Abstract
Vascular endothelial growth factor (VEGF) was originally discovered as a tumor-derived factor that is able to induce endothelial cell behavior associated with angiogenesis. It has been implicated during wound healing for the induction of endothelial cell proliferation, tube formation and blood vessel remodeling. However, previous investigations into the biological effect of VEGF concluded that a particular range of growth factor concentrations are required for healthy vasculature to form, motivating recent studies to regulate VEGF activity via molecular sequestering to biomaterials. Numerous VEGF sequestering strategies have been developed, and they have typically relied on extracellular matrix mimicking moieties that are not specific for VEGF and can affect many growth factors simultaneously. We describe here a strategy for efficient, specific VEGF sequestering with poly(ethylene glycol) (PEG) microspheres, using peptides designed to mimic VEGF receptor type 2 (VEGFR2). By immobilizing two distinct peptides with different serum stabilities, we examined the effect of serum on the specific interaction between peptide-containing PEG microspheres and VEGF. We addressed the hypothesis that VEGF sequestering in serum-containing solutions would be influenced by the serum stability of the VEGF-binding peptide. We further hypothesized that soluble VEGF could be sequestered in serum-containing cell culture media, resulting in decreased VEGF-dependent proliferation of human umbilical vein endothelial cells. We show that soluble VEGF concentration can be effectively regulated in serum-containing environments via specific molecular sequestering, which suggests potential clinical applications.
Keywords: Angiogenesis, VEGF, Sequestration, Poly(ethylene glycol), Extracellular matrix
1. Introduction
During wound healing, vascular endothelial growth factor (VEGF) is released by activated platelets, neutrophils and macrophages [1], and stimulates an angiogenesis cascade that ultimately leads to new blood vessel formation [2]. Events in the angiogenesis cascade including induction of endothelial cell proliferation are highly dependent on local VEGF activity, motivating numerous studies to deliver VEGF and induce blood vessel formation in a healing wound. Clinical trials focused on VEGF protein and gene delivery show that leaky, immature vasculature often forms below and above an optimal VEGF concentration range [3,4], demonstrating a need to carefully regulate the VEGF dosage. Recent investigations have concluded that maintaining VEGF levels in an optimal concentration range results in mature blood vessel formation in vivo [5,6], which motivates the need for developing biomaterials that regulate VEGF activity.
One mechanism by which nature regulates VEGF-dependent signaling involves sequestering and release from the extracellular matrix (ECM). Natural ECMs are composed of numerous VEGF-binding molecules, including collagens [7–9], glycoproteins (e.g. fibronectin [10]) and proteoglycans [11]. Sequestered VEGF165, the most commonly studied VEGF isoform due to its role in wound healing, can be released from the ECM by proteolytic remodeling of ECM components [12]. Investigators have recently mimicked the function of the natural ECM using synthetic biomaterials that bind to VEGF via affinity interactions. For example, Tan et al. [13] demonstrated that heparan sulfate proteoglycans incorporated into collagen microspheres can bind to VEGF and regulate VEGF-dependent endothelial cell behavior in vitro, and Chung et al. [14] showed that microspheres with immobilized heparin and heparin-fibrin conjugates [15] can influence VEGF release kinetics in vitro and induce neovascularization in vivo. These studies and others [9,16–20] clearly show that ECM mimicking molecules (e.g. heparin, proteoglycans) can bind to numerous growth factors, providing an adaptable mechanism for growth factor regulation [21]. In addition, our group recently showed that peptide mimics of VEGF receptor type 2 (VEGFR2) immobilized within a synthetic hydrogel can specifically sequester VEGF, and thereby selectively regulate VEGF-dependent endothelial cell behavior in vitro [22,23]. This receptor mimicking approach is particularly attractive, as it allows one to regulate activity of a specific growth factor, even in heterogeneous environments like biological fluids. However, previous studies have not explored in detail the context-dependence of growth factor sequestering, and little is known about the role of biological fluids in growth factor regulation in vitro or in vivo. Biological fluids such as blood serum contains significant quantities of growth factors and other proteins [24,25], which may decrease the specificity and affinity of growth factor sequestering. Therefore, there is a need to more clearly understand the serum dependence of growth factor sequestering biomaterials.
Here we examined the effect of serum on VEGF binding to polymeric microspheres containing specific, VEGF-binding peptides. These peptides were derived from VEGFR2 [26,27] and were chosen based on their differing serum stability, which allowed us to characterize the influence of serum on VEGF binding and associated VEGF regulation. Specifically, we explored a wild-type VEGF-binding peptide as well as a derivative of this peptide that included four D-substituted amino acids, which provide enhanced peptide stability against protease-mediated degradation [27]. We hypothesized that peptide stability in serum would influence VEGF sequestering. Specifically, we reasoned that the increased serum stability of the D-substituted VEGF-binding peptide (VBP) would increase VEGF sequestering relative to the wild-type VEGF-binding peptide (VBPWT). In addition, we hypothesized that VEGF-binding microspheres would reduce VEGF-dependent human umbilical vein endothelial cell (HUVEC) proliferation in culture, resulting in a novel, biology-inspired mechanism for “knocking down” VEGF signaling in vitro.
2. Materials and methods
2.1. Peptide synthesis and characterization
Two peptides identified from a previous study, VBP sequence CEFdAdYdLdIDFNWEYPASK and the wild-type VBPWT sequence CEL-NVGIDFNWEYPASK [26,27], and a peptide with the same amino acids but in a scrambled sequence (Scramble), CDAdPYNFdEFA-WEYdVISLdK, were synthesized using standard Fmoc solid-phase peptide synthesis on MBHA Rink amide resin, as previously described [23]. All amino acids (EMD Novabiochem), were protected at the N-terminus with an Fmoc protecting group. Initial deprotec-tion was performed with 20% piperidine (Sigma-Aldrich) in sequence-grade dimethylformamide (DMF; Fisher Scientific). Subsequent amino acid coupling was performed by first activating the C-terminus with 0.5 M hydroxybenzotriazole (Creosalus) in DMF, then deprotecting the N-terminus of the resin initially and later the growing peptide chain using 20% piperidine. After synthesis, cleavage was performed by reacting acetone-washed resin with 10 ml of 95 vol.% trifluoroacetic acid (TFA; Sigma Aldrich), 2.5 vol.% triisopropylsilane (Sigma Aldrich) and 2.5 vol.% deionized water (DI water); peptides were thereafter washed with diethyl ether (Fisher Scientific), dried, dissolved in DI water and lyophi-lized. Peptide identity was determined by dissolving a small sample at 10 mg mr−1 in 70% acetonitrile (ACN; Sigma Aldrich), 30% DI water and 0.1% TFA; samples were subsequently subjected to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker) using a 2,5-dihydroxybenzoic acid matrix (Sigma Aldrich) at 20 mg ml−1 in 70% ACN. Peptide purity of >85% was determined with C18 reverse-phase high performance liquid chromatography (Shimadzu, Supelco silica column). The peptide content, meaning the percentage of dry powder mass containing peptide, was determined by using Ellman’s assay to detect free thiol groups (Thermo Fisher).
