Abstract
Campylobacter and antimicrobial-resistant Campylobacter are frequently isolated from broiler chickens worldwide. In Canada, campylobacteriosis is the third leading cause of enteric disease and the regional emergence of ciprofloxacin-resistant Campylobacter in broiler chickens has raised a public health concern. This study aimed to identify, critically appraise, and synthesize literature on sources of Campylobacter in broilers at the farm level using systematic review methodology. Literature searches were conducted in January 2012 and included electronic searches in four bibliographic databases. Relevant studies in French or English (n = 95) conducted worldwide in any year and all study designs were included. Risk of Bias and GRADE criteria endorsed by the Cochrane collaboration was used to assess the internal validity of the study and overall confidence in the meta-analysis. The categories for on-farm sources were: broiler breeders/vertical transfer (number of studies = 32), animals (n = 57), humans (n = 26), environment (n = 54), and water (n = 63). Only three studies examined the antimicrobial resistance profiles of Campylobacter from these on-farm sources. Subgroups of data by source and outcome were analyzed using random effect meta-analysis. The highest risk for contaminating a new flock appears to be a contaminated barn environment due to insufficient cleaning and disinfection, insufficient downtime, and the presence of an adjacent broiler flock. Effective biosecurity enhancements from physical barriers to restricting human movement on the farm are recommended for consideration to enhance local on-farm food safety programs. Improved sampling procedures and standardized laboratory testing are needed for comparability across studies. Knowledge gaps that should be addressed include farm-level drug use and antimicrobial resistance information, further evaluation of the potential for vertical transfer, and improved genotyping methods to strengthen our understanding of Campylobacter epidemiology in broilers at the farm-level. This systematic review emphasizes the importance of improved industry-level and on-farm risk management strategies to reduce pre-harvest Campylobacter in broilers.
Introduction
Molecular epidemiologic studies have identified poultry as the most important source of human campylobacteriosis in industrialized countries [1]–[3]. However, Campylobacter in broilers has received little attention in comparison to other microbial hazards, e.g. Salmonella that have more severe sequelae in humans. In Canada, the current estimated annual incidence of campylobacteriosis is 447.23 cases per 100,000 in the population, making campylobacteriosis the third leading cause of enteric diseases [4]. Frequent isolation of Campylobacter by surveillance programs and primary research surveys in Canada and around the world have highlighted chicken as an important source of Campylobacter [5]–[9]. More recently Canadian antimicrobial resistance surveillance has detected regional increases in fluoroquinolone-resistant Campylobacter in chicken, which is a public health concern as fluoroquinolones are a class of antimicrobials considered “critically-important” to human medicine [10].
Campylobacter risk management in poultry starts at the farm-level to reduce downstream dissemination [11]. Many risk factors for broiler colonisation with Campylobacter and the emergence of antimicrobial resistant Campylobacter have been identified in the literature: environment, livestock, pests, wildlife, equipment and farm workers, catching crews, antimicrobial use (AMU) and to a lesser extent vertical/pseudovertical transfer [12]–[15]. There has been an increasing amount of research done on Campylobacter in broilers and much advancement in the microbiological and molecular methods for identification and quantification of Campylobacter in a variety of samples. Previously conducted reviews on Campylobacter sources for poultry identify and prioritize control options, inform Campylobacter performance objectives and summarize evidence for vertical and horizontal transfer of Campylobacter in the poultry industry [5], [12], [16]–[18]. Studies investigating on-farm sources and risk factors in Canadian broiler flocks are limited, thus this systematic review (SR) examines the global evidence for sources and risk factors of Campylobacter and antimicrobial resistant-Campylobacter in broilers at the farm-level (excluding processing) to determine prevalence estimates and characterise epidemiological linkages described in available primary research.
Systematic review (SR) is a transparent and replicable methodology to identify, critically appraise and synthesize the literature on a clearly formulated question [19], [20]. Meta-analysis (MA) is a statistical method to combine results from similar studies identified in a SR, that measure the same outcome, into an overall average estimate of effect [20]. This SR tabulates the global evidence and presents meta-analytic and molecular summaries on sources of Campylobacter for broilers, which has not been done to our knowledge. In addition, the SR updates 7 years of research from a previous SR [12] and identifies agreement or changes in our research knowledge since other reviews and predictive models have been conducted [13], [16], . Results from this SR will be used to identify potential sources and control points/measures that may be relevant to the Canadian broiler industry to reduce Campylobacter and antimicrobial resistant Campylobacter on-farm. Knowledge gaps and future research priorities to address knowledge gaps for Campylobacter in broilers are also highlighted.
The objectives of this present SR-MA are: 1) to identify and characterise the global evidence for sources of Campylobacter and/or resistant Campylobacter in broilers at the farm-level (excluding processing), 2) to estimate the average prevalence and agreement across epidemiological studies for various sources and, 3) assesses laboratory methods used to isolate and characterise Campylobacter in primary research. For the purpose of this SR, a “source” pertains to any potential exposure route (e.g. environment, animals, water, vertical transfer) for Campylobacter infection in broilers identified by microbial detection or as a risk factor.
Methods
Team, question, protocol and definitions
The systematic review research team included expertise in the following area: microbiology/molecular microbiology, poultry production and processing, food safety, epidemiology, bibliographic, and synthesis research (e.g. systematic review and meta-analysis). The team defined the broad research question to include all French and English primary research investigating any source of on-farm Campylobacter spp. for broiler chickens during grow out. General categories included sources related to vertical transmission, domestic and wild animals, humans, water, environment and farm equipment. We were interested in capturing any potential source of Campylobacter infection to broilers identified by microbial detection and/or as a risk factor; all study designs and all types of broiler production (e.g. conventional, organic, and free-range) were included. A priori developed and pre-tested SR protocol included the study question, sub-questions, definitions, procedure for literature search, study inclusion/exclusion criteria and checklists for conducting relevance screening, basic characterization, methodological assessment and data extraction on relevant primary research, following the general principles of SR methodology [19].
Search strategy
A broad list of search terms was developed by the research team in order to retrieve all citations pertaining to sources of Campylobacter in broiler chickens. The searches were conducted by combining the following terms: Campylobacter (n = 1 term) AND broilers (n = 5) AND on-farm sources, e.g., animals, environment (n = 63). At this stage, no restrictions or filters were imposed in terms of study design, publication language, origin of study, and publication date. The search was conducted in four electronic bibliographic databases (PubMED, Scopus, Current Contents, Food Safety and Technology Abstracts) to increase the probability of identifying all potentially relevant publications. The literature search was performed in January 2012. Citations retrieved from all databases were imported into a reference management software (“RefWorks-COS”, ProQuest LLC, Ann Arbor, MI) and de-duplicated. Search verification included hand-searching of reference lists of review articles [12], [16], [22] and 3 recent relevant primary research articles [23]–[25].
Abstract and article-level relevance screening and study characterization
Through initial abstract-based screening (Figure 1) potentially relevant primary research in English or French investigating Campylobacter spp. in broiler chickens on-farm and sources of Campylobacter spp. of interest were identified (Appendix S1a). Non-primary research studies (e.g. narrative reviews) or primary research studies outside of the scope were intentionally excluded. All citations deemed relevant at the abstract-based screening level were procured as full articles. At the next level, the full paper was used to confirm relevance, assess and categorize the main themes (e.g. vertical transmission, animal, human, water, environmental, or equipment sources of Campylobacter on-farm) and various descriptive characteristics (Appendix S1b). During the second level screening articles were assessed for two exclusion criteria: 1) minimum sufficient reporting of data and, 2) replicable laboratory protocols. Minimum sufficient data was defined as reporting of sufficient (e.g. proportion and number of samples) extractable raw or unadjusted data. For studies which employed multivariable modeling, effect estimates with reported sample size and measure of variability or exact p-values were considered essential. Studies were also excluded if the laboratory methods and materials sections lacked sufficient detail or did not reference a paper with sufficient detail to permit replication. Both screening levels were conducted by two independent reviewers using standardized and pre-tested checklists. Reviewer agreement (κ≥0.8) was evaluated using 30 abstracts and 5 full articles for abstract and full paper screening, respectively. Conflicts were resolved through consensus between respective reviewers and if this was not possible, by a third team member.
