Abstract
Background and purpose
Hydrogen sulfide (H2S) is a highly toxic gas for which no effective antidotes exist. It acts, at least in part, by binding to cytochrome c oxidase, causing cellular asphyxiation and anoxia. We investigated the effects of three different ligand forms of cobinamide, a vitamin B12 analog, to reverse sulfide (NaHS) toxicity.
Methods
New Zealand white rabbits received a continuous intravenous (IV) infusion of NaHS (3 mg/min) until expiration or a maximum 270 mg dose. Animals received six different treatments, administered at the time when they developed signs of severe toxicity: Group 1—saline (placebo group, N = 9); Group 2—IV hydroxocobalamin (N = 7); Group 3—IV aquohydroxocobinamide (N = 6); Group 4—IV sulfitocobinamide (N = 6); Group 5—intramuscular (IM) sulfitocobinamide (N = 6); and Group 6—IM dinitrocobinamide (N = 8). Blood was sampled intermittently, and systemic blood pressure and deoxygenated and oxygenated hemoglobin were measured continuously in peripheral muscle and over the brain region; the latter were measured by diffuse optical spectroscopy (DOS) and continuous wave near infrared spectroscopy (CWNIRS).
Results
Compared with the saline controls, all cobinamide derivatives significantly increased survival time and the amount of NaHS that was tolerated. Aquohydroxocobinamide was most effective (261.5 ± 2.4 mg NaHS tolerated vs. 93.8 ± 6.2 mg in controls, p < 0.0001). Dinitrocobinamide was more effective than sulfitocobinamide. Hydroxocobalamin was not significantly more effective than the saline control.
Conclusions
Cobinamide is an effective agent for inhibiting lethal sulfide exposure in this rabbit model. Further studies are needed to determine the optimal dose and form of cobinamide and route of administration.
Keywords: Heart, Lung, Muscle, CNS/Psychological
Introduction
Hydrogen sulfide (H2S) is a highly toxic, flammable, colorless gas that is produced naturally by decaying organic matter and by many industrial processes.1 H2S is the second most common cause of fatal gas inhalation in the workplace in the United States, second only to carbon monoxide.2 H2S has unique features that make poisoning toxicologically unusual and distinctive.
In most incidents, casualties occur in multiple victims, with high risk to first responders who do not recognize the need to protect themselves with a self-contained breathing apparatus.3 The exposure–response curve for lethality is extremely steep for H2S. Concentration of the gas is much more important than duration of exposure,4 and, compared with other inhaled toxic substances, H2S gives little margin of safety.3
H2S is highly reactive. Its major mechanism of toxicity is inhibition of cytochrome c (CytC) oxidase, which has generally been proposed to cause effects analogous to cyanide exposure.1 It is classified as a cellular asphyxiant, together with carbon monoxide, cyanide, and azide. It appears to act more quickly than oxygen deprivation with regard to physiologic cellular anoxia effects,3 causing impaired adenosine triphosphate production, anaerobic metabolism with lactate accumulation and metabolic acidosis.7 H2S may lead to sulfhemoglobin generation at high levels of sulfide exposure.
In acute cases of H2S toxicity, sodium nitrite has been used with limited success, and its mechanism of action is unclear. Nitrite treatment is based on the observation that sulfide resembles cyanide in that both bind reversibly to cytochromes.8 The theory is that methemoglobin (MetHb) generated by nitrite binds sulfide, as it does with cyanide, to regenerate active cytochrome oxidase.9
Pretreatment with several agents, including pyruvate, α-ketocarboxylic acids, and bicarbonate, increases survival rates in animals. Unfortunately, these pretreatments have not led to successful therapeutic post exposure interventions.10,11 Therefore effective therapies that can be administered rapidly are needed for H2S exposure, particularly for mass casualties.
Hydroxocobalamin binds cyanide and is approved for cyanide poisoning, serving as a cyanide scavenger. Hydroxocobalamin also binds sulfide, and has been shown to decrease plasma sulfide concentrations, but it has not been shown to be clinically effective for H2S poisoning.12 In addition, hydroxocobalamin has only limited solubility, so it must be administered in large volumes intravenously. For mass casualty H2S exposure scenarios, intramuscular injection of small volumes of more effective antidotes will be needed.
We are developing the cobalamin analog cobinamide as a novel therapy for mass casualty cyanide poisoning.13 Cobinamide is highly water-soluble, can be rapidly absorbed across muscle, and has a high binding affinity for cyanide. In addition to cyanide, cobinamide also has a high affinity for sulfide. Therefore, we hypothesized that cobinamide could potentially be used to treat H2S poisoning in vivo. In this study, we investigated the ability of cobinamide to reverse lethal H2S exposure in a rabbit model.
Because H2S inhibits CytC oxidase, we are able to use previously described non-invasive optical technologies—diffuse optical spectroscopy (DOS) and continuous wave near infrared spectroscopy (CWNIRS)—to rapidly and noninvasively monitor toxicity and reversal. The approach is analogous to methods we have demonstrated of monitoring cyanide poisoning and treatment.13-16 Inhibiting CytC oxidase decreases oxygen extraction from circulating blood manifested by alterations in circulating blood tissue oxyhemoglobin(OHb) and deoxyhemoglobin (RHb). concentrations.17-19 This increases circulating hemoglobin oxygenation with resultant changes in absorption spectra. As toxicity is reversed, these changes return toward baseline.