2.2. PEG-norbornene synthesis
Four-arm poly(ethylene-glycol) (PEG; Mn = 20,000, Jenkem) was functionalized with norbornene moieties at each arm in order to utilize thiolene photopolymerization, as introduced by Lin and Anseth [28] and described previously [23]. Briefly, 4-arm PEG, terminated at each arm with a hydroxyl functional group, was allowed to react under constant stirring in a sealed argon-purged flask with 10-fold excess (with respect to the number of PEG arms) of 5-norbornene-2-carboxylic acid (Sigma-Aldrich) in dichloro-methane (Fisher), five molar equivalents of N,N’-dicyclohexylcar-bodiimide (Sigma), 0.5 molar equivalents 4-dimethylamino pyridine (Sigma-Aldrich) and five equivalents of pyridine (Sigma-Aldrich). Derivatization was determined as >90% using 1H nuclear magnetic resonance, and quality control was established by ensuring that the final product was able to form a 3 wt.% PEG gel of 4-arm norbornene-derivatized PEG (PEG-NB) and PEG3400 dithiol (Laysan Bio) with equal molar ratios of norbornene and thiol in the reaction.
2.3. Microsphere synthesis
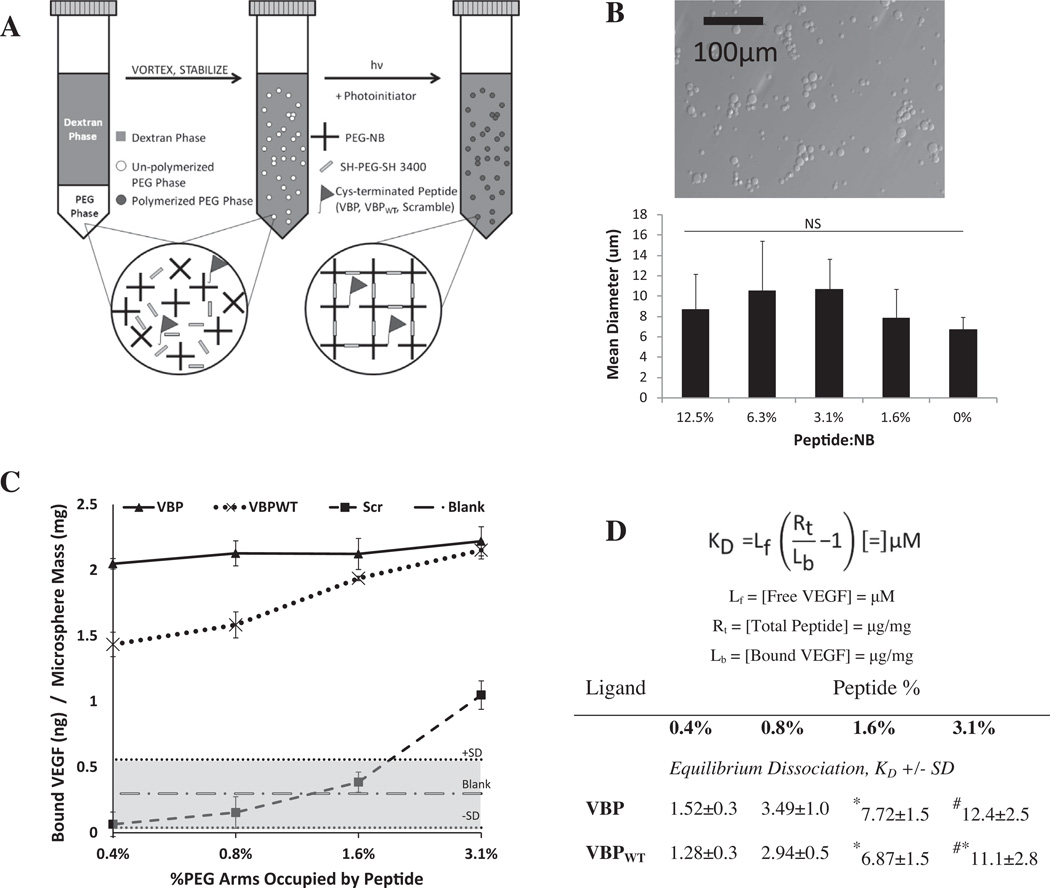
Microspheres with and without covalently linked peptide were synthesized using a water-in-water emulsion [29], as described previously [22,23] and demonstrated in Fig. 1A Microspheres were synthesized by first preparing a PEG-rich phase. In this phase, PEG-NB was mixed with a half molar equivalent of PEG3400 dithiol along with a peptide solution and photoinitiator (at a final concentration of 0.05 wt.%). The peptide concentration to achieve a molar per cent with respect to norbornene groups of 3.1%, 1.6%, 0.8%, 0.4% or 0% (denoted “Blank” microspheres) was prepared and mixed into the PEG phase. The PEG phase was degassed briefly with argon and vortexed. The final weight per cent of PEG (including PEG-NB and PEG3400 dithiol) was 10 wt.% for a total of 500 ml in DI water. A dex-tran-rich phase was prepared by dissolving dextran T-40 (Sigma-Aldrich) at 40 wt.% in a pH 8 buffer containing 0.22 M KCl and 10 mM NaPO4, degassed with argon before use. Subsequently, 3 ml of a dextran-rich phase was added to the PEG-rich phase in a conical tube. The tube containing both phases was then purged with argon, vortexed for 1 min and allowed to sit at room temperature for 20 min. The suspension was UV polymerized for 6 min, then the reaction mixture was diluted 25-fold in fresh DI water. Microspheres were washed by centrifuging at 2500 rpm, followed by decanting and refilling with fresh DI water a total of three times. After the final wash, microspheres were suspended in DI water, frozen and lyophilized. The peptide content was determined by bicinchoninic acid assay (Thermo Scientific), subtracting the signal from that of Blank microspheres for analysis of peptide content per weight of microsphere. The microsphere size distribution was determined by staining a microsphere suspension with Trypan blue (Fisher) and imaging with an Olympus IX51 epifluorescence microscope at 20× magnification, with image processing done by manually measuring the microsphere area and back-calculating the diameter using ImageJ software. Peptide incorporation did not significantly impact on the microsphere size (Fig. 1B). The peptide density is hereafter reported as the percentage of PEG-NB arms occupied by peptide in the coupling reaction.
Fig. 1.