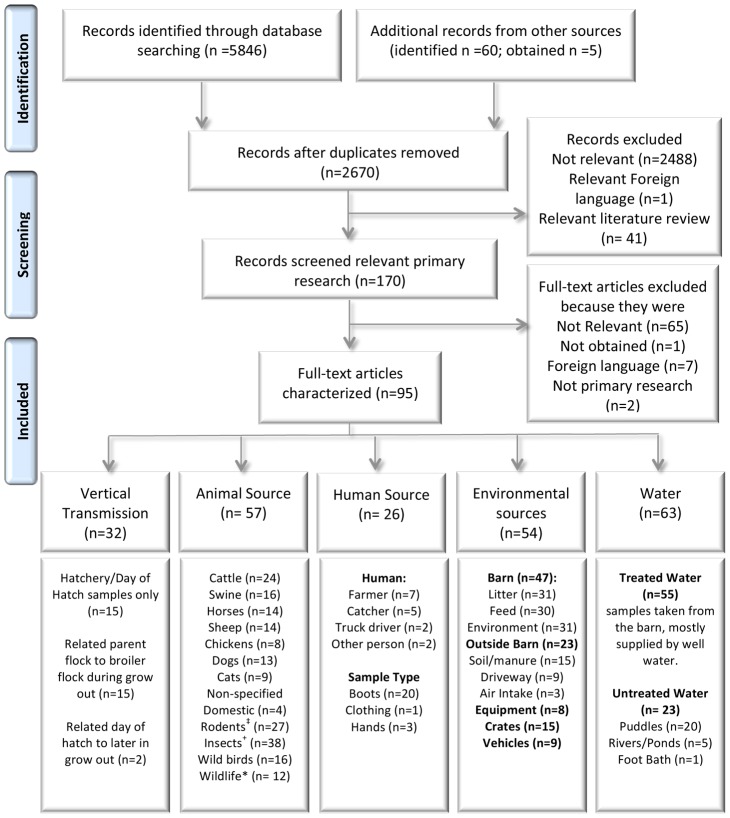
Figure 1. Flow of abstracts and articles through different steps of the systematic review.
95 articles were relevant. ‡rodents identified were mainly mice, also included voles, rats and guinea pig. + Insects identified were mainly flies and beetles with only a few examining mealworm, cockroach, caterpillar and snails. *Wildlife identified included rabbit, raccoon, possum, skunk, deer, mink, fox, hedgehog, shrew and Pukeko.
Risk of Bias, GRADE and Data Extraction
On prioritized subsets of data the review implemented further assessment of groups of studies (Appendix S1c, S1d). A direct modification of the Risk of Bias and GRADE criteria endorsed by the Cochrane collaboration was used to assess each study and evaluate the GRADE for groups of similar studies [26], [27]. The risk of bias assessment aims to assess the internal validity of the study which informs one of the GRADE criteria. GRADE criteria are summarized across groups of like studies to indicate the level of confidence in the current evidence. Essentially the one to four star grading system indicates that future studies are **** unlikely to change the conclusions of the current evidence; *** may have some impact on the conclusions; ** will likely change the conclusions; * the current evidence is very weak [28]–[30].
The data extraction part of this tool (Appendix S1e) aimed to efficiently capture the pertinent information and results needed for meta-analysis. Any studies where it was noted at the full paper relevance and characterization step that there was “no data to extract” did not progress to this level of evaluation.
Study management and data analysis
All SR stages; relevance screening at the abstract and paper level, methodological assessment and data extraction were conducted in a web-based electronic systematic review management software DistillerSR (Evidence Partners Inc., Ottawa, Canada). The data were analyzed descriptively (STATA 12, Microsoft Excel 2010) followed by random effect meta-analysis (MA) of selected sub-groups of data in Comprehensive Meta-analysis Software V2 (Biostat, Englewood, NJ). Where appropriate, homogenous meta-analyses with more than 10 lines of data were evaluated for publication bias by Begg's and Egger's test [31], [32]. If publication bias was detected, Duval and Tweedie's trim and fill method was used to estimate the potential impact on the estimate and conclusions of the MA. Results with high heterogeneity are still reported in this manuscript as the authors felt that these results were more informative than reporting a range of results. However, we caution readers to use heterogeneous results carefully as the source of heterogeneity is unknown and future research may dramatically change the results of the MA.
Results
Description of studies
The electronic search yielded 2670 citations after de-duplication, including 60 citations identified through search verification (Figure 1). Out of 2530 abstracts excluded through RS1, 2488 were not relevant to sources of Campylobacter spp. in broiler chickens, one was considered relevant but in a foreign language and 41 were considered relevant literature reviews or commentaries. Among 170 abstracts considered potentially relevant after abstract screening, five had been identified only through search verification. At the second level screening of the full paper (n = 170), an additional 75 articles were excluded for reasons described in Figure 1.
The 95 articles (Appendix S2) evaluated for descriptive characteristics were published in English and originated from United States of America (USA)/Canada 19.1%, Europe 63.8%, South and Central America 2.0%, Asia 8.5%, Africa 1.0% and Australia and New Zealand 5.3%. The first relevant study was published in 1983 and 75% of the studies captured were published 2000–2012. Studies were mainly observational (92%), with the most common study design being cross-sectional (42.4%) and cohort (29.3%). Thirty seven (38.9%) studies included molecular epidemiology data with respect to the Campylobacter spp. samples taken. Conventional broiler production was the focus of 89% of studies, with 4.0% and 6.0% reporting results for organic and free-range production systems, respectively and 3% did not report production type. All studies investigated Campylobacter spp. in broiler chickens however, only 3 of these also reported antimicrobial susceptibility results and 9 reported antimicrobial drug usage in the flocks studied.
There were four exclusion criteria in our methodological assessment at the second level of screening with the full paper. Six studies were excluded from further analysis because they did not provide sufficient data for at least one outcome of interest based on the guidelines provided to the reviewers. No studies were excluded based on the other three criteria. Eighty-seven percent appropriately described the methods to allow the study to be reproduced with the remaining 13% considered sufficient because the missing details were referenced to other sources. Seventeen percent sufficiently described the Campylobacter spp. isolation and characterization methods and 83% of the 95 studies had referenced pertinent information in their laboratory methods. The final criterion, all 8 experimental studies (e.g., control and quasi-experimental trials) were considered to have selected an appropriate control group.
Assessment of farm sources of Campylobacter spp
Studies were divided into categories based on the source(s) of Campylobacter spp. investigated. Many studies investigated more than one: vertical transmission (32 studies), domestic and wild animal sources (57), human sources (26), environmental sources and equipment (54) and water (63) (Figure 1). The outcomes reported are summarized in Tables 1 to 5 and include 3 types of outcomes: 1) Campylobacter spp. prevalence information from all source categories, 2) Association measure [Odds Ratio (OR)], reporting the odds that a sample would be Campylobacter -positive if it originated from a farm where the broiler flock was Campylobacter -positive, 3) Association measure (OR) reporting the odds that a broiler flock is Campylobacter -positive when a risk factor (e.g., animal, pest or environmental) is present. Studies that used molecular methods to link broiler isolates from sources were also noted.
Table 1. Summary of meta-analysis, genotyping results and GRADE for domestic animals on farm or adjacent to farms.
| Category | # studies (lines of data) | MA # studies (lines of data) | Outcome : : |
Risk Factor | MA results (95% CI) | Heterogeneity (I2) | Molecular linkage to broiler flock (# of studies)§ | Adapted GRADE¥ | ||
| Prevalence | #tested | Reported matched | Reported not related | |||||||
| Odds (sample) | ||||||||||
| Odds (questionnaire) | ||||||||||
| Adjacent Broilers | 8 (13) | 6 (9) | Prevalence | 46.2% (26.6–67.0) | High (82.4%) | 8 | 8 | NA | ** | |
| 3 (5) | Odds (sample) | Adjacent broilers | 124.78 (3.6–288.2) | High (79.7%) | ** | |||||
| Laying Hens | 3 (3) | 2 (2) | Prevalence | 87.1% (78.4–92.6) | Low (9.84%) | 1 | 1 | ** | ||
| Cats | 2 (2) | 1 (1) | Prevalence | 7.1% (1.0–37) | 1 | NA | 1 | ** | ||
| Cattle | 15 (22) | 10 (14) | Prevalence | 50.0% (43.1–56.8) | Low (11.1%) | 12 | 8 | 1 | *** | |
| 3 (3) | Odds (sample) | Cattle | 0.71 (0.30–1.73) | Moderate (48.4%) | ** | |||||
| 2 (2) | Odds (questionnaire) | Cattle on farm | 1.44 (0.97–2.15) | Low (0.0%) | ** | |||||
| Dogs | 8(10) | 6 (8) | Prevalence | Dogs | 28.0% (9.4–59.4) | Moderate (66.7%) | 7 | 1 | 4 | ** |
| 2 (2) | Odds (questionnaire) | Dogs on farm | 1.76 (1.23–2.56) | Low (0.0%) | * | |||||
| Horses | 7 (9) | 3 (4) | Prevalence | 29.4% (14.8–49.9) | Low (0.0%) | 7 | 1 | 5 | ** | |
| 1 (1) | Odds (questionnaire) | horses on farm | 0.90 (0.64–1.27) | Low (0.0%) | * | |||||
| Pigs | 7 (8) | 6 (7) | Prevalence | 62.4% (37.8–81.9) | High (84%) | 5 | 1 | 1 | ** | |
| 1 (1) | Odds (sample) | Pigs | 2.03 (0.30–13.9) | NA | * | |||||
| 3 (3) | Odds (questionnaire) | Pigs on farm | 1.44 (0.92–2.27) | Low (0.0%) | ** | |||||
| Sheep | 7 (9) | 4 (5) | Prevalence | 29.3% (5.6–74.5) | High (85.5%) | 6 | 2 | 2 | ** | |
| 2 (2) | Odds (questionnaire) | Sheep on farm | 1.55 (0.85–1.56) | Moderate (42.9%) | * | |||||
| Animals, unspecified | 5 (6) | 5 (6) | Odds (questionnaire) | Animals on farm | 2.02 (1.07–3.79) | Moderate (44.0%) | 1 | 1 | ** | |
| Livestock, unspecified | 2 (2) | 2 (2) | Odds (questionnaire) | Livestock on farm | 0.61 (0.35–1.05) | Low (0.0%) | * | |||
| Other birds (e.g., bantam) | 1 (1) | 1 (1) | Odds (questionnaire) | Other birds on farm | 2.10 (0.94–4.68) | Low (0.0%) | 1 | * | ||

Outcomes include prevalence of Campylobacter ssp. in the sample, association measures (Odds Ratio based on sampling and Odds Ratio based on questionnaires to establish the presence or absence of risk factors.).