Specifically, we investigated the ability of several cobinamide derivatives given by intramuscular or intravenous (IV) injection to reverse H2S poisoning in a highly-monitored rabbit model.
Materials and methods
General preparation: Pathogen-free New Zealand White rabbits weighing 3.5–4.5 kg (Western Oregon Rabbit Supply) were used. All procedures were reviewed and approved by the University of California, Irvine, Institutional Animal Care and Use Committee. General methods and DOS monitoring have been described previously,17 and are summarized here.
Animal preparation
Animals were anesthetized with an intramuscular injection of a 2:1 ratio of ketamine HCl (100 mg/ml, Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, MI): Xylazine (20 mg/ml, Anased, Lloyd Laboratories, Shenandoah, IA) at a dose of 0.75 cc/kg, using a 23gauge 5/8-inch needle. After the intramuscular injection, a 23gauge 1-inch catheter was placed in the animals’ marginal ear vein to administer continuous IV anesthesia. The animals were intubated with a 3.0 cuffed endotracheal tube secured by a gauze tie and were mechanically ventilated (dual phase control respirator, model 32A4BEPM-5R, Harvard Apparatus, Chicago, IL, USA) at a respiratory rate of 20–22 breaths/min, a tidal volume of 60 cc, and FiO2 of 100% during the initial preparation portion of the surgery. A pulse oximeter (Biox 3700 Pulse Oximeter, Ohmeda, Boulder, CO) with a probe placed across the cheek was used to measure SpO2 and heart rate. Blunt dissection was performed to isolate the femoral artery and vein on the left thigh for blood sampling, sulfide infusion, and systemic pressure monitoring. Following placement of the femoral lines, FiO2 was reduced to 21% (room air source) for the remainder of the experiment.
Sodium Hydrosulfide poisoning
Sodium Hydrosulfide (NaHS) (Sigma #161527, NaHS xH20) was dissolved in 90 ml of 0.9% saline in to a concentration of 3 mg/ml, and was given intravenously by a syringe pump at the rate of 3 mg (1cc) NaHS per min (Kent Scientific Genie Plus syringe pump, Torrington, Ct). The solution was continuously infused until the animal expired or had received a full 270 mg dose over 90 min. On completion of the experiment, surviving animals were euthanized with an IV injection of 1.0 cc Euthasol (390 mg pentobarbital sodium, 50 mg phenytoin sodium (Virbac AH, Inc, Fort Worth, Texas) administered through the marginal ear vein.
Data collection
Blood was collected at baseline, at 5 min after the start of the sulfide infusion, at the time of antidote injection, and at 2.5, 5, 7.5, 10, 15, 30, 45, 60, and 90 min after injecting the antidote, with each measurement set requiring ~30 sec. During this time, DOS and CWNIR measurements were taken continuously.
Study and control groups
The NaHS dose was calculated to achieve 100% fatality within ~30 min of exposure, with infusion performed intravenously through a femoral line. A total of 42 animals were studied: 9 animals received an IM injection of saline, and 33 animals received an IV or IM injection of hydroxocobalamin acetate, aquohydroxocobinamide, sulfitocobinamide, or dinitrocobinamide. Injections were performed when CWNIRS monitoring demonstrated development of a terminal phase (defined below as plateau of phase ii oxyhemoglobin levels). The dose and number of animals are shown in Table 1.
Table 1.
Experiment summary.
| Group # | Treatment Group | N | mg of NaHS (mean)± SEM | range (mg) | p vs. control | p vs. IV cobalamin |
|---|---|---|---|---|---|---|
| 1 | Control (NaHS alone) | 9 | 93.8 ± 6.2 | 67.5 – 123 | – | 0.1 = NS |
| 2 | IV Hydroxocobalamin | 7 | 121.7 ± 13.9 | 54 – 153 | 0.1 = NS | – |
| 3 | IV Aquohydroxocobinamide | 6 | 261.5 ± 2.4 | 252 – 270 | < 0.0001 | < 0.0001 |
| 4 | IV Sulfi tocobinamide | 6 | 170 ± 8.8 | 138 – 195 | < 0.0001 | < 0.02 |
| 5 | IM Sulfi tocobinamide | 6 | 133 ± 4.4 | 114 – 144 | < 0.0003 | 0.48 = NS |
| 6 | IM Dinitrocobinamide | 8 | 165.4 ± 19.4 | 87 – 267 | < 0.01 | 0.09 |
Non-invasive measurements using diffuse optical spectroscopy
DOS measurements were obtained through a fiberoptic probe with a light diode emitter and detector at a fixed distance (10 mm) from the source fiber, which was placed on the shaved surface of the right inner thigh of the animal. The broadband DOS system we constructed17 combines multi-frequency domain photon migration with time-independent near infrared spectroscopy to accurately measure bulk tissue absorption and scattering spectra. It employs six laser diodes at discrete wavelengths (661, 681, 783, 805, 823, and 850 nm), and a fiber-coupled avalanche photo diode (APD) detector (Hamamatsu high-speed APD module C5658, Bridgewater, NJ, USA) for the frequency domain measurements. The APD detects the intensity-modulated diffuse reflectance signal at modulation frequencies between 50 and 550 MHz after propagation through the tissue. Absorption and reduced scattering coefficients are measured directly at each of the six laser diode wavelengths using frequencydependent phase and amplitude data. Reduced scattering coefficients are calculated as a function of wavelength throughout the NIR region by fitting a power-law to six reduced scattering coefficients. Steady-state acquisition is accomplished using a broadband reflectance measurement from 650 to 1000 nm that follows frequency domain measurements using a tungsten-halogen light source (Ocean Optics HL-2000, Dunedin, Fl, USA) and a spectrometer (BWTEK BTC611E, Newark, DE, USA). Intensity of the steady-state reflectance measurements are calibrated to the frequency domain values of absorption and scattering to establish the absolute reflectance intensity18,20,21 Tissue concentrations of OHb and RHb are calculated by a linear least squares fit of the wavelength-dependent extinction coefficient spectra of each chromophore. We used OHb and RHb absorption spectra reported by Zijlstra et al.22 for data fitting and analysis.