(A) Schematic of PEG-NB microsphere synthesis for covalent peptide incorporation. Shown is the emulsion procedure for synthesizing PEG microspheres using thiolene chemistry. (B) (top) Bright-field image of Blank PEG microspheres; the scale bar represents 100 µm. (bottom) Graph of mean microsphere diameter ±1 SD at a range of incorporated peptide concentrations, as determined using ImageJ software. (C, D) Comparison of peptide density and affinity, and their effect on VEGF binding at 1 mg ml−1 microspheres and 10 ng ml−1 recombinant human VEGF in 0.1% BSA in PBS loading solution. (C) Amount of VEGF bound per weight of microspheres with covalently tethered Scramble microspheres (dashed line, boxes), VBPWT microspheres (dotted line, Xs) and VBP microspheres (solid line, triangles). Sequestered VEGF for Blank microspheres is shown (alternating dashes and dots), with the gray area outlining the average ±1 SD. (D) Effective dissociation constant, KD, of microsphere binding to soluble VEGF. Results are indicated as KD ± SD, with symbols indicating a significant difference compared to 0.4% (*), 0.8% (#) and 1.6% (&) of each respective peptide at a p < 0.05.
2.4. In vitro VEGF binding assays
For all studies, microspheres were incubated in 10 ng ml−1 VEGF because this concentration has been shown to result in maximal endothelial cell proliferation in vitro [30] and has been observed during in vivo wound healing [31]. The microsphere conditions were analyzed in triplicate. Before incubation, 1.5 ml microcentrifuge tubes were blocked with a solution of 0.1 wt.% bovine serum albumin (BSA; Fisher Scientific) in phosphate-buffered saline (PBS) at pH 7.4 (prepared from powdered form, Fisher Scientific) overnight, followed by two washing steps in DI water and subsequent freezing and lyophilizing. Microspheres were weighed out and incubated at 1 mg ml−1 in 9.9 ng ml−1 recombinant human vascular endothelial growth factor-165 (rhVEGF165, hereafter referred to as VEGF; R&D Systems) and 0.1 ng ml−1 of iodine-1250tagged rhVEGF165 (hereafter referred to as [125I]VEGF; Perkin-Elmer). The microspheres were then incubated for 4 h at 37 °C and 95% relative humidity. After incubation, they were centrifuged at 11,000 rpm, and 1 ml of supernatant was collected from each sample. Supernatant counts per minute were determined with a γ-counter (Perkin-Elmer, Cobra II Auto-Gamma) and compared to a [125I]VEGF standard curve to determine the radiolabeled VEGF concentration in the supernatant. Measurement of the loading solution before the microsphere incubation allowed back-calculation of the amount of VEGF bound to microspheres. Significance was determined using a two-tailed Student’s t-test, assuming unequal variance unless otherwise stated, and reported at a p-value of <0.05.
2.5. VEGF binding in serum- and heparin-containing solutions
VEGF binding was measured in solutions containing different protein content and serum concentrations in order to elucidate the serum-dependence of VEGF sequestering. Initial experiments with various microsphere peptide concentrations were performed in 0.1% BSA in PBS at pH 7.4. Various concentrations of fetal bovine serum (FBS; Gibco) − 25%, 10% and 2% – were prepared in pH 7.4 PBS. Above 25% serum, microspheres were unable to be segregated by centrifugation, most likely due to viscosity effects at such a high protein content; microspheres in 5 wt.% BSA in PBS (50 mg ml−1, similar to the total protein concentration of serum [24]) were similarly unable to be segregated following centrifugation. In order to recapitulate the total protein concentration in 25% FBS, a BSA solution was prepared at 1.25 wt.% in pH 7.4 PBS (denoted albumin-only solution). For analysis, the 1.6% and 0.4% peptide concentrations were se-lected to represent the high and low peptide conditions, respectively. For protease activity binding and release assays, 100× HALT protease inhibitor cocktail (Thermo) was diluted to working concentration (1×) in 25% FBS in pH 7.4 PBS. For heparin binding studies, 1.6% peptide microspheres were incubated in soluble porcine unfractionated heparin (UFH; Sigma) at 10 µg ml−1 in a solution containing 0.1% BSA in PBS and 9.9 ng ml−1 VEGF with 0.1 ng ml−1 [125I]VEGF. Preincubation studies were performed by preincubating 1.6% peptide microspheres in 0.1% BSA in PBS with or without 10 µg ml−1 UFH for 4 h. Subsequently, microspheres were incubated in 0.1% BSA in PBS containing 9.9 ng ml−1 VEGF and 0.1 ng ml−1 [125I]VEGF for 4 h. Binding results were tabulated as described above. For serum preincubation experiment, microspheres were incubated in 25% FBS for 4 h, washed three times in 0.1 wt.% BSA in PBS and subsequently incubated in 9.9 ng ml−1 VEGF, 0.1 ng ml−1 [125I]VEGF in 0.1% BSA in PBS. For heparin binding, significance was determined using a two-tailed Student’s t-test, assuming unequal variance unless otherwise stated, and reported at a p-value of <0.05.
2.6. Calculation of equilibrium dissociation constant, KD
An equilibrium dissociation constant, KD was calculated for select VEGF binding conditions. The calculation was based on previous work [32] describing fractional receptor occupancy (the receptor here is defined as VBP, VBPWT or Scramble peptide) as a function of the equilibrium dissociation constant and ligand (VEGF) concentration. Rearrangement of the given equation, B = K × L/ (1+K × L), where B is the fractional occupancy of the receptor, K is 1/KD and L is the ligand concentration [33], yields a dissociation constant given by KD = L/(1/B – 1). The fractional occupancy B is defined by moles of bound ligand divided by the moles of total receptor (peptide), B = RL/(R + RL), where RL is defined as the concentration of receptor-ligand (peptide-VEGF) complex, which is approximated as the moles of bound ligand. In this context, the equilibrium dissociation constant is valid under the assumptions that all peptide binding sites are equally accessible (as suggested by confocal images of fluorescently tagged peptide in PEG microspheres, data not shown). Significance was determined using a two-tailed Student’s t-test, assuming unequal variance unless otherwise stated, and reported at a p-value of <0.05.