GRADE, Adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, see methods for details.
all 37 studies that reported molecular epidemiologic data, number tested include all that obtained samples (positive and negative culture).
MA- meta-analysis, 95% CI – 95% confidence interval, I2- measure of heterogeneity in the meta-analysis, NA- Not applicable or not isolated or no matching genotype found or not reported.
Table 5. Summary of meta-analysis, genotyping results and GRADE for drinking water and water treatment.
| Category | # studies (lines of data) | MA # studies (lines of data) | Outcome : : |
Risk Factor | MA results (95% CI) | Heterogeneity (I2) | Molecular linkage to broiler flock (# of studies)§ | Adapted GRADE¥ | ||
| Prevalence | #tested§ | Reported matched | Reported not related | |||||||
| Odds (sample) | ||||||||||
| Odds (questionnaire) | ||||||||||
| Drinking water from the barn | 31 (56) | 18 (37) | Prevalence | 10.8% (7.8–14.7) | High (88.5%) | 21 | 12 | 7 | ** | |
| 4 (5) | Odds (sample) | Drinking water from the barn | 6.19 (1.06–36.24) | Moderate (50%) | ** | |||||
| Municipal Water | 8 (10) | Odds (questionnaire) | Municipal Water vs. well water | 1.36 (0.7–2.61) | High (77%) | ** | ||||
| Acidified Water | 2 (2) | Odds (questionnaire) | Acidified Water vs. well water | 1.84 (0.43–7.91) | Moderate (67%) | * | ||||
| Filtered Water | 1 (1) | Odds (questionnaire) | Filtered Water vs. well water | 0.45 (0.16–1.28) | Low (0%) | * | ||||
| Treated Water | 3 (3) | Odds (questionnaire) | Treated Water vs. well water | 1.02 (0.51–2.07) | Low (0%) | ** | ||||
| Untreated Water (lakes) | 1 (1) | Odds (questionnaire) | Untreated water vs. municipal water | 3.42 (1.01–11.55) | ||||||

Outcomes include prevalence of Campylobacter ssp. in the sample, association measures (Odds Ratio based on sampling and Odds Ratio based on questionnaires to establish the presence or absence of risk factors.).
GRADE, Adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, see methods for details.
all 37 studies that reported molecular epidemiologic data, number tested include all that obtained samples (positive and negative culture).
MA- meta-analysis, 95% CI – 95% confidence interval, I2- measure of heterogeneity in the meta-analysis.
Overall, the prevalence of Campylobacter in broilers increased with the age of the flock (data not shown) with peak shedding occurring between days 21 to 42 of grow out.
Vertical transmission
This route was investigated in 32 studies included in this SR, and is defined as any study that examined the transmission of Campylobacter spp. from broiler breeder flocks to their offspring. The number of studies investigating transmission was the same in Europe and North America and all studies were observational in design: cohort, cross-sectional and prevalence. The overall GRADE of the evidence for these 32 studies was two stars (results not shown), indicating we have little confidence the overall conclusions from current research will remain the same with the addition of future studies. Observational study designs are downgraded compared to highly controlled trials, and in this case additional down-grading occurred due to conflicting results between studies. The 32 studies captured on vertical transmission were all published in peer-reviewed journals, 18 studies were published since (n = 10) or were not captured (n = 8) by Adkin et al [12]. Of these 32 studies, only 11 suggest their results support the possibility of vertical transmission. One of two studies [33], [34] within this subset investigating antimicrobial resistant Campylobacter supported the potential for vertical transmission.
From 32 studies, 15 related the breeding flock to subsequent progeny Campylobacter status between hatching and the pre-slaughter stage. Fifteen studies only sampled at the hatchery and/or day-old chicks for Campylobacter. Twenty seven of 32 studies attempted to isolate Campylobacter in birds less than two weeks or in hatch material, however only five studies successfully cultured positive results from hatch materials [35], day old chicks [36]–[38] and one week old chicks [39]. Very few studies followed sufficiently similar designs, or reported comparable results and 3 studies did not have extractable data, thus MA was not possible for these results.
Other evidence investigating vertical transmission included risk factor and genotyping studies. Two articles reported a significant association to the hatchery [40], [41]; the MA results (forest plot not shown) of these two studies (9 trials) was homogenous and reported an increased odds of 4.8 (95% CI 2.9–8.1), p<0.001 depending on the hatchery. Overall, large-scale molecular data for vertical transfer was unavailable. Only 4 of the 13 studies that used genotyping detected at least one broiler flock-matching genotype from breeders by flagellin A gene (flaA) short variable region (SVR) sequencing [42], flaA Polymerase Chain Reaction (PCR)-Restriction Fragment Length Polymorphism (RFLP) [38], Randomly Amplified Polymorphic DNA (RAPD)/Pulsed Field Gel Electrophoresis (PFGE) [34] and/or by Multi-Locus Sequence Typing (MLST) [43]; the other 9 studies found no matching genotypes and concluded that vertical transmission was unlikely.
Animals
Pests, domestic and wild animals investigated are listed (Figure 1). Poultry and livestock were commonly found on-farm or in close proximity to the broiler farms. Recovery rates of Campylobacter from adjacent broilers, layers, cattle and pigs were relatively high; in dogs, horses and sheep, recovery rates were low to moderate. The results of animal specific meta-analyses can be found in Table 1. More studies reported the detection of broiler flock matching genotypes [e.g., flaA-types, RAPD-types, Amplified Fragment Length Polymorphism (AFLP)-types and PFGE Kpn/SmaI-types] from adjacent broiler flocks (n = 8) and cattle (n = 8) compared to pigs (n = 1), sheep (n = 2), horses (n = 1) and dogs (n = 1). Cattle appear to be the most frequently identified non-broiler animal with broiler-flock matching isolates compared to other domestic animals found on-farm/adjacent to farm. In some cases, cattle and dogs are colonized with Campylobacter species uncommon to chickens such as C. hyointestinalis [43]–[45] and C. upsaliensies [46], [47], respectively. Random effect MA of association measures (i.e., odds ratio) indicates that only adjacent broilers are more likely to be Campylobacter-positive when the broiler flock in question is Campylobacter-positive (i.e., likely bi-directional spread), although Campylobacter was found in many domestic animals around the farm, and in few studies, the isolate matched the Campylobacter from the broiler flock. The only exception was the presence of dogs on the farm was related to an increased odds (OR = 1.76, 95% CI 1.23–2.56) that the flock would be Campylobacter positive, but no isolate matched the broiler flock, Table 1. There was insufficient data available to extensively examine directionality of spread within broiler and livestock production units. One study noted intermittent bidirectional spread between cattle and broilers [48], and 2 studies identified positive animals before chicks were placed in the broiler house [49], [50], suggesting that animals can be a source of Campylobacter.
Insects including beetles, flies and pests such as rodents were examined in 21 studies and their prevalence results are shown in Table 2. There was a lot of variability between species depending on the degree of contact with the broilers or their feces and the stage of production the samples were taken. Several studies identified matching genotypes to the broiler flock, but samples were only positive when the broiler flocks were present. The MA of four studies indicated increased odds of Campylobacter colonization in the presence of pests (OR = 2.38, 95% CI 1.33–4.27).