Antidote injection
The effects of continuous NaHS infusion on hemoglobin oxygenation are tri-phasic with time (Fig. 1A). Initially, the tissue OHb concentration decreases, as animals become hypotensive due to H2S exposure (phase i). Reflex tachycardia with increased perfusion, together with inhibition of CytC oxidase and inability of tissues to extract oxygen from the circulating blood increases tissue OHb concentrations (phase ii). As sulfide poisoning progresses, the animals develop terminal phase cardiovascular collapse with a precipitous drop in OHb and rise in RHb concentrations (phase iii) as the animals expire. Opposite effects are seen in RHb. If an effective antidote is not administered when the tissue OHb curve begins to decrease in phase ii, animals rapidly expire. Thus, we used this as the point of antidote injection.
Fig. 1.
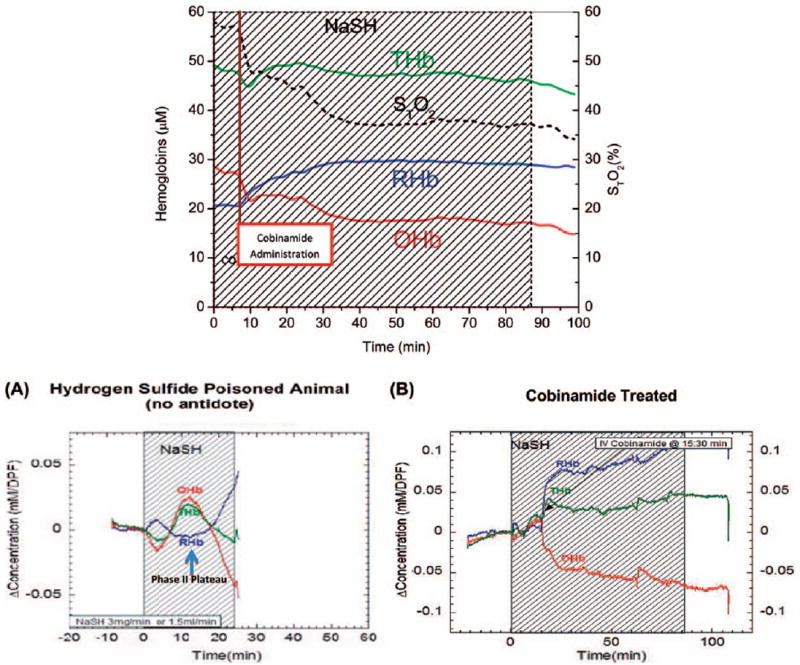
Effects of Cobinamide on Tissue Oxy-, Deoxy-, and Total Hemoglobin Concentrations in H2S -poisoned Rabbits. DOS-Muscle Tissue oxyhemoglobin (OHb, red lines), deoxyhemoglobin (RHb, blue lines), total hemoglobin (THb, green lines) and tissue oxygen saturation (STO2) were measured by Diffuse Optical Spectroscopy (DOS). (A) Continuous wave near infrared spectroscopy (CWNIRS)-Brain The effects of lethal dose H2S (NaHS). As H2S poisoning progresses, the animals develop terminal phase cardiovascular collapse with precipitous drop in OHb and rise in RHb as untreated animals expire. (B) CWNIRS-Brain The effects of lethal dose H2S infusion are blocked by cobinamide. Cobinamide-treated animals tolerate almost three times the lethal control dose of H2S infusion.
Continuous wave near infrared spectroscopy
CWNIRS over the forearm muscle was used to assess the effects of sulfide on tissue OHb and RHb concentrations. CWNIRS provides rapid, real time measures of tissue OHb and RHb concentration changes and penetrates more deeply into tissues than DOS;23 it can, therefore, be used to assess regions such as the CNS and can collect data more frequently which is important in the study of pharmacokinetics. CWNIRS, however, does not account for scattering effects, and provides only relative information on changes in the concentrations of molecular species (as opposed to absolute concentrations obtained by DOS).