2.7. Assays of VEGF biological activity
HUVECs (Lonza) were expanded and plated between population doublings 5 and 7 on 75 cm2 tissue culture flasks (Corning) at 2500 cells cm−2 in “growth medium”, consisting of M199 medium (with Earle’s salts and L-glutamine; CellGro) with added penicillin/ streptomycin (HyClone) and EGM2 supplement (Lonza). On day 0, before adding cells to 96-well plates (Costar), the wells were incubated in 0.1 wt.% porcine gelatin (Sigma) in DI water for 1 h. Cells were grown to ~70% confluence in the growth medium and subsequently removed from the plastic by trypsinization on day 0. Briefly, flasks were rinsed with sterile pH 7.4 PBS, and a 0.05% buffered trypsin solution (Lonza) was added for 2 min until the cells were completely detached. Upon detachment, trypsin was diluted 4-fold in medium containing 2% FBS in M199, hereafter referred to as “low-serum medium”. Cells were added to a 15 ml centrifuge tube and centrifuged at 200g for 5 min. Counting was performed by first aspirating low-serum medium, adding back 1 ml of the low-serum medium and adding 10 µl of the cell suspension diluted 1:1 in Trypan blue to a hemacytometer. Cells were then suspended at 40,000 cells ml−1 in low-serum medium, providing a condition for cell-cycle synchronization consistent with the literature [34,35]. Subsequently, wells were aspirated and cells were added at 100 µl per well, then incubated overnight at 37 °C, 95% relative humidity and 5% CO2. On day 1, the medium was diluted with an equal volume of medium containing 2%, 20% or 50% FBS (to dilute the conditions down to 2%, 10% and 25% FBS, respectively) with 1 mg ml−1 microspheres (with 1.6% peptide or 0% peptide in the case of Blank) and containing 0, 1 or 10 ng ml−1 VEGF. At day 3, 5 µl of 5-ethynyl-2’-deoxyuridine (EdU; Invitrogen) in low-serum medium was added to give a final concentration of 10 µM in the wells, and cells were incubated for an additional 12–15 h. The per cent EdU incorporation was determined for all conditions by following the standard Click-iT EdU assay procedure for tagging S-phase nuclei as well as for staining all cell nuclei with Hoechst (Invitrogen); imaging was accomplished using a Ti Eclipse inverted epifluorescence microscope (Nikon) with NIS Elements v3.2 software; a threshold was applied uniformly across all plates and counted using the built-in object counting in the NIS Elements software. The fractions of EdU-positive and Hoechst-positive cells were calculated for each condition, and subsequent data analysis was performed using a two-tailed Student’s t-test, assuming unequal variance unless otherwise stated, and reported at a p-value of <0.05.
3. Results
Binding of VEGF to microspheres was specific and dependent on the peptide content. Microspheres containing either of the peptides VBP or VBPWT, which are designed to mimic VEGFR2, sequestered significantly higher amounts of soluble VEGF than microspheres containing a scrambled version of VBP (Scramble) or no peptide (Blank). In addition, VBP microspheres sequestered significantly more VEGF than VBPWT microspheres at each peptide density, except for the highest density tested (3.1%). Non-specific binding to Scramble microspheres was significantly higher than to Blank microspheres only at the highest peptide density tested (3.1%, Fig. 1C), indicating that there was a range of peptide densities that allowed for specific VEGF binding between 0.4% and 1.6%. In this range, the significant VEGF binding only to VBP and VBPWT microspheres and not to Scramble or Blank microspheres indicated that VEGF sequestering was peptide sequence specific.
3.1. VEGF sequestering in serum is specific
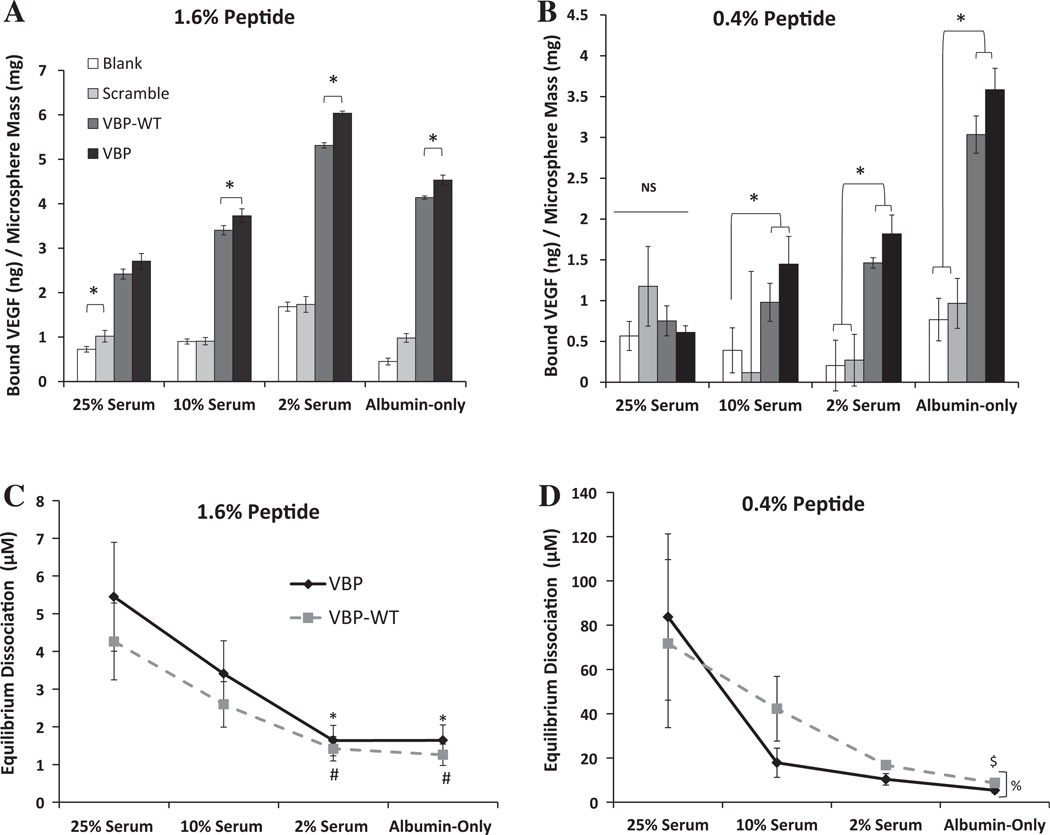
VBP and VBPWT microspheres also exhibited significant VEGF sequestering in the presence of serum. At higher peptide density (1.6%), VBP and VBPWT microspheres bound significantly more VEGF than Blank or Scramble microspheres in all serum concentrations tested (Fig. 2A). At lower peptide density (0.4%), VBP and VBPWT microspheres sequestered significantly more VEGF than Blank or Scramble microspheres in all serum conditions except 25% serum, where there was no significant sequestering observed (Fig. 2B). Increasing the concentration of serum decreased the amount of VEGF binding to VBPWT and VBP microspheres (Fig. 2A). Specifically, VEGF binding to VBP and VBPWT microspheres was significantly higher in the albumin-only solution when compared to the 25% serum solution for each peptide density tested (Fig. 2A and B). This suggests that VEGF sequestering was influenced not only by the total protein amount in solution, but also by the identity of the proteins in solution, as the total protein amount was the same in the albumin-only and 25% serum solutions. VBP microspheres sequestered more VEGF than VBPWT microspheres only at higher peptide concentration (1.6%) in all solutions except 25% serum (Fig. 2A).
Fig. 2.
Comparison of VEGF binding to microspheres at higher and lower peptide density in various FBS-containing solutions (25 vol.% in PBS, 10 vol.% in PBS, 2 vol.% in PBS) and in albumin-only solution (1.25% w/v BSA in PBS), which mimics the total protein amount of 25% serum). (A) Bound VEGF for 1.6% peptide density. (B) Bound VEGF for 0.4% peptide density microspheres. Statistical significance in (A) and (B) is reported at p<0.05 and indicated by an asterisk. (C) Equilibrium dissociation constants for 1.6% microspheres. Significance is indicated by symbols representing a difference compared to VBP in 25% serum (*) or VBPWT in 25% serum (#). (D) Equilibrium dissociation constants for 0.4% microspheres. Significance is reported compared to VBP in 2% serum (&), and between VBP and VBPWT in albumin-only solution (%). Error bars represent 1 SD about the mean. Statistical significance is reported at p < 0.05.