Table 2. Summary of meta-analysis, genotyping results and GRADE for hygiene barriers, pests, wildlife and humans.
| Category | # studies (lines of data) | MA # studies (lines of data) | Outcome : : |
Risk Factor | MA results (95% CI) | Heterogeneity (I2) | Molecular linkage to broiler flock (# of studies)§ | Adapted GRADE¥ | ||
| Prevalence | #tested | Reported matched | Reported not related | |||||||
| Odds (sample) | ||||||||||
| Odds (questionnaire) | ||||||||||
| Hygiene barriers | 3 (6) | 3 (6) | Odds (questionnaire) | Use of hygiene barriers around broiler barn | 0.23 (0.12–0.44) | Low (0.0%) | ** | |||
| Insects/Beetles | 8 (13) | 5 (10) | Prevalence | 28.9% (12.1–54.8) | High (73%) | 6 | 3 | 3 | ** | |
| Insects/Fly | 9 (12) | 7 (10) | Prevalence | 7.1% (1.6–26.0) | High (98%) | 3 | 3 | 3 | * | |
| Insects/all | 16 (27) | 8 (15) | Prevalence | 11.8% (4.3–28.2) | High (88%) | 10 | 4 | 5 | ** | |
| Insects/litterbug | 1 (3) | 1 (1) | Prevalence | 83.3% (19.4–99.0) | 1 | 1 | ** | |||
| Rodents | 18 (26) | 7 (10) | Prevalence | 49.6% (24.0–75.5) | High (88.7) | 10 | 2 | 4 | ** | |
| 5 (5) | Odds (questionnaire) | Presence of rodents/insects | 2.38 (1.34–3.27) | Moderate (51%) | ** | |||||
| Wildlife/deer | 1 (2) | NA | Culture negative | 1 | * | |||||
| Wildlife/racoon | 1 (1) | One isolate | Isolated once | 1 | 1 | * | ||||
| Wildlife/rabbit | 1 (1) | 1 (1) | Prevalence | 10.0%(0.6–67.4) | 3 | 2 | * | |||
| Wild Birds | 9 (15) | 4 (9) | Prevalence | 37.2%(28.4–47) | High (71%) | 9 | 3 | 4 | ** | |
| Humans, Boots | 13 (2) | 9 (20) | Prevalence | 14.1% (8.7–22.0) | High (69%) | 10 | 9 | ** | ||
| 3 (4) | Odds (sample) | Worker boots positive | 39.8 (8.0–196) | Low (13.6%) | ||||||
| Humans, hands | 3 (5) | 3 (5) | Prevalence | 11.6% (6.9–18.9) | Moderate (48%) | 2 | 2 | ** | ||
| Humans, lunch bag | 1 (1) | 1 (1) | Prevalence | 60% (29.7–84.2) | 1 | 1 | * | |||

Outcomes include prevalence of Campylobacter ssp. in the sample, association measures (Odds Ratio based on sampling and Odds Ratio based on questionnaires to establish the presence or absence of risk factors.).
GRADE, Adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, see methods for details.
all 37 studies that reported molecular epidemiologic data, number tested include all that obtained samples (positive and negative culture).
MA- meta-analysis, 95% CI – 95% confidence interval, I2- measure of heterogeneity in the meta-analysis, NA- Not applicable or not isolated or no matching genotype found or not reported.
The role of wildlife was also assessed and detected few isolates from wild waterfowl (e.g., wild geese) [51] and wild bird (e.g., starlings) [44], [46], [51]–[54] populations, which are common on-farm and carriers of multiple pathogens affecting commercial birds. The average prevalence in these species was 37.2% (95% CI 28.4–47.0) although the data was heterogeneous. The wild bird samples collected yielded negative results in a few studies [43], [48] and where detected, isolates were largely unrelated to broiler flocks [44], [46], [52]; only 3 studies detected flock-matching genotypes on the basis of flaA sequencing [53], [54] and combined flaA sequencing and MLST (i.e., same clonal complex and sequence types) [51]. There were few samples from other wildlife (e.g., deer, raccoons and rabbits) and those from culture-positive rabbits did not match the broiler flock Campylobacter genotypes [44], [48], [54].
Hygiene barriers around the broiler barn to keep out other animals, pests and insects were investigated in three studies, the MA [55] indicated this practice was highly protective (OR = 0.23, 95% CI 0.12–0.44). Two studies examined the association between pest prevention interventions and Campylobacter colonization of the broiler flock. Insecticide and rodent control measures were not significantly associated with Campylobacter status [56], but fly screens may significantly prevent colonization (p = 0.002) or delay colonization (p<0.0001) [57].
Humans
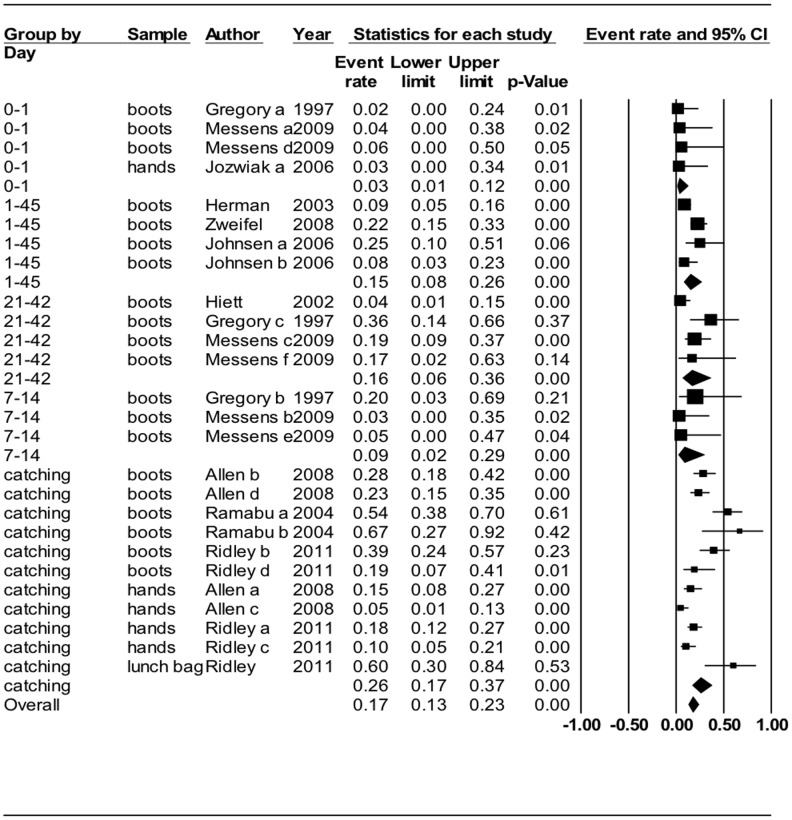
The role that people on the broiler farm may play in the contamination of the broiler flock with Campylobacter was investigated in 26 observational studies, mainly from Europe, that equally examined the risk of personnel directly involved (e.g., farmer, hired farm workers) and indirectly involved (e.g., catching crew) in flock-rearing. Random effect MA of the prevalence of Campylobacter on farm workers' boots, Figure 2, was homogenous and the prevalence increased with the age of the flock: day 0–1, 3.2% (95% CI 0.8–12.0); days 7–14, 8.5% (95% CI 2.1–29.0), and; days 21–42, 16.3% (95% CI 6.4–35.7). In agreement with these data, the prevalence found on the catchers at the end of grow-out was 25.50% (95% CI 16.7–36.9), however this data was heterogeneous. Random effect MA of 3 studies (4 trials) indicates high odds of workers' boots/footwear testing positive for Campylobacter if the flock is Campylobacter positive (OR = 39.8, 95% CI 8.0–196.0). Nine of 10 studies that genotyped Campylobacter isolated from farm workers and catching crew boots/footwear reported that the genotypes matched the broiler flock. Risk factors related to the frequency and number of farm workers were assessed in four studies; conflicting results were found for the number of visits per day [56], [58], and increased odds of the flock being Campylobacter positive when there are many farm workers was indicated in two studies [59], [60]. No association was reported between thinning activities and the broiler flock Campylobacter status [61].
Figure 2. Random effect meta-analysis of Campylobacter prevalence by sampling day on human samples.
Data from swabs of personal protective equipment, hand surfaces and other personal effects grouped by flock age: day 0–1 (or day of chick placement) I2 = 0%, days 1–45 (unspecified age of flock upon sampling) I2 = 60%, days 7–14 (early grow) I2 = 56%, 21–42 (mid grow to late grow) I2 = 0%, and catching (slaughter age) I2 = 80%.
Environmental sources
There were 54 studies that investigated environmental sources, 6 of these were experimental studies mainly focusing on cleaning and disinfection practices. Environmental sources were mainly investigated in Europe (n = 38) compared to other regions of the world (n = 16). Barn, production inputs (e.g., feed and litter), and the equipment to move these inputs were examined in many studies (Figure 1). Sampling of the external environment, the stored manure, vehicles entering the barn area, movable devices (e.g., crates and modules) were also examined in a number of studies.
Heavier equipment, usually kept on-farm such as forklifts and tractors, yielded a lower Campylobacter prevalence of 21.1% (95% CI 14–30.6) and 15.2% (95% CI 9.4–23.5), respectively, however there were few studies contributing to these estimates. Campylobacter detection from major production inputs such as clean litter and feed was low, Table 3. There was a high prevalence of Campylobacter on crates (48.1%, 95% CI 44.7–51.6), on other catching equipment (35.3%, 95% CI 30.4–40.6) and transport equipment (27.3%, 95% CI 24.8–29.9%), all subsets were moderately to highly heterogeneous, Table 3. There is evidence that the genotypes detected from pre-transport crates end up in residual flocks, the remaining birds in the barn, post-thinning [43], [52], [62], [63]. At the abattoir, genotypes from crates were detected from the cloaca [53], [64], [65], feathers and neck skin [64], [66], [67], and in the final carcass/post chill water [53], [67], [68]. None of these studies attempted to trace the main source (e.g., farm or processor origin) of Campylobacter contaminating the crates and catching equipment, but the use of the same catching crew was identified as a vehicle for the dissemination of Campylobacter within an integrated operation [62]. Other factors such as residual crate contamination and travel time (i.e., related to the time of exposure to the contaminated crate) were investigated in a few studies. In one study, crate washing and immersion in 10% quaternary ammonium compound or 100 ppm hypochlorite solution did not eliminate but slightly reduced the number of Campylobacter-positive crates by 40% and 60%, respectively [67]. In another study, exposure to reused crates yielded higher prevalence of Campylobacter in the cloaca and neck skin of birds sampled post-transport. Combined cleaning, disinfection and 12 hours of crate/transport equipment drying time was ineffective in reducing the Campylobacter load in flocks at the time of slaughter [66]. Travel time to the abattoir and additional data (i.e., pre- and post-transport Campylobacter load) were inconsistently reported, hence, it has not been possible to determine the impact of travel time on contamination. Thus, transport equipment and catching personnel pose a risk of transferring new Campylobacter to residual flocks and increasing the Campylobacter contamination on birds prior to arrival at the abattoir, but these activities were not associated with the Campylobacter status of the flock.