The CWNIRS system consists of a light source (HL 2000, Ocean Optics, FL), a CCD spectrometer (USB4000, Ocean Optics, FL), and customized optical fiber guides (24). Continuous wave near infrared light was delivered to the rabbit forearm using a fiber optic probe (9 mm source-detector separation), and transmitted light intensities at five wavelengths (732, 758, 805, 840, 880 nm) were measured using the CCD spectrometer every second. We quantified changes in OHb and RHb concentrations throughout the experiment using a modified Beer–Lamberts’ law and those changes are displayed in real time using Labview software (version 7.1, National Instrument, TX, USA).24
Blood pressure was recorded at the same time points and every minute for the first 10 min after the antidote injection
Histology: At death or sacrifice, the lungs were removed en bloc and inflated with formalin (20 cm pressure) for histologic preparation in representative animals from the groups. The lung sections were prepared at 0.2 to– 0.4 cm thickness and embedded in paraffin, sectioned, and slides were stained with hematoxylin–eosin and studied by light microscopy. A pathologist blinded to experimental data reviewed coded histopathology specimens.
Study design
Six groups of animals were studied as follows. In all groups, NaHS infusion (3 mg/min) was given until the animal expired; antidotes were administered when the plateau of phase ii (see Fig. 1A) poisoning developed.
Group 1 (N = 9): 1 ml saline by IM injection (control).
Group 2 (N = 7): 1 ml 100 mM hydroxocobalamin by IV injection.
Group 3 (N = 6): 1 ml 100 mM aquohydroxocobinamide by IV injection.
Group 4 (N = 6): 1 ml 100 mM sulfitocobinamide by IV injection.
Group 5 (N = 6): 1 ml 100 mM sulfitocobinamide by IM injection.
Group 6 (N = 8): 1 ml 100 mM dinitrocobinamide by IV injection.
Statistical methods
Baseline statistics across groups were compared using analysis of variance (ANOVA). Specific two-group comparison testing was accomplished using two-sample t-test. Response to treatment (maximal tolerated dose) across the groups was compared using ANOVA. A two-tailed p value < 0.05 was considered significant. All data were analyzed using a standard statistical package (Systat-12, Systat Software, Inc., Chicago, IL).
Results
IV NaHS rapidly caused pronounced physiological effects: animals showed an initial stabilization of total tissue perfused hemoglobin, but this rapidly decreased after approximately 15 min of NaHS administration (3.0 mg/min) (Fig. 1A). Tissue OHb concentrations decreased rapidly over time and the concentrations of RHb rise, consistent with severe tissue hypoxia. Mean blood pressure decreased ~30% from baseline during the first 15 min of NaHS infusion.
In animals treated with any one of the three different cobinamide derivatives (Fig. 1B), optically measured levels of both OHb and RHb stabilized as NaHS was continuously administered. The concentration of total tissue hemoglobin was higher than at the beginning of sulfide administration (cobinamide sulfide that is formed after cobinamide administration has some optical interference affecting the readings of OHb and RHb, so these comparisons must be interpreted with caution).
Six groups of rabbits were studied: nine animals in the control group, eight animals in the IM dinitrocobinamide group because this is the leading derivative for IM injection, and six animals in each of the other four treatment groups. Animals were injected with saline or antidote when they showed physiologic optical evidence of decompensation of OHb and RHb using continuous optical spectroscopic monitoring (plateau of phase ii), which presages irreversible toxicity. The survival times and corresponding total NaHS dose received prior to death or termination of the experiment at 90 min determine the effective dose relative to controls for each of the antidotes. The time from initiation of NaHS infusion to the injection of antidote (time to reach phase ii) was similar in all groups (a mean of 13.1 ± 0.4 min after the start of NaHS, range 10.6–14.9 min, p > 0.2 for all groups).
Results are summarized in Table 1 and Fig. 2. Control animals had the shortest survival time and tolerated the lowest amount of NaHS, with a mean of 93.8 ± 6.2 mg SEM and range of 67.5–123 mg of NaHS before death.
Fig. 2.