3.2. Microspheres exhibit high-affinity VEGF binding
The equilibrium dissociation constants for the VEGF interaction with VEGF binding microspheres were calculated based on the measured amount of VEGF sequestered combined with the mea-sured amount of peptide present in the microspheres. We assumed that the VEGF binding had reached equilibrium by 4 h, based on previous results showing equilibrium after less than 30 min in the same buffer [23]. We calculated the fraction of peptide occupied by VEGF, known as the fractional occupancy, denoted “B”. Using the experimental value for the total peptide concentration in the microspheres, L, the equilibrium dissociation constant was found by [32]. Comparison of the equilibrium dissociation constants gave a useful measure of the relative affinity of the interaction between the peptide-containing microspheres and the VEGF.
The affinity of the VEGF-microsphere interaction was influenced by both peptide density and serum concentration. VBP and VBPWT microspheres exhibited sequestering of VEGF from solution, with equilibrium dissociation constants in the range of 1–10 µM (Fig. 1C). The equilibrium dissociation constants of the VEGF-VBP and VEGF-VBPWT interactions increased with increasing peptide concentration (Fig. 1D), suggesting decreasing VEGF-peptide affinity at increasing peptide concentration in the microspheres. Serum decreased the affinity of the VEGF-peptide interaction, as the equilibrium dissociation constants of the VBP-VEGF and VBPWT-VEGF interactions increased with increasing serum concentration and were lowest in the albumin-only solution (Fig. 2C and D). VBPWT and VBP microspheres did not exhibit significantly different equilibrium dissociation constants except at lower peptide density (0.4%) in the albumin-only solution, where the VBP-VEGF interaction exhibited a lower KD than VBPWT-VEGF (Fig. 2D).
3.3. Protease dependence of VEGF binding
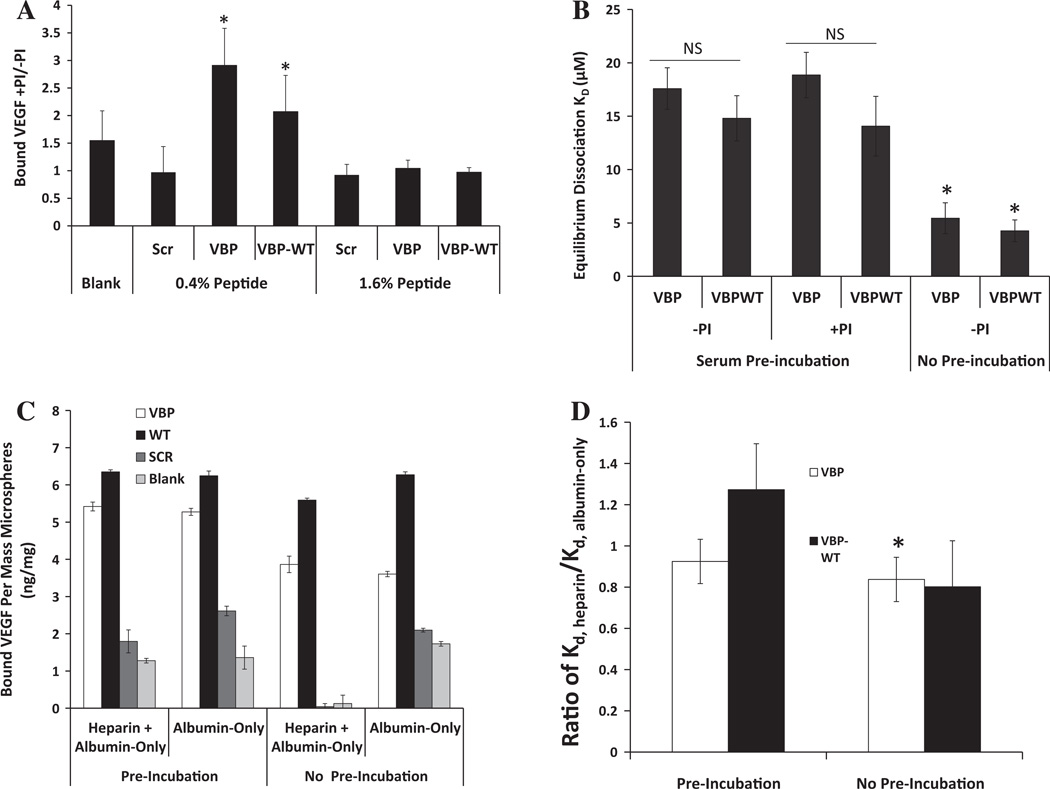
Protease activity influenced VEGF binding in serum, but only at lower peptide density. VBP and VBPWT microspheres with higher peptide density (1.6%) were incubated in 25% serum solution with a protease inhibitor cocktail and VEGF, resulting in increased VEGF sequestering to VBP and VBPWT microspheres relative to 25% serum solution without protease inhibitor cocktail (Fig. 3A). To further study the effect of serum on the VBP and VBPWT peptides, we first preincubated microspheres with 1.6% peptide density in 25% serum without supplemented VEGF, washed them thoroughly with an albumin-only solution and then measured the VEGF sequestering in the albumin-only solution. The VEGF binding affinities of VBP and VBPWT microspheres decreased after serum preincubation when compared to the no-serum preincubation (Fig. 3B). Again, the presence of protease inhibitor in this preincubation step did not influence VEGF-VBP and VEGF-VBPWT binding affinities at higher peptide density. Interestingly, these data suggest that serum decreased the affinity of VBP and VBPWT microspheres for VEGF in a protease-independent fashion at higher peptide density and in a protease-dependent manner at lower peptide density. Therefore, the serum stability of the VEGF-binding peptides is unlikely to be the sole determinant of the strong binding in serum, and other variables, such as the intrinsic peptide-VEGF affinity, may also strongly influence VEGF sequestering.
Fig. 3.
Comparison of VEGF binding with VBP and VBPWT microspheres in the presence and absence of protease inhibitor. (A) Microspheres at 1.6 and 0.4% peptide densities, as well as Blank microspheres, were incubated in 25% FBS with and without protease inhibitor cocktail (PI). Data are presented as bound VEGF (ng VEGF per mg microspheres) with protease inhibitor (+PI) divided by bound VEGF without protease inhibitor (—PI). A one-tailed Student’s t-test was performed to test if these values were significantly greater than 1, with asterisks denoting p < 0.05. (B) Comparison of effective dissociation constant for VBP and VBPWT microspheres preincubated in 25% serum with or without protease inhibitor. A two-tailed Student’s t-test was performed between VBP and VBPWT microspheres with no preincubation vs. each respective condition with serum preincubation. Asterisks denote p < 0.05. (C) Comparison between different UFH-containing solutions both in a UFH or albumin-only preincubation step or with UFH or albumin-only in the presence of VEGF. Data are presented as bound VEGF per mass of 1.6% peptide microspheres in ng mg−1 for VBP (white bars), VBPWT (black bars), Scramble (dark gray bars) or Blank (light gray bars) microspheres. No statistical significance existed between preincubation conditions, and in all cases VBP and VBPWT microspheres bound significantly more VEGF than Scramble and Blank. (D) Comparison of VEGF-binding dissociation constants of heparin-containing medium, KD,heparin, divided by the same of albumin-only medium, KD,albumin-only, for both preincubated microspheres and for no preincubation. Statistical significance is depicted as significantly less than 1 with a one-tailed Student’s t-test, with asterisks denoting p < 0.05.