Table 3. Summary of meta-analysis, genotyping results and GRADE for catching equipment and other environmental-type samples.
| Category | # studies (lines of data) | MA # studies (lines of data) | Outcome : : |
Risk Factor | MA results (95% CI) | Heterogeneity (I2) | Molecular linkage to broiler flock (# of studies)§ | Adapted GRADE¥ | ||
| Prevalence | #tested§ | Reported matched | Reported not related | |||||||
| Odds (sample) | ||||||||||
| Odds (questionnaire) | ||||||||||
| Catching equipment | 3 (7) | 3 (7) | Prevalence | 35.3% (30.4–40.6) | High (71%) | 3 | 3 | * | ||
| Crates | 12(16) | 13 (19) | Prevalence | 48.1% (44.7–51.6) | High (89%) | 12 | 11 | * | ||
| Transport equipment | 4 (11) | 4 (11) | Prevalence | 27.3% (24.8–29.9) | High (87%) | 3 | 3 | * | ||
| Forklift | 3 (3) | 3 (3) | Prevalence | 21.1% (14.0–30.6) | Low (0%) | 2 | 2 | * | ||
| Tractor | 3 (3) | 3 (3) | Prevalence | 15.2% (9.4–23.5) | Low (0%) | 2 | 2 | * | ||
| Feed | 22 (32) | 13 (23) | Prevalence | 4.2% (2.3–7.6) | Low (26%) | 11 | 2 | 8 | *** | |
| Litter | 4 (8) | 3 (3) | Prevalence | Clean | 10.4% (2.1–38.8) | Moderate (62%) | 9† | 4 | 4 | ** |
| 3 (3) | 3 (3) | Prevalence | Day 0–1 broilers | 8.1% (1.6–31.5) | High (79%) | ** | ||||
| 3 (6) | 3 (4) | Prevalence | Day 7–14 broilers | 10.5% (3.1–29.6) | High (67.5%) | ** | ||||
| 10 (14) | 7 (8) | Prevalence | Day 21–42 broilers | 11.1% (7.5–16.2) | High (90%) | ** | ||||

Outcomes include prevalence of Campylobacter ssp. in the sample, association measures (Odds Ratio – samples and Odds Ratio – questionnaire).
GRADE, Adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, see methods for details.
all 37 studies that reported molecular epidemiologic data, number tested include all that obtained samples (positive and negative culture).
all age sub-groups.
MA- meta-analysis, 95% CI – 95% confidence interval, I2- measure of heterogeneity in the meta-analysis.
There was a wide range of outdoor environmental samples commonly reported; concrete apron and driveways, sample of water from puddles, ditches, and surrounding grass and soil, Table 4. Heterogeneity due to flock age (i.e. association with the Campylobacter status of the broiler flock) was investigated in a random effect MA of these outdoor environmental samples (not shown). Flock age did not explain the heterogeneity in the dataset, Campylobacter was isolated from day 0 of broiler placement and the prevalence in outdoor samples was relatively constant throughout the grow-out period. However none of the studies were able to demonstrate the direction of spread between flock and environment. Six studies reported detection prior to chick placement/or flock colonization in conventional flocks [43], [48], [65], [69], flocks reared under organic [70] and free-range production systems [71]. Early exposure to the outdoor environment appears to influence the onset of colonization in these production systems [70], [71]. Two studies reported that adjacent flocks remained negative throughout the duration of the growing period despite the presence of Campylobacter-positive flocks on the same farm [44], [53], suggesting that biocontainment measures can prevent within-farm dissemination of Campylobacter.
Table 4. Summary of meta-analysis, genotyping results and GRADE for exterior and interior barn environment.
| Category | # studies (lines of data) | MA # studies (lines of data) | Outcome : : |
Risk Factor | MA results (95% CI) | Heterogeneity (I2) | Molecular linkage to broiler flock (# of studies)§ | Adapted GRADE¥ | ||
| Prevalence | #tested§ | Reported matched | Reported not related | |||||||
| Odds (sample) | ||||||||||
| Odds (questionnaire) | ||||||||||
| Air Samples | 11 (21) | 10 (20) | Prevalence | 7.3% (3.5–14.6) | High (83%) | 7 | 5 | 1 | ** | |
| 1 (1) | Odds (sample) | Air sample | 24.05 (1.1–553.3) | * | ||||||
| Concrete around shed and driveway | 5 (7) | 4 (6) | Prevalence | 21.9% (11–38.8) | High (78%) | 5 | 5 | * | ||
| 1 (1) | Odds (sample) | Concrete | 1.80 (0.06–54.6) | * | ||||||
| Shed exterior | 7 (13) | 6 (11) | Prevalence | 18.3% (12.7–25.7) | High (91%) | 6 | 5 | 1 | ** | |
| 2 (4) | Odds (sample) | Shed exterior | 4.96 (3.49–7.04) | Low (0%) | ** | |||||
| Shed Interior | 29 (84) | 25 (56) | Prevalence | 15.0% (11.5–19.3) | High (93%) | 16 | 13 | 1 | ** | |
| 4 (8) | Odds (sample) | Shed interior | 9.95 (4.76–20.78) | High (69%) | ** | |||||
| Dead stock | 3 (5) | 2 (4) | Prevalence | 15.1% (2.2–58.2) | Moderate (64%) | 3 | 2 | ** | ||
| 1 (1) | Odds (sample) | Dead stock | 3.46 (12.0–100.6) | * | ||||||
| Foot bath | 1 (3) | 1 (3) | Prevalence | 58% (25.0–85.0) | High (74%) | 1 | 1 | * | ||
| Manure pile | 1 (3) | 1 (3) | Prevalence | 13.7% (4.4–35.1) | Low (9%) | 1 | 1 | * | ||
| 1 (1) | Odds (sample) | Manure pile | 1.67 (0.04–63.8) | * | ||||||
| Soil | 3 (9) | 2 (6) | Prevalence | 14.5% (6.8–28.2) | Low (0%) | 3 | 3 | ** | ||
| 2 (3) | Odds (sample) | Soil | 0.57 (0.08–4.03) | Low (0%) | ** | |||||
| Puddle/ditch | 12 (25) | 9(20) | Prevalence | 21.4% (16.6%–27.0%) | Low (26%) | 11 | 7 | 2 | ** | |
| 4 (5) | Odds (sample) | Puddle/ditch | 1.6 (0.71–3.54) | Low (0%) | ** | |||||
| Pond/river | 2 (3) | 2 (3) | Prevalence | 64.8% (7.3–79.0) | Moderate (50%) | 2 | 0 | 1 | * | |

Outcomes include prevalence of Campylobacter ssp. in the sample, association measures (Odds Ratio based on sampling and Odds Ratio based on questionnaires to establish the presence or absence of risk factors.).
GRADE, Adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, see methods for details.
all 37 studies that reported molecular epidemiologic data, number tested include all that obtained samples (positive and negative culture).
MA- meta-analysis, 95% CI – 95% confidence interval, I2- measure of heterogeneity in the meta-analysis.
Detection of Campylobacter in the indoor barn environment (e.g., wall, floor, posts, feeders, drinkers) (15.0%, 95% CI 11.5–19.3) and in air samples (7.3%, 95% CI 3.5–14.6) were moderately and highly heterogeneous, respectively, Table 4. Flock-matching genotypes were detected in indoor environment samples from prior to placement to 24 hours after placement, and more frequently around mid-grow out when flock colonization typically becomes evident.
While carry-over was not an objective of this SR, there were 5 longitudinal prevalence studies and one cohort study that demonstrated carry-over of Campylobacter genotypes through two or more flock cycles and also isolated matching genotypes in samples taken in the indoor barn environment demonstrating residual contamination acted as a source for the next broiler cycle [49], [70], [72]–[75].