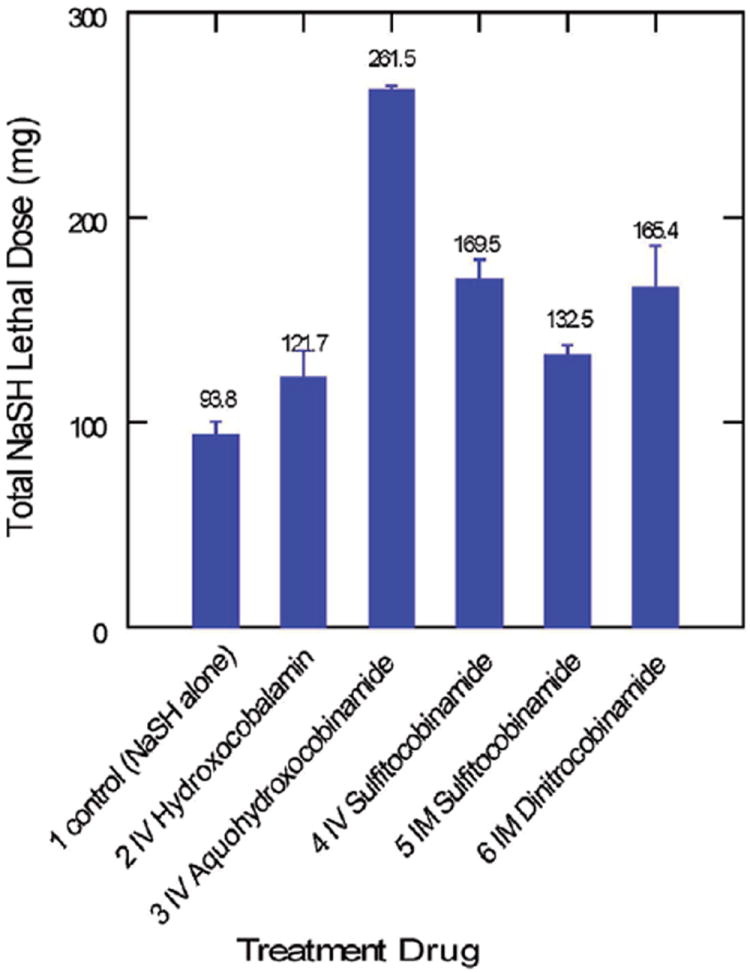
Total tolerated NaHS dose with antidote administration. Animals in the control group received no antidote.
In the animals that received IV hydroxocobalamin, the mean lethal dose of 121.7 ± 13.9 mg SEM of NaHS (range 54–153 mg) was not statistically significantly different from controls (p = 0.1). Hydroxocobalamin cannot be administered intramuscularly due to low solubility.
Group 3 animals received aquohydroxocobinamide antidote intravenously, because this form of cobinamide is poorly transported across muscle and cannot be administered intramuscularly. The water bound to the cobinamide can easily be displaced by sulfide, and thus this cobinamide form would be predicted to be the most effective in reversing sulfide/NaHS toxicity. These six animals tolerated a mean of 261.5 ± 2.4 mg SEM of NaHS, with 5/6 of the animals surviving the full 90 min until they were euthanized (p < 0.001 compared with controls).
Two ligand bound forms of cobinamide were studied; sulfitocobinamide (a sulfite group bound to the cobalt atom) and dinitrocobinamide (two nitrite groups bound to the cobalt atom). The ligand(s) facilitate absorption after intramuscular injection. The sulfite ligand has a higher affinity for the cobalt atom than the nitrite groups (Ka ≈ 1 × 1011 versus ≈ 1 × 107, respectively).
Animals treated with IV sulfitocobinamide died at a mean dose of 170 ± 8.8 mg SEM of NaHS (range 138–195 mg) (p < 0.001 compared with controls), while animals treated with intramuscular sulfitocobinamide died at a mean dose of 133 ± 4.4 mg SEM NaHS (P < 001 compared with controls). While both delivery modes were effective against NaHS, IV administration resulted in a statistically significantly higher NaHS lethal dose compared with IM administration (p < 0.01).
Animals treated with dinitrocobinamide administered intramuscularly tolerated NaHS doses equivalent to IV sulfitocobinamide, dying at a mean dose of 165.4 ± 19 mg NaHS (p < 0.01 compared with controls) (Nitrite treatment alone showed no significant increase in survival in this model, surviving a mean of 112 mg of NaHS in three animals tested).
Survival curves (Fig. 3) show the increased survival of animals receiving antidote compared with the survival of controls. IV aquohydroxocobinamide and IM dinitrocobinamide resulted in the longest survival. It is important to emphasize that the NaHS infusion continued throughout the study, so animal groups with the highest survival curves received the highest dose of NaHS.
Fig. 3.

Survival curve for animal groups receiving continuous infusion of NaHS at 3 mg/min until expiration.
Blood Pressure
Mean systolic blood pressure decreased with NaHS infusion to <70% of baseline in all groups, from 72 ± 1.8 mmHg at baseline to 46 ± 1.8 mmHg immediately prior to antidote injection (p < 0.0001). The treatment groups had higher blood pressure after the injection of antidotes, with blood pressure rising to 52 ± 3.0 mmHg compared with the control group, in which blood pressure decreased to 41 ± 2.6 mmHg (p < 0.01).
The initial rise in blood pressure was then followed by a decline in blood pressure in the treatment groups as the NaHS infusion continued. In the groups with most effective treatment, the slope of the blood pressure decline stabilized. Notably, the early blood-pressure rise did not occur with dinitrocobinamide, despite its effectiveness in increasing survival time and total NaHS dose. Blood pressure in the dinitrocobinamide-treated animals was 47 ± 2.4 mmHg just prior to antidote injection and fell to 41 ± 2.4 at 5 min post treatment. This may be because of some nitric oxide generation from the nitrite.
Pulmonary edema development
In some animals, clinical evidence of pulmonary edema developed during NaHS infusion, manifested by audible rales and frothy pulmonary secretions. On post-mortem examination, these animals showed varying degrees of pulmonary edema (Fig. 4), with evidence of airway epithelium detachment, edema under the epithelium, and some regions of alveolar edema.