3.4. Heparin modulation of specific VEGF sequestering
Soluble heparin increased the specificity of VEGF binding in the albumin-containing solution. Microspheres containing 1.6% peptide density were incubated in an albumin-only solution with or without soluble unfractionated heparin. Levels of VEGF binding to the Scramble and Blank controls were significantly lower in the presence of heparin vs. albumin-only (Fig. 3C), demonstrating that heparin reduced non-specific binding of VEGF. VBP microspheres in the presence of heparin bound VEGF with higher affinity relative to the albumin-only control (Fig. 3D). The presence of heparin in a preincubation did not significantly affect VEGF binding to VBP and VBPWT microspheres (Fig. 3C), suggesting that soluble heparin interacted directly with VEGF to increase binding affinity to VBP microspheres. Therefore, serum-borne heparin likely did not reduce binding in serum.
3.5. Microspheres influence VEGF-dependent HUVEC proliferation
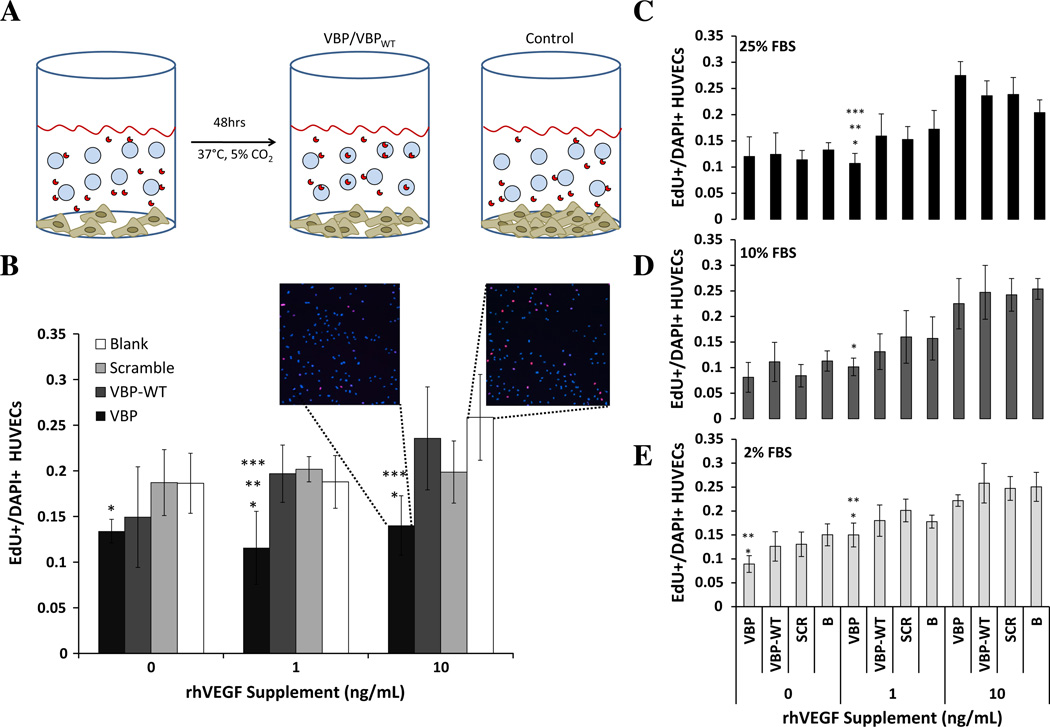
VEGF-binding microspheres reduced VEGF-dependent HUVEC proliferation. HUVECs were cultured in a low-serum medium to provide cell-cycle synchronization, after which medium containing the specified VEGF supplement and microspheres was added (Fig. 4A). VEGF sequestering to VBP microspheres significantly reduced HUVEC proliferation relative to Blank microspheres, both in the presence and in the absence of recombinant human VEGF supplements (Fig. 4B). VBP microspheres also significantly decreased the per cent of proliferating HUVECs compared to Scramble microspheres at 1 ng ml−1 VEGF supplement and compared to VBPWT microspheres at both 1 and 10 ng ml−1 VEGF supplement (Fig. 4B). Thus, at relatively low serum concentration (2% serum), VBP microspheres significantly decreased the activity of soluble VEGF in culture during the 48 h incubation, likely by sequestering soluble VEGF and decreasing the effective soluble VEGF concentration. The effects of VEGF binding microspheres on VEGF activity were influenced by the serum concentration in culture. The reduction in HUVEC proliferation was highly reproducible for VBP microspheres in 2% serum (Fig. 4E). In addition, VBP microspheres significantly reduced VEGF-dependent HUVEC proliferation at 1 ng ml−1 VEGF relative to Blank microspheres at 10% serum (Fig. 4D) and relative to both Scramble and Blank microspheres at 25% serum (Fig. 4C). Finally, VBP microspheres reduced VEGF-dependent proliferation relative to VBPWT at 25% serum (Fig. 4C), suggesting that VBP’s higher VEGF-binding affinity and serum stability enhanced VEGF sequestering when compared to the VBPWT peptide with no D-substitutions.
Fig. 4.
(A) Schematic of HUVEC proliferation assay. Briefly, HUVECs were seeded on gelatin-coated 96-well plates with 1 mg ml−1 microspheres and 0,1 or 10 ng ml−1 VEGF for 48 h. (B) HUVECs were cultured in 2% FBS in M199 medium with or without supplemented VEGF and microspheres, either Blank (white bars) or 1.6% peptide density of Scramble (light gray bars), VBPWT (dark gray bars) and VBP (black bars) microspheres. (C–E) HUVECs were cultured in 25% FBS (C, black bars), 10% FBS (D, dark gray bars) or 2% FBS (E, light gray bars). Error bars represent 1 SD from the mean. Statistical significance is reported at p < 0.05 and indicated by asterisks, denoting that the condition is statistically different from *Blank, **Scramble, and ***VBPWT in each respective VEGF concentration and serum concentration.