Water
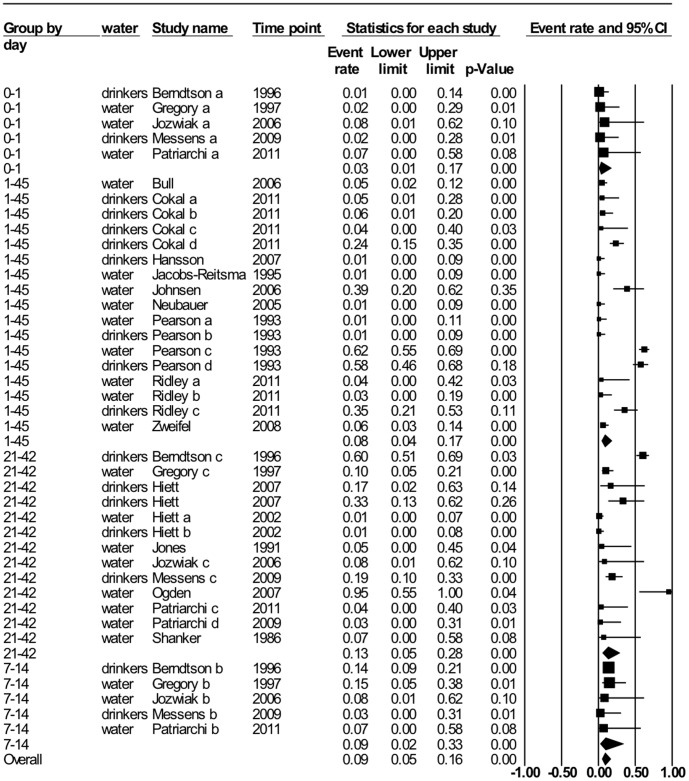
Drinking water is a major input into the broiler barn and would be a major source of Campylobacter if present. Drinking water samples were taken from a variety of places within the barn and in many cases there is the risk that the Campylobacter isolated was from broiler contamination as opposed to the drinking water supply being contaminated. In Figure 3 the temporal MA shows an increase in Campylobacter positive water samples with age of the broiler flock. As well, studies that examined the odds of drinking water testing positive in a positive flock was 6.19 (95% CI 1.1–36.2), with moderate heterogeneity (I2 = 50%), but the total number of studies was very low. Twelve of 21 studies that genotyped the isolates found flock-matching genotypes in water and water lines in colonized flocks. In two studies, the PFGE patterns of isolates from the drinking line [76] and sequence types from header tank [77] were indistinguishable to isolates from subsequent flocks (i.e., both drinking water system and in bird feces), suggestive of carryover. Less explored niches within the drinking water system such as ciliates and flagellate protozoa also yielded positive Campylobacter results [78], [79] and based on the available data drinking water as a source of Campylobacter cannot be ruled out. One study also raised the issue of detection limits by culture, as they had immunoflorescent test positives on Campylobacter culture negative samples [79]. The studies that examined the water source or treatment of water as a risk factor for flocks testing Campylobacter positive did not significantly show an association with the exception of untreated water (e.g. lakes) that had an increased odds of the broiler flock being Campylobacter positive OR 3.42 (95% CI 1.01–11.55), Table 5.
Figure 3. Random effect meta-analysis of Campylobacter prevalence by sampling day on water samples.
Data from water samples collected from drinkers/water lines or unspecified locations within the barn are grouped by flock age: day 0–1 (or day of chick placement) I2 = 0%, days 1–45 (unspecified age of flock upon sampling) I2 = 92%, days 7–14 (early grow) I2 = 87% and 21–42 (mid grow to late grow) I2 = 0%.
Characterization of antimicrobial-resistant Campylobacter and risk factors
There were 10 studies that reported farm-level antimicrobial drug use data. Three studies reported resistance profiles for Campylobacter isolates (Table 6), of which two also provided drug-use information. Within these 2 studies, resistant isolates were broiler-flock matched. Idris [34] found resistance patterns in broilers reflective of the medication administered to breeders (i.e., ciprofloxacin, a flouroquilonone antimicrobial). The broiler PFGE and RAPD types matched the breeder genotypes isolated, which suggests vertical transfer occurred. Subsequent exposure of the breeders' descendants to the same antimicrobial resulted in a high Minimum Inhibitory Concentration which implies reduced susceptibility to the antimicrobial. Messens [44] detected isolates from the drinking water, farmer's footwear and other barns, which were resistant to one or more of the drugs that were administered to the flock, suggestive of within-flock (via drinking water) and within farm (via farmers footwear) dissemination of antimicrobial-resistant Campylobacter. Skov [80] found nalidixic-acid resistant C. jejuni in beetles, but prevalence in beetles and drug use history for the broiler flock were unavailable; the insects were found only while birds were present in the barn and not during downtime.
Table 6. Sources of antimicrobial-resistant Campylobacter.
| Authors | Source/transfer mechanism | Susceptibility test and profiles | Prevalence | Genotyping/Antimicrobial use history/comments |
| Skov et al, 2004 [80] | Pests (beetle) | Unknown: Nal | Not reported | Resistance profile of beetles matched broiler genotypes;Drug use data unavailable;Detected in beetles during growing period but not during downtime. |
| Idris et al, 2006 [34] | Broiler breeders/vertical transfer | Agar Dilution: Cip, Cip/Tet | Descendants of broiler breeders :C. jejuni = 60% (Cip)
C. coli = 23.5% (Cip) :C. jejuni = 60% (Cip)
C. coli = 23.5% (Cip)
|
Genotypes and resistance profiles of descendants matched the broiler breeder genotypes;Resistance related to drug use in breeders;Detected in descendants of broiler breeders 6 weeks post-medication. |
| Messens et al, 2009/Herman et al 2003 [44], [45] | Adjacent flock, drinking water, footwear | E-test: Ery/Tet Tet,Cip/Nal/Tet, Nal/Tet, Cip/Nal | 54% (resistant to 1 or more antimicrobials) | Some genotypes and resistance profiles of adjacent flocks, drinking water and footwear matched broiler flock genotypes;Resistance related to drug use: 12 out of 18 flocks medicated 1–4 times with: Quinolone-Fluoroquinolone (n = 5 flocks); Ampicillin (n = 1); Sulfonamide (n = 1); Fluoroquinolone-Trimethoprim-Sulfonamide (n = 1); Fluoroquinolone-Sulfonamide-Tetracycline (n = 1); Macrolides-Lincosamides-Polypeptide (n = 1); Lincosamides-Aminoglycosides-Quinolone (n = 1); Lincosamides-Aminoglycosides (n = 1);Isolates exhibited resistance to more than one antimicrobial; profile of some isolates detected from the same source changed through time. |

Same broiler integrator that supplied the breeder flock in the study.
Ery – erythromycin, Tet – Tetracycline, Cip – ciprofloxacin, Nal – nalidixic acid.
Assessment of laboratory methods
The laboratory methods captured in the 95 studies were summarized in Table 7. The goal of this exercise was to identify primary isolation protocols, typing/speciation and genotyping tools to link broiler Campylobacter from potential sources. This SR found extensive variations in the method of detection for Campylobacter and genotyping to establish epidemiological linkages. PCR was the most commonly used subtyping tool, but biotyping and/or serotyping were also used. Agar dilution and E-test methods were used for characterizing resistant profiles. Genotyping was done in 37 studies (38.9%) using one test method (18 studies), 2 test methods (16 studies), and 3 test methods (3 studies). The use of more than one test method enhanced the over-all discriminatory power (i.e., ability to differentiate between strains) or confirmed the genotype groups/clusters, however, concordance (i.e., agreement between results of two independent typing systems [81]) were not well described. Overall, PFGE alone or in combination with other tests (Table 7) was the most frequently used technique, but there were variations in the number of enzymes used (i.e., SmaI, 10 studies and SmaI+KpnI, 8 studies), and analytical method used to interpret results (i.e., visual observation vs. mathematical). The most common analytical method to determine similarity between genotypes was Dice coefficient (i.e., algorithm that assigns strains to the same group [82]); arbitrary cut-off values ranged from 94 to 99% similarity (i.e., 100% minus 1 to 6%). Genotypes were also assessed if these clustered to the same group, using Unweighted Paired Group Matrix Analysis (UPGMA), Neighbor Joining algorithm and HKY85 method. Most studies interpreted the genotyping results based on the convention for PFGE band interpretation developed by Tenover and others [83] that categorized the genotypes as “indistinguishable”, “closely related”, “possibly related” and “unrelated”. In this study, the term “flock-matching/flock-matched”, “identical” and “indistinguishable” was used synonymously to describe linkages from broilers and the different sources.
