations of the current study include: 1) this is a post-hoc analysis of a phase III clinical trial; 2) comparisons between M protein-reduction groups at later landmark time points may be less meaningful due to diminishing sample sizes on subsequent cycles of therapy; and 3) as the comparators in the trial were PLD + bortezomib and bortezomib alone, standard agents, such as dexamethasone,12 were not included.
Taken together, our data support the possibility that an early disease response, as defined by an at least 50% M protein reduction after cycle 2 to bortezomib alone and especially to PLD + bortezomib, identifies a group of patients with superior long-term outcomes. Moreover, these data also provide a rationale for individualization of therapy based on such early outcomes, in which those patients with at least a 50% M protein response after cycle 2, and a 75% response after cycle 4, would continue on bortezomib or PLD + bortezomib. Furthermore, this analysis provides a pathway for additional study, including the investigation of the effects of adding other agents to the combination therapy of PLD + bortezomib or to bortezomib alone when patients experience a less than adequate M protein response (<25% reduction) in early therapy.
In summary, our results suggest that the combination of PLD + bortezomib had significant benefit over bortezomib alone in extending TTP of early responders in cycle 2 and 4 landmark analyses similar to the overall study. An early response of a >50% reduction in M protein at cycle 2 resulted in a significant risk reduction for progression. These data suggest that early reductions in M protein may provide better outcomes with PLD + bortezomib combination therapy. More research is needed to investigate the effect of early responses and M protein reduction on outcomes in multiple myeloma.
Acknowledgments
Supported by Centocor Ortho Biotech Services, LLC, Horsham, PA.
The study was supported by Centocor Ortho Biotech Services, LLC. Dr. Bladé is a consultant to Janssen-Cilag and Celgene. Dr. Sonneveld is a consultant to Johnson and Johnson. Dr. Londhe is an employee of Centocor Ortho Biotech Services, LLC. Dr. Lowery and Mr. Lantz are former employees of Centocor Ortho Biotech Services, LLC.
Footnotes
All other authors had no disclosures.
Contributor Information
Jatin Shah, The University of Texas M. D. Anderson Cancer Center, Houston, TX.
Joan Bladé, Hematology Department, Hospital Clinic, IDIBAPS, Barcelona, Spain.
Pieter Sonneveld, Erasmus Medical Center, Rotterdam, The Netherlands.
Jean-Luc Harousseau, University Hospital Hotel-Dieu, Nantes, France.
Keith Lantz, formerly with Centocor Ortho Biotech Services, LLC, Horsham, PA.
Anil Londhe, Centocor Ortho Biotech Services, LLC, Horsham, PA.
Colin Lowery, formerly with Centocor Ortho Biotech Services, LLC, Horsham, PA.
Robert Z. Orlowski, The University of Texas M. D. Anderson Cancer Center, Houston, TX.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Durie BG. New approaches to treatment for multiple myeloma: durable remission and quality of life as primary goals. Clin Lymphoma Myeloma. 2005;6(3):181–190. doi: 10.3816/CLM.2005.n.045. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Sonneveld P, Schuster MW, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137(5):429–435. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- 6.Ross DM, To LB, Horvath N. Assessment of early paraprotein response to vincristine-doxorubicin-dexamethasone chemotherapy may help guide therapy in multiple myeloma. Intern Med J. 2004;34(9-10):576–578. doi: 10.1111/j.1445-5994.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- 7.Schaar CG, Kluin-Nelemans JC, le Cessie S, Franck PF, te Marvelde MC, Wijermans PW. Early response to therapy and survival in multiple myeloma. Br J Haematol. 2004;125(2):162–166. doi: 10.1111/j.1365-2141.2004.04884.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Kamiya O, Hirabayashi N, et al. Posttreatment M-protein nadir level is a significant prognostic factor associated with survival in multiple myeloma. Nagoya Myeloma Cooperative Study Group. Jpn J Cancer Res. 1999;90(3):355–360. doi: 10.1111/j.1349-7006.1999.tb00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu K, Nagura E, Hirabayashi N, et al. Posttreatment nadir M-protein level is a stronger discriminator of survival following plateau attainment than is percent reduction in M-protein in patients with IgG myeloma. Int J Hematol. 2001;74(2):205–208. doi: 10.1007/BF02982006. [DOI] [PubMed] [Google Scholar]
- 10.van Rhee F, Bolejack V, Hollmig K, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110(3):827–832. doi: 10.1182/blood-2007-01-067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagannath S, Richardson PG, Barlogie B, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91(7):929–934. [PubMed] [Google Scholar]
- 12.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™): Multiple Myeloma. [Accessed October 19, 2010];2011 1 Available at: http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. [Google Scholar]