4. Discussion
Regulation of growth factor activity is an important function of the extracellular matrix, which is composed of numerous growth-factor-binding proteins, such as collagens [7–9], glycoproteins (e.g. fibronectin [10]) and proteoglycans (e.g. perlecan [11]). This regulation is particularly important during angiogenesis, during which growth factors stimulate endothelial cells to migrate, proliferate and eventually undergo tube formation [2,36]. Many previous approaches have used soluble molecules (e.g. peptides, proteins or small molecules) to bind and thereby inhibit growth factor activity [17,37,38]. In contrast, biomaterials with immobilized growth factor-binding molecules have largely been used for the incorporation and controlled delivery of biologically active growth factors rather than the inhibition of growth factor activity via sequestering [39–42]. Here we have demonstrated that synthetic hydrogel microspheres derivatized with VEGF-binding molecules can bind and down-regulate VEGF in a highly specific manner. We focused on the role of serum in VEGF sequestering to microspheres conjugated with different VEGF-binding peptides. The ability of these microspheres to bind VEGF and influence VEGF activity differs, and may be dependent on their VEGF binding affinity and serum stability. The ability to regulate VEGF activity specifically using binding ligands designed to mimic VEGFR2 may enable our approach to be generalized to regulation of a variety of other growth factors by mimicking other growth factor receptors.
Here we focused particularly on studying the serum-dependence of VEGF sequestering, as serum is a component of blood. Serum is also a ubiquitous component of cell culture media due to its high concentration of vital nutrients and proteins [24,25]. We showed that, in the presence of serum, microspheres containing VEGFR2-mimicking peptides [26,27] sequestered VEGF. Though serum reduced the affinity of VEGF sequestering, the influence of protease inhibition was negligible. Finally, a physiologic level of soluble heparin [43–45] increased VEGF binding affinity, consistent with previous studies demonstrating heparin-mediated VEGF binding to VEGFR2 [46–48]. Taken together, these results demonstrated the ability to specifically target VEGF even in a solution containing a diversity of proteins [24,25]. Interestingly, a previous study demonstrated that the serum stability and VEGF binding affinity of soluble VBP were higher than that of soluble VBPWT [27], and our binding results here for the same peptides tethered to PEG microspheres show more efficient VEGF sequestering to VBP microspheres when compared to VBPWT microspheres. Collectively, these observations indicate that VBP targets VEGF with high affinity both in solution and when conjugated to PEG hydrogel microspheres. Further, we can speculate that affinity ligands for other target molecules (e.g. other growth factors) may similarly benefit from strategies to increase their target binding affinity and serum stability, ultimately leading to more efficient sequestering in heterogeneous serum-containing solutions.
Other studies utilizing peptides to decrease VEGF-dependent HUVEC proliferation have shown efficacy in soluble form by either binding to and blocking VEGF [49,50] or by antagonizing VEGFR2 [51–54]. These studies demonstrate, using soluble molecules, potent inhibition of HUVEC proliferation of 30–60% [49,50,52], consistent with our observed 30–50% reduction in VEGF-dependent HUVEC proliferation in both low (Fig. 4B) and high (Fig. 4C) serum conditions. Previous studies with VEGF supplement to endothelial cells demonstrated that proliferation is maximal at 1–1.2 ng ml−1 VEGF, and that proliferation is decreased at VEGF concentrations below 1 ng ml−1 [30]. Our observed reduction in proliferation in medium supplemented with 1 ng ml−1 VEGF can be explained by considering our binding studies in 2% serum, which demonstrated 30–50% VEGF binding and a resulting soluble VEGF concentration of 0.5–0.7 ng ml−1 in culture. Such a reduction in soluble VEGF would be expected to reduce HUVEC proliferation below that observed at 1 ng ml−1 VEGF, as we observed. Finally, decreased HUVEC proliferation in low-serum conditions without VEGF supplement suggest sequestering of endogenous VEGF present either as serum-borne VEGF or cell-secreted VEGF. Capture of serum-borne VEGF has been demonstrated previously by our group using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on serum-incubated microspheres, which sequestered proteins with distinct bands between 10 and 15kDa consistent with the typical PAGE band for monomeric VEGF (Fig. 1S). In addition, results from VEGF binding in 2% serum relative to albumin demonstrated an increase in binding (Fig. 2A) and similar VEGF binding affinities of both VBP and VBPWT microspheres (Fig. 2C). Taken together, these results suggest the ability to leverage both supplemented and endogenous VEGF using a specific affinity-based approach.
5. Conclusion
In the current study, we have investigated the serum-dependence of VEGF sequestering to biomaterials containing VEGFR2-mimicking peptides. We observed high-affinity binding in the presence of serum, which significantly reduced VEGF-dependent HUVEC proliferation in culture. Consequently, this strategy of incorporating receptor-mimicking peptides into a biomaterial is effective for demonstrating specific high-affinity binding of a growth factor. Although many biomaterial formulations currently employ covalently immobilized peptides, many of the investigations have not studied the effect of complex biological environments such as serum. Previous studies investigating the serum stability of peptides in solution indicate that the half-life of many peptides is on the order of hours when in soluble form [55]. Strategies such as incorporating D-substituted amino acids and amidat-ing the carboxy-terminus, both methods employed in this study, have been shown to increase peptide stability against protease-mediated degradation [56,57]. Numerous other strategies for improving peptide stability in solution, such as amino-terminus acetylation, cyclization, glycosylation and the addition of protein motifs, have also been shown to increase peptide half-life in complex biological environments [56–58]. In addition, as a previous study has shown with the VBP and VBPWT peptides, iteratively engineering peptide derivatives has been shown to increase peptide serum stability as well as target binding affinity [27]. In future studies it may be possible to use these techniques, and combinations thereof, to further enhance peptide stability and improve growth factor sequestering in biological solutions.
Supplementary Material
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.actbio.2013.06.033.
Appendix B
Certain figures in this article, particularly Figure 4, are difficult to interpret in black and white. The full color images can be found in the on-line version, at http://dx.doi.org/10.1016/j.actbio.2013.06.033.