Table 7. Summary of Campylobacter isolation and genotyping protocols.
| Recovery steps (%, 95 studies) | Types | # of studies |
| Farm-collected broiler samples (73.6%) | droppings: fecal, caecal, fecal/caecal swabs | 53 |
| cloacal swabs | 13 | |
| boot socks/overshoe | 3 | |
| sand sample | 1 | |
| other farm-collected but non-broiler samples | (8) | |
Abattoir level and/or laboratory collected broiler samples (45.2%)
|
caecal content/caecal contents swab | 30 |
| cloacal swabs | 11 | |
| intestinal pool | 2 | |
| Combined with above samples: feathers, neck skin, bile | (4) | |
| Used transport media (25.2%) | Cary Blair | 7 |
| Phosphate Buffered Saline | 5 | |
| Maximum Recovery Diluent | 4 | |
| Amies Charcoal | 4 | |
| Buffered Peptone Water | 3 | |
| Used enrichment media to transport specimen | (10) | |
| Incorporated enrichment step (75.7%) | Preston | 29 |
| Bolton | 10 | |
| Campylobacter Selective Enrichment | 10 | |
| Exeter | 8 | |
| Hunt's | 5 | |
| Less commonly used (1–3 studies): Modified/Double Strength Enrichment, Tryptic Soy Broth, Supplemented Nutrient Broth (5% horse blood), Brucella, THAL Campy | 10 | |
| Culture plating, raw and enriched samples (95.7%) | mCCDA, charcoal- based | 30 |
| CCDA, charcoal- based | 12 | |
| Campy-Cefex Agar, charcoal- based | 10 | |
| Preston, charcoal- based | 10 | |
| Campylobacter Selective Agar, blood-free | 7 | |
| Campylobacter Selective Agar, blood-based | 6 | |
| Less commonly used (1–4 studies): Virion, Karmali, Butzler, CAT agar, Brucella, Mueller Hinton with Preston Agar, Campy-FDA | 16 | |
| Direct molecular detection, raw samples or enriched samples (4.2%) | PCR | 4 |
| Antimicrobial sensitivity testing (3.1%) | Agar dilution | 1 |
| E-test | 1 | |
| Unknown | 1 | |
| Speciation (81.0%) | PCR | 41 |
| Biotyping and serotyping | 24 | |
| PCR combined with biotyping and serotyping | 12 | |
| Genotyping (molecular linkage) (38.9%)¥ | ||
| 1 method | flaA (sequencing or PCR-RFLP) | 5 |
| RAPD | 5 | |
| PFGE | 4 | |
| Less frequently used: AFLP, MLST, ribotyping, sequencing of other genes | 4 | |
| 2 methods | flaA+PFGE | 8 |
| flaA+MLST | 4 | |
| Less frequently used: flaA+ribotyping, PFGE+RAPD, flaA/flaB+23S rRNA spacer) | 3 | |
| 3 methods | flaA+PFGE+AFLP | 2 |
| flaA+PFGE+MLST | 1 |

some studies may have also collected samples on-farm (21 studies collected at the farm and abattoir).
Genotyping to link broiler, animal and environmental type sample matrices; methods were also used for nomenclature/arbitrary genotype assignment.
( ) not counted, mCCDA-modified charcoal cefoperazone deoxycholate agar, PCR-Polymerase Chain Reaction, flaA-flagellin A, RAPD- Randomly Amplified Polymorphic DNA, PFGE-Pulsed Field Gel Electrophoresis, AFLP-Amplified Fragment Length Polymorphism. MLST – Multi-locus Sequence Typing, REA-Restriction Endonuclease Analysis.
Discussion
There has been an increasing interest over the last decade in controlling Campylobacter in broiler chickens and minimizing drug-resistant Campylobacter in the human food chain. In Canada, campylobacteriosis is the third most common enteric pathogen and recent surveillance data has identified regional increases in Ciprofloxacin-resistant Campylobacter, which is a public health concern [4], [10]. Campylobacter contaminated broiler meat is a food safety risk and evaluation of the global literature on Campylobacter sources is relevant to many countries as general industry-operational factors (e.g., catching, thinning, transport, processing) that may contribute to contamination are similar across countries; though in an integrated system, there may be less complexity in the coordination of monitoring and implementation of control programs throughout the poultry production chain. The Canadian broiler industry is a vertically-coordinated industry (i.e., sectors are closely-linked operationally but not owned by a single company) unlike in many European countries [84] and in the USA [85] which are heavily integrated. However, the product process flow, from hatching egg production to retail in Canada [86], is largely similar to many countries [84], [85]. Canadian broiler hatching eggs/chicks are sourced domestically and approximately 21% are imported from the USA in compliance with trade agreements [87]. This industry practice of international exchange of live products is also practiced in some European countries [88], [89] and may have relevance to the introduction of new Campylobacter strains. Broilers are grown out in cycles so there is an all in-all out approach and farms within Canada vary in size and number of barns [90], as in other countries [84], [85].
Similar to other synthesis and evaluation of the literature[12], [13], [16], this SR highlights the need for improved disinfection of the broiler house between flocks and improved biosecurity practices to stop the potentially self-perpetuating cycle of flock contamination. The meta-analysis of quantitative outcomes in this SR improves our understanding of the degree of variation across studies and covariates that account for some of the heterogeneity. The increasing use of molecular methods over the last decade has been highlighted in this review and adds more evidence to substantiate the overall findings and recommendations. This information, used in conjunction with context specific knowledge of the local broiler industry, can identify critical control points for prevention of broiler flock Campylobacter contamination.
One of the findings from this SR is that Campylobacter can be found on the farm prior to a new flock arriving at the broiler house. Inadequate cleaning and disinfection and short downtime of the broiler house between flocks may be a major source of Campylobacter carryover as studies confirmed Campylobacter in dust swabs from the interior of the barn and the drinking water system prior to or during flock placement [43], [44], [70], [71], [91]. The cycle of contaminated flocks is self-perpetuating if chicks are being placed in an already contaminated environment, in this SR 83% of the studies that reported carryover concluded persistent Campylobacter genotypes could be identified through several cycles [49], [70], [72]–[75]. In agreement with Adkin et al [12] and Newell et al [16] inputs such as clean litter and feed into the barn were not likely sources of Campylobacter to young broilers. Drinking water was found to be a potential vehicle for flock-level contamination; in agreement with our meta-analyses the consumption of untreated water was associated with an increased odds of a Campylobacter positive broiler flock. There was some evidence for vertical transmission or pseudovertical transfer (e.g. egg surface contamination) however it seems that this research is still hampered by the sensitivity of isolation and genotyping techniques, which will be discussed later. Meta-analysis of two studies associated the hatchery source to Campylobacter colonization at the broiler level (OR = 4.8) [40], [41] and Idris et al demonstrated identical resistance patterns in the breeders and broilers [34]. Previous reviews that looked at vertical transmission concluded this was a low risk exposure route for Campylobacter in broilers [12], [13], however based on the evidence identified in this SR there is evidence that vertical transfer occurs and due to well documented isolation issues, we cannot conclude on the relative importance of this source compared to others. There have been many investigations into potential biological mechanisms related to the survival of Campylobacter from egg to post-hatch stages [18], but more research is required to investigate this potentially under-recognized transmission route. It appears that any of these sources could result in an infected broiler flock as only a few susceptible birds are sufficient to result in flock-level colonization by the end of grow out [21], [92].
Domestic animals and their environment were positive for Campylobacter in several studies, but they had very small sample size and often these isolates did not match the Campylobacter that subsequently colonized the flock. This SR-MA identified adjacent broiler flocks (n = 8 studies) as a source of flock-matching genotypes (8/8 studies) and the MA (OR = 124.78) suggests that the presence of adjacent broilers may pose a significant risk for Campylobacter transmission to a new flock in agreement with other synthesis papers [16], [21]. The same was not seen for other animals (e.g., cattle) on the farm, despite high prevalence and the detection of flock-matching genotypes in 66% (8/12) of studies. Overall, there was very little wildlife evidence and the 9 wild bird studies reported an average prevalence of 37.2% with flock matching genotypes reported in 33% of studies and no epidemiological analysis. These were also considered low risks for Campylobacter infection in broilers in the UK [12]; and were not prioritized for intervention relative to other vectors and production factors in recent guidance documents [13]. There is Canadian surveillance evidence that indicates where farming is dense and species are diverse, Campylobacter in particular, can be very high [93] and is likely being shared among operations [94], [95]. The potential for transmission of Campylobacter via other infected animals or pests highlights the importance of biosecurity, including hygiene barriers between animal production units. A number of biosecurity measures have been highlighted in previous reviews [12], [13], [16] including restrictions of other animals on the farm [21] and the use of pest control methods such as fly screens or insecticides [57], however the effectiveness of these interventions still needs to be evaluated for Canadian broiler farms.
Our examination of the evidence on resistance profiles of Campylobacter isolated from multiple sources netted three studies; two related the resistance to broiler antimicrobial drug use [34], [44] and one related the antimicrobial-resistant Campylobacter to drug use in the breeders [34], implying vertical transfer is possible. With only three studies, it is not possible to draw any conclusions from this data, however understanding the potential transmission routes through the broiler production hierarchy is important to assess the potential role that the international and interprovincial exchange of hatching eggs and chicks may have in the emergence of antimicrobial-resistant Campylobacter in Canadian broilers. Farm sources of drug-resistant Campylobacter should also be explored such as transmission from beef, dairy and swine herds, although the molecular epidemiology and broiler colonization (e.g., of bovine origin) studies have yet to be conducted.
There were several potential sources of Campylobacter that showed a temporal correlation with the flock becoming Campylobacter positive. These included pests (e.g. fly and rodents), humans (e.g., visitors, number of workers that enter the barn, frequency of access to barns), farm equipment, and water, which may indicate that these potential sources are actually becoming contaminated by the broiler flock as it is colonized and not vice versa.