Fig. 4.
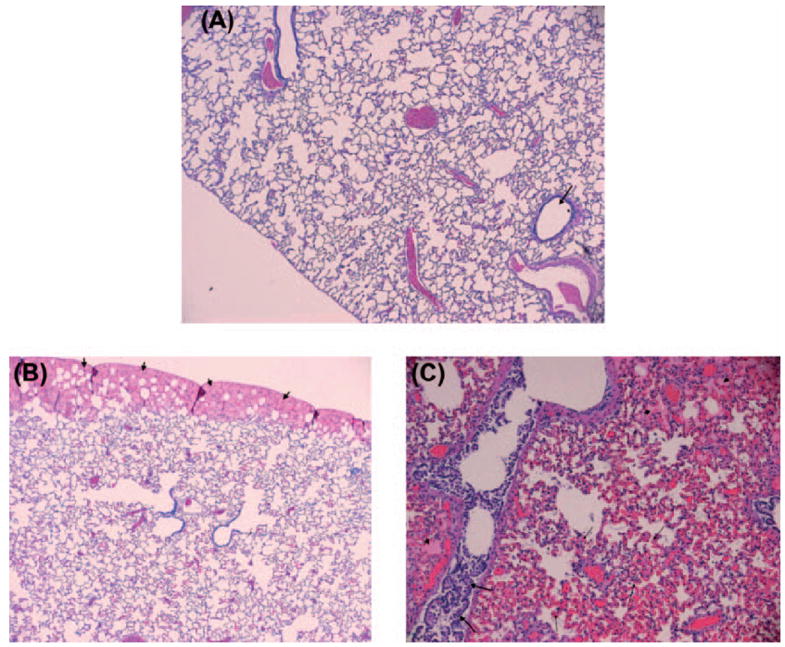
(A) Normal lung with arrow pointing to a bronchiole. The lining epithelium of the bronchiole is intact. There is no evidence of congestion or edema (X4). (B and C) NaHS-treated lungs show varying degrees of edema, congestion, and damage to bronchiolar epithelium. Arrows in image B shows subpleural edema. The airway epithelium is intact. Image C shows a NaHS-treated animal with marked congestion (thin arrows) and edema (small arrows). The bronchiolar epithelium is injured with separation from the basement membrane and curling of the epithelium in the lumen of the airway (X4 and X10).
Discussion
This work was done in response to the unmet need for an effective H2S antidote, particularly a treatment that could be applied in mass exposure scenarios. In this intensively monitored rabbit model, we measured multiple real-time parameters of the effects of H2S exposure and rate and extent of treatment response. The concurrent measurement of systemic physiology and optical measures of tissue OHb and RHb concentrations, in addition to survival information, provide a comprehensive picture of the metabolic events and responses.
Our prior research with cobinamide for treating cyanide poisoning,16,25,26 and in-vitro and murine studies (manuscript in preparation) provide evidence that cobinamide should be effective in reversing H2S toxicity and served as the basis for the hypothesis of the current study.
We found that continuous infusion of NaHS results in fatal H2S poisoning within a relatively narrow dose range. DOS and CWNIRS monitoring demonstrated marked changes in the OHb and RHb concentrations during induction of H2S poisoning. The initial response to exposure revealed a decrease in tissue and brain region hemoglobin oxygen saturation associated with decreased blood pressure. Reflex tachycardia and progressive inability of tissues to extract oxygen led into a second phase of increased OHb and decreased RHb concentrations. This was followed by a third, terminal phase of cardiovascular collapse and death. These changes were blocked by administration of cobinamide. Interestingly, the optical and some of the physiologic changes were not fully reversed back to baseline, though this is in the face of ongoing administration of H2S. Furthermore, we observed a component of optical interference of the DOS and CWNIRS readings from cobinamide sulfide which is formed after administering cobinamide, and that to some degree affects the DOS OHb and RHb measurements. However, the demonstration of treatment effectiveness was further confirmed by concurrent hemodynamic and survival improvements. Cobinamide increased the tolerated lethal NaHS exposure by more than two-fold.
We investigated different ligand forms of cobinamide and different modes of administration. In Group 3 animals, aquohydroxocobinamide was administered intravenously. In this form, a water and a hydroxyl group are coordinated to the cobalt atom, and these can be readily replaced by sulfide (Fig. 1B). However, this form of cobinamide is not well absorbed across muscle, limiting its potential utility for mass casualty settings. Binding of ligands to the cobalt site prior to administration facilitates transfer across muscle, and possibly other membranes. These improved properties appear proportional to the binding affinity of the ligand to the cobalt site. However, they may result in competition of the active site for sulfide binding, and may reduce effectiveness as an antidote agent. We found that IV sulfitocobinamide was reasonably effective in NaHS treatment, though less so than IV aquohydroxocobinamide. Even though IM sulfitocobinamide is absorbed relatively quickly, it does not achieve the plasma concentrations obtained after IV injection, and thus, one would not expect intramuscular administration to be as effective as IV injection, which was the case.