References
- 1.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. J Surg Res. 2009;153:347. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Annu Rev Med. 2003;54:17. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Nat Med. 2000;6:389. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. J Clin Invest. 2004;113:516. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Wound Repair Regen. 2008;16:585. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Pharm Res. 2005;22:1110. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 7.Nagai N, Kumasaka N, Kawashima T, Kaji H, Nishizawa M, Abe T. J Mater Sci. 2010;21:1891. doi: 10.1007/s10856-010-4054-0. [DOI] [PubMed] [Google Scholar]
- 8.Chan TR, Stahl PJ, Yu SM. Adv Funct Mater. 2011;21:4252. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang AY, Leong S, Liang Y-C, Huang RCC, Chen CS, Yu SM. Biomacromolecules. 2008;9:2929. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 10.Mosesson MW. J Thromb Haemost. 2005;3:1894. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee T-Y, Folkman J, Javaherian K. PLoS One. 2010;5:e9945. doi: 10.1371/journal.pone.0009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N. Mol Biol Cell. 2010;21:687. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Q, Tang H, Hu J, Hu Y, Zhou X, Tao Y, et al. Int J Nanomed. 2011;6:929. doi: 10.2147/IJN.S18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung HJ, Kim HK, Yoon JJ, Park TG. Pharm Res. 2006;23:1835. doi: 10.1007/s11095-006-9039-9. [DOI] [PubMed] [Google Scholar]
- 15.Chung Y-I, Kim S-K, Lee Y-K, Park S-J, Cho K-O, Yuk SH, et al. J Controlled Release. 2010;143:282. doi: 10.1016/j.jconrel.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Wong C, Inman E, Spaethe R, Helgerson S. Thromb Haemostasis. 2003;89:573. [PubMed] [Google Scholar]
- 17.Kim SH, Kiick KL. Peptides. 2007;28:2125. doi: 10.1016/j.peptides.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zisch AH, Lutolf MP, Hubbell JA. Cardiovasc Pathol. 2003;12:295. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 19.Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im GI, et al. Exp Mol Med. 2011;43:622. doi: 10.3858/emm.2011.43.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Biomaterials. 2010;31:4630. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Integr Biol. 2011;3:832. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toepke MW, Impellitteri NA, Lan Levengood SK, Boeldt DS, Bird IM, Murphy WL. Adv Healthcare Mater. 2012;1:457. doi: 10.1002/adhm.201200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL. Biomaterials. 2012;33:3475. doi: 10.1016/j.biomaterials.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E. Biotechnol Prog. 2006;22:1294. doi: 10.1021/bp060121o. [DOI] [PubMed] [Google Scholar]
- 25.Issaq HJ, Xiao Z, Veenstra TD. Chem Rev. 2007;107:3601. doi: 10.1021/cr068287r. [DOI] [PubMed] [Google Scholar]
- 26.Piossek C, Schneider-Mergener J, Schirner M, Vakalopoulou E, Germeroth L, Thierauch KH. J Biol Chem. 1999;274:5612. doi: 10.1074/jbc.274.9.5612. [DOI] [PubMed] [Google Scholar]
- 27.Germeroth L, Piossek C, Thierauch K-H, Schneider-Mergener J, Volkmer-Engert R, Bachmann MF, et al. Thromb Haemost. 2003;501 doi: 10.1160/TH03-02-0106. [DOI] [PubMed] [Google Scholar]
- 28.Lin C-C, Anseth KS. Adv Funct Mater. 2009;19:2325. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franssen O, Hennink WE. Int J Pharm. 1998;168:1. doi: 10.1016/s0378-5173(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, Henzel WJ. Biochem Biophys Res Commun. 1989;161:851. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 31.Howdieshell TR, Riegner C, Gupta V, Callaway D, Grembowicz K, Sathyanarayana , et al. Ann Surg. 1998;228:707. doi: 10.1097/00000658-199811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michaelis L, Menten ML. Biochem Z. 1913;49:352. [Google Scholar]
- 33.Klotz IM, Rosenberg R. Chemical thermodynamics. 5th ed. New York: John Wiley & Sons; 1994. [Google Scholar]
- 34.Cooper S. FASEB J. 2003;17:333. doi: 10.1096/fj.02-0352rev. [DOI] [PubMed] [Google Scholar]
- 35.Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Nat Cell Biol. 2001;3:950. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 36.Maharaj ASR, D’Amore PA. Microvasc Res. 2007;74:100. doi: 10.1016/j.mvr.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serratì S, Margheri F, Pucci M, Cantelmo AR, Cammarota R, Dotor J, et al. Biochem Pharmacol. 2009;77:813. doi: 10.1016/j.bcp.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharjee PS, Huq TS, Mandal TK, Graves RA, Muniruzzaman S, Clement C, et al. PLoS One. 2011;6:e15905. doi: 10.1371/journal.pone.0015905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vulic K, Shoichet MS. J Am Chem Soc. 2012;134:882. doi: 10.1021/ja210638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Furst EM, Kiick KL. J Control Release. 2006;114:130. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-elbert SE. J Biomed Mater Res, Part A. 2006;80:13. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- 42.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Biomaterials. 2006;27:5836. doi: 10.1016/j.biomaterials.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Engelberg H, Dudley A. Circulation. 1961;23:578. doi: 10.1161/01.cir.23.4.578. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann-Kiefer K, Kemming G, Chappell D, Flondor M, Kisch-Wedel H, Hauser A, et al. Eur J Med Res. 2009;14:526. doi: 10.1186/2047-783X-14-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama H, Sato K, Okudaira M, Morita C, Takahashi C, Suzuki D, et al. Kidney Int. 1999;56:650. doi: 10.1046/j.1523-1755.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- 46.Tesslers S, Rockwell P, Hicklinb D, Cohenln T, Levin B, Witte L, et al. J Biol Chem. 1994;269:12456. [PubMed] [Google Scholar]
- 47.Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. J Biol Chem. 1992;267:6093. [PubMed] [Google Scholar]
- 48.Soker S, Goldstaub D, Svahn CM, Vlodavsky I, Levi B-Z, Neufeld G. Biochem Biophys Res Commun. 1994;203:1339. doi: 10.1006/bbrc.1994.2329. [DOI] [PubMed] [Google Scholar]
- 49.Fairbrother WJ, Christinger HW, Cochran AG, Fu G, Keenan CJ, Quan C, et al. Biochemistry. 1998;37:17754. doi: 10.1021/bi981931e. [DOI] [PubMed] [Google Scholar]
- 50.Héroult M, Bernard-Pierrot I, Delbé J, Hamma-Kourbali Y, Katsoris P, Barritault D, et al. Oncogene. 2004;23:1745. doi: 10.1038/sj.onc.1206879. [DOI] [PubMed] [Google Scholar]
- 51.Papo N, Silverman AP, Lahti JL, Cochran JR. Proc Natl Acad Sci U S A. 2011;108:14067. doi: 10.1073/pnas.1016635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. J Am Chem Soc. 2008;130:5744. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 53.Hetian L, Ping A, Shumei S, Xiaoying L, Luowen H, Jian W, et al. J Biol Chem. 2002;277:43137. doi: 10.1074/jbc.M203103200. [DOI] [PubMed] [Google Scholar]
- 54.Koepsel JT, Nguyen EH, Murphy WL. Integr Biol. 2012;4:914. doi: 10.1039/c2ib20055d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powell MF, Grey H, Gaeta F, Sette A, Colon S. J Pharm Sci. 1992;81:731. doi: 10.1002/jps.2600810802. [DOI] [PubMed] [Google Scholar]
- 56.Hong SY, Oh JE, Lee K-H, Peptide M. Biochem Pharmacol. 1999;58:1775. doi: 10.1016/s0006-2952(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 57.Powell M, Stewart T, Otvos L, Urge L, Gaeta FCA, Sette A, et al. Pharm Res. 1993;10:1268. doi: 10.1023/a:1018953309913. [DOI] [PubMed] [Google Scholar]
- 58.Walker JR, Altman RK, Warren JW, Altman E. J Pept Res. 2003;62:214. doi: 10.1034/j.1399-3011.2003.00085.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.