Nitrite binds less tightly to the cobalt than sulfite, and although absorption after intramuscular injection may not be as efficient as with the sulfite ligand, displacement of the nitrite by sulfide would be easier. We found that IM dinitrocobinamide was more effective than sulfitocobinamide, suggesting dinitrocobinamide is a reasonable candidate agent for further study. We are testing other ligands to further optimize overall effectiveness.
Blood pressure decreased during NaHS infusion, likely due to vasodilation induced by H2S.27 A secondary reflex tachycardia occurred, but could not compensate for the vasodilation. Administration of cobinamide caused a very rapid increase in blood pressure (see Fig. 5). However, as the H2S infusion continued, the blood pressure decreases again, and, in the animals receiving the most effective antidotes (IV aquohydroxocobinamide and IV sulfitocobinamide), this second progressive decrease in blood pressure leveled off as animals survive to the end of NaHS infusion. In the animals that received dinitrocobinamide, no initial rise in blood pressure occurred following intramuscular injection, despite improved survival. Dinitrocobinamide releases a nitrite molecule for each sulfide molecule bound; the nitrite could be reduced to nitric oxide, which is a potent vasodilator and would counteract any increase in blood pressure from reversal of H2S poisoning.
Fig. 5.

Blood pressure changes with NaHS infusion and antidote treatment.
In some of the animals that died from NaHS, pulmonary edema developed clinically and was observed histologically. While pulmonary edema has been well described during H2S inhalation, IV NaHS has not previously been described as causing pulmonary edema to our knowledge. The mechanisms for the pulmonary edema in this IV NaHS infusion model are uncertain. It is possible that fluid overload may have contributed. However, a direct pulmonary or cardiovascular toxic effect of H2S may have occurred.
There are a number of limitations to the study. First, NaHS was given by IV infusion; whether inhalation of H2S gas would have led to similar results is not known. Second, NaHS was given at a single rate and a single antidote dose was used. Third, this was a short-term survival study, and potential long-term effects were not investigated.
Despite these limitations, this study demonstrates that cobinamide shows considerable promise as an antidote for H2S poisoning. Cobinamide has the potential to be administered intravenously or intramuscularly depending on the clinical scenario. Cobinamide has a relatively long half-life of 32.3 min using a one-phase decay model,28 suggesting it could potentially be used for prophylaxis in high-risk scenarios such as first responders, and for stabilization until victims can be removed from exposure sites.
Acknowledgments
Supported by Funding Sources: CounterACT NIH # 1U54 NS079201, CounterACT R21 NS72105, NIH U01-NS058030, LAMMP, LAMMP #445474-30136, AMRMC W81XWH-12-2-0098.
Abbreviations
- DOS
diffuse optical spectroscopy
- FD
frequency-domain
- SS
Steady-state
- H2S
hydrogen sulfide
- NaHS
sodium Hydrosulfide
- CWNIRS
continuous wave near infrared spectroscopy
- MetHb
methemoglobinemia
- Ohb
oxyhemoglobin
- RHb
deoxyhemoglobin
- CNS
central nervous system
- CytC
cytochrome c
- IM
intramuscular
- IV
intravenous
Footnotes
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Munday S. Hydrogen sulfide. In: Olsan KR, editor. Poisoning and Drug Overdose. 6. New York: McGraw-Hill; 2012. [Google Scholar]
- 2.Center PbtWaHSBatLAJRI. New York: 2008. [2013 9/22/13]. Hydrogen sulfide: a potential first responder hazard. updated 9/26/2008; Available from: http://www.broomefire.com/news/otherNews/2008/081005a.pdf. [Google Scholar]
- 3.Guidotti TL. Hydrogen sulfide: advances in understanding human toxicity. Int J Toxicol. 2010;29:569–581. doi: 10.1177/1091581810384882. [DOI] [PubMed] [Google Scholar]
- 4.Prior MG, Sharma AK, Yong S, Lopez A. Concentration-time interactions in hydrogen sulphide toxicity in rats. Can J Vet Res. 1988;52:375–379. [PMC free article] [PubMed] [Google Scholar]
- 5.Roth SH, Skrajny B, Bennington R, Brookes J. Neurotoxicity of hydrogen sulfide may result from inhibition of respiratory enzymes. Proc West Pharmacol Soc. 1997;40:41–43. [PubMed] [Google Scholar]
- 6.Guidotti T. Occupational exposure to hydrogen sulfide in the sour gas industry: some unresolved issues. Int Arch Occup Environ Health. 1994;66:153–160. doi: 10.1007/BF00380773. [DOI] [PubMed] [Google Scholar]
- 7.Gresham C, LoVecchio F. Industrial toxins. In: Tintinalli J, Stapczynski J, Cline D, Ma O, Cydulka R, Meckler G, editors. Tintinalli ’ s Emergency Medicine: A Comprehensive Study Guide. 7. New York: McGraw-Hill; 2011. [Google Scholar]
- 8.Smith L, Kruszyna H, Smith RP. The effect of methemoglobin on the inhibition of cytochrome c oxidase by cyanide, sulfide or azide. Biochem Pharmacol. 1977;26:2247–2250. doi: 10.1016/0006-2952(77)90287-8. [DOI] [PubMed] [Google Scholar]
- 9.Beck JF, Bradbury CM, Connors AJ, Donini JC. Nitrite as antidote for acute hydrogen sulfide intoxication? Am Ind Hyg Assoc J. 1981;42:805–809. doi: 10.1080/15298668191420738. [DOI] [PubMed] [Google Scholar]
- 10.Dulaney M, Jr, Hume AS. Pyruvic acid protects against the lethality of sulfide. Res Commun Chem Pathol Pharmacol. 1988;59:133–136. [PubMed] [Google Scholar]
- 11.Almeida AF, Guidotti TL. Differential sensitivity of lung and brain to sulfide exposure: a peripheral mechanism for apnea. Toxicol Sci. 1999;50:287–293. doi: 10.1093/toxsci/50.2.287. [DOI] [PubMed] [Google Scholar]
- 12.Fujita Y, Fujino Y, Onodera M, Kikuchi S, Kikkawa T, Inoue Y, et al. A fatal case of acute hydrogen sulfide poisoning caused by hydrogen sulfide: hydroxocobalamin therapy for acute hydrogen sulfide poisoning. J Anal Toxicol. 2011;35:119–123. doi: 10.1093/anatox/35.2.119. [DOI] [PubMed] [Google Scholar]
- 13.Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, et al. Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med. 2010;55:352–363. doi: 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JG, Lee J, Mahon SB, Mukai D, Patterson SE, Boss GR, et al. Noninvasive monitoring of treatment response in a rabbit cyanide toxicity model reveals differences in brain and muscle metabolism. J Biomed Opt. 2012;17:105005. doi: 10.1117/1.JBO.17.10.105005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Keuter KA, Kim J, Tran A, Uppal A, Mukai D, et al. Noninvasive in vivo monitoring of cyanide toxicity and treatment using diffuse optical spectroscopy in a rabbit model. Mil Med. 2009;174:615–621. doi: 10.7205/milmed-d-02-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner M, Mahon SB, Lee J, Kim J, Mukai D, Goodman S, et al. Comparison of cobinamide to hydroxocobalamin in reversing cyanide physiologic effects in rabbits using diffuse optical spectroscopy monitoring. J Biomed Opt. 2010;15:017001. doi: 10.1117/1.3290816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Armstrong J, Kreuter K, Tromberg BJ, Brenner M. Noninvasive in vivo diffuse optical spectroscopy monitoring of cyanide poisoning in a rabbit model. Physiol Meas. 2007;28:1057–1066. doi: 10.1088/0967-3334/28/9/007. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Cerussi AE, Saltzman D, Waddington T, Tromberg BJ, Brenner M. Hemoglobin measurement patterns during noninvasive diffuse optical spectroscopy monitoring of hypovolemic shock and fluid replacement. J Biomed Opt. 2007;12:024001. doi: 10.1117/1.2715189. [DOI] [PubMed] [Google Scholar]
- 19.Merritt S, Gulsen G, Chiou G, Chu Y, Deng C, Cerussi AE, et al. Comparison of water and lipid content measurements using diffuse optical spectroscopy and MRI in emulsion phantoms. Technol Cancer Res Treat. 2003;2:563–569. doi: 10.1177/153303460300200608. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Mukai D, Kreuter K, Mahon S, Tromberg B, Brenner M. Potential interference by hydroxocobalamin on cooximetry hemoglobin measurements during cyanide and smoke inhalation treatments. Ann Emerg Med. 2007;49:802–805. doi: 10.1016/j.annemergmed.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Pan T, Rasmussen JC, Lee JH, Sevick-Muraca EM. Monte Carlo simulation of time-dependent, transport-limited fluorescent boundary measurements in frequency domain. Med Phys. 2007;34:1298–1311. doi: 10.1118/1.2710549. [DOI] [PubMed] [Google Scholar]
- 22.Zijlstra WG, Buursma A, Assendelft OW. Visible and Near-infrared Absorption Spectra of Human and Animal Hemoglobin. AH Zeist, Netherlands: VSP BV; 2000. [Google Scholar]
- 23.Kim JG, Liu H. Investigation of biphasic tumor oxygen dynamics induced by hyperoxic gas intervention: the dynamic phantom approach. Appl Opt. 2008;47:242–252. doi: 10.1364/ao.47.000242. [DOI] [PubMed] [Google Scholar]
- 24.Kim JG, Lee J, Roe J, Tromberg BJ, Brenner M, Walters TJ. Hemodynamic changes in rat leg muscles during tourniquet-induced ischemia-reperfusion injury observed by near-infrared spectroscopy. Physiol Meas. 2009;30:529–540. doi: 10.1088/0967-3334/30/7/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, et al. Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med(Maywood) 2006;231:641–649. doi: 10.1177/153537020623100519. [DOI] [PubMed] [Google Scholar]
- 26.Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, et al. Intramuscular Cobinamide Sulfite in a Rabbit Model of Sublethal Cyanide Toxicity. Ann Emerg Med. 2010 doi: 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 28.Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, Bigby TD. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol (Phila) 2010;48:709–717. doi: 10.3109/15563650.2010.505197. [DOI] [PMC free article] [PubMed] [Google Scholar]