Catching crews and independent operators (e.g., truckers, drivers) were identified as a potential source of new genotypes in the flock during late grow-out when Campylobacter shedding in the contaminated flocks peaks, increasing the chances of within-farm (i.e., through contaminating the environment) and industry-wide Campylobacter dissemination (e.g., through catching and transport equipment). Similarly, flock thinning, practiced by approximately 11% of Canadian broiler producers [96], has been noted as particularly high risk exposure for the remaining flock as Campylobacter introduction as low as 2 colony forming units could lead to pen-level colonization within a 7-day period [97]. Katsma et al suggested that banning the practice of thinning may reduce slaughter level Campylobacter prevalence [21]. Contamination of the birds on route to slaughter may result in increased external contamination and internal colonization if the birds remain in the contaminated crates for more than 6 hours [43], [64], [66]; bird contamination/colonization may lead to processing plant contamination, which could subsequently contaminate batches of Campylobacter-negative flocks [45], thus undermining overall industry-level reduction efforts. Some studies captured in this SR linked crates and catching crew related sources of Campylobacter with external contamination of the birds upon entering the abattoir, but none of the studies were appropriately designed to establish the direction of spread. Improvement in biosecurity compliance, such as provision of farm-specific clean boots and clothes to catching crew, was practiced by only 38% of broiler producers in Canada [96]. And improvement in biosecurity interventions such as effective cleaning and disinfection of crates and equipment are needed as current research indicates these methods are ineffective [13] and drying time (<24 hours) may be too short [66]. Factors such as production expectations (i.e., to achieve projected kilograms/quota), related management practices (i.e., thinning) and producer education may have an impact on Campylobacter colonization prevention. These are likely to vary widely by region and country, thus the local context should be considered when developing recommendations.
Biosecurity improvements were highlighted throughout this SR and are consistent with the findings of previous reviews [12], [13], [16]. The focus of this review was on sources as opposed to evaluating the effectiveness of various biosecurity interventions, however other reviews have indicated more work needs to be done to assess how effective biosecurity interventions may be in reducing Campylobacter positive flocks and within flock prevalence [21]. Current industry and national requirements for biosecurity and the level of compliance by individual producers should be taken into account when modernizing local biosecurity practices. Biosecurity programs generally involve access management (e.g., restriction to rearing areas, hygiene barriers), animal health management and general farm operational/structural improvements to reduce disease transmission [98]. In Canada, biosecurity-associated risks such as rodent control and proximity of the barn to manure piles have been identified as risk factors for Campylobacter colonization in turkeys, the same is likely true for broilers [99]. Canadian avian biosecurity recommendations address a number of biosecurity risk reduction practices including installing hygiene barriers, manure management, designating manure storage areas that are distant to the main rearing area, and maintenance and modernization of facilities such as fly screen installation and drainage improvement to reduce pests [98], [100]. Restriction of personnel movement (i.e., in multi-barn facilities) may not be practical when there is a limited number of farm workers hired by the producer, but any of the following management practices could reduce the risk of human movement contributing to Campylobacter contamination of the broiler flock. Minimizing the frequency of access by farm workers or following a strict movement protocol (e.g., from young to old flocks in multi-barn facilities), limiting visitors, provision of farm/barn-designated clothing and footwear, decontamination (e.g., proper hand washing), and other structural changes or modernization of facilities (e.g., anteroom and hygiene barriers/fences, separate entrances in multiple barns). In Canada, biosecurity protocols and producer education programs need to be improved as a recently conducted Canadian survey of producers about their knowledge and attitudes toward food safety and good production practices indicates low to moderate compliance for hand washing (36%) and visitors changing clothing before entering barn (50%) [96]. Another Canadian study found farm workers often commit mistakes related to bio-containment (i.e., barn entry and exit procedures) [101]. It is recommended that on-going producer education emphasize the importance of biosecurity, improved compliance with on-farm food safety program recommendations and monitoring within the context of their local broiler industry [98], [100], [102], [103].
Few studies were appropriately designed to establish the direction of Campylobacter exchange or to understand the reservoir vs. vector potential of identified sources. In many cases the potential sources are not mutually exclusive e.g., environment, human, equipment could all contribute to both the dissemination and maintenance of Campylobacter and once the farm is contaminated, the cycle may be self-perpetuating even with all-in-all-out practices and stringent biosecurity. Forty percent of the studies in this review were cross-sectional, which cannot inform the direction of spread. Additionally, sampling inconsistencies including frequency of sampling, age at sample collection and variations of genotyping techniques (i.e., insensitive to genetic recombination) limited the ability to establish direction of spread in other study designs. Host-preference [48], invasive ability/gut colonization, and competitive performance within the gut [104] are also factors that complicate our understanding of host preference among Campylobacter, but are beyond the scope of this SR. The detection of flock-matching genotypes in livestock may be suggestive of an increased proportion of highly host-adaptive subpopulations [105] that result from frequent genetic interchange (i.e., interspecies and intraspecies) [106]. On the other hand, niche segregation among Campylobacter has also been described [105] and may explain the presence of highly host-specific/non-flock matching Campylobacter subpopulations such as those that colonize wild birds [107]. Host-related genomic markers for source-tracking of Campylobacter, has yet to be identified [106]. Future host-association and Campylobacter dynamics studies should adopt a more structured sampling methodology and utilize high-performance genotyping techniques that are sensitive to intra-and inter-genomic recombination [108] in addition to MLST [106], [109], [110].
The inability to culture Campylobacter from birds less than 2 weeks old presents a major barrier in researching Campylobacter in broilers and has led to inconsistent study results within this SR. Several hypotheses have been suggested to explain researchers' inability to isolate Campylobacter during the first two weeks of placement. First, Campylobacter may be in a non-culturable form as there were several studies that successfully detected Campylobacter DNA, but have failed to culture [34], [111]–[113]. Thus, there is a need to explore the use of a more reliable molecular technique for detecting viable or “potentially infectious units” of Campylobacter [114] from hatchery and chick samples. Second, different isolation techniques have highly variable sensitivity that may affect results if Campylobacter concentration is below the detection limits [113]. Because of the inherently low number of cells in eggs/eggshells, embryos, yolk sac, and neonatal intestines enhanced recovery techniques (e.g., combining membrane filtration and enrichment) [115], [116] need to be explored to improve our detection limits in these samples. Third, the type of sample may be important, for example, Campylobacter may not be present in the cecal or fecal samples during early rearing because it is still colonizing the small intestine [34], [117].
The studies captured in this SR used various techniques to sample isolates and evaluate Campylobacter. Different sample matrices (e.g., fecal, cecal, cloacal swabs, boot socks), isolation protocols, and genotyping methods were identified and tabulated; all have different sensitivity and specificity, which could influence the prevalence results, genotype diversity and be a source of between study heterogeneity [118]–[120]. Each of the genotyping techniques reported has certain limitations [24], [121], and may not be suitable for longitudinal investigations (i.e., multiple broiler cycles) across wide geographical areas. Two of the older methods RAPD and ribotyping are rarely used now because of typeability issues [122], [123]. Only a few studies identified by this SR used more recent techniques such as MLST. PFGE was the most common among captured studies and is considered the “gold standard” for many priority enteric pathogens. For Campylobacter, however, PFGE fingerprints tend to evolve rapidly [83], and interpretation may change based on the number of enzymes used [70], [71]. A Canadian Campylobacter outbreak demonstrated that PFGE failed to detect clusters of human cases because the PFGE fingerprints changed during the course of the outbreak [124], same in a Canadian Salmonella outbreak [125], which suggests this technique may not be suitable for longitudinal studies (i.e., several broiler production cycles). The use of PFGE, in combination with other methods was described in most studies in this SR. Fla typing was the second most frequently used, but it has the potential for recombinational exchange of alleles: unrelated strains may end up with the same fla type whereas related strains may end up with different fla types [43]. Detailed assessment of the potential impact of these laboratory factors is beyond the scope of this SR, but we have highlighted the variability and quantity of research that is underpinned by each method. Future research should work towards developing an internationally accepted genotyping technique for Campylobacter that is inexpensive and meets most of the performance criteria including stability, typeability, reproducibility, discriminatory power and concordance in order to validate the genetic and epidemiological linkages [81].
In conclusion, identifying farm-level sources for contamination of Campylobacter in broiler chickens can help identify and prioritize control points and recommendations for biosecurity improvements on-farm and industry-wide and highlights information gaps in understanding the epidemiology of Campylobacter in broiler production. This systematic review meta-analysis assessed multiple outcomes (prevalence and risk factors) to identify and summarize the evidence for sources of Campylobacter on-farm. The highest risk for contaminating a new flock appears to be a contaminated barn environment due to inadequate disinfection and cleaning, insufficient downtime and the presence of an adjacent broiler flock. Gaps still exist in understanding the role of other domestic and wild animals and vertical transfer in the epidemiology of Campylobacter and antimicrobial-resistance profiles of isolates from the different source categories. This study highlights the potential impact of enhanced biosecurity and the need for improved compliance particularly in bio-containment, personnel decontamination measures, and equipment cleaning and disinfection. Further research, such as advanced genotyping methods, sensitive to intra-and intergenomic recombinations are required to improve on-farm source attribution and to provide recommendations for further refinement of food safety programs in Canada.
Supporting Information
PRISMA checklist.
(DOC)
Study Protocol. Outlines the protocol for relevance and abstract screenings, data characterization, Risk of Bias (RoB), and GRADE, and; data extraction forms.
(DOCX)
List of the 95 references used in the study.
(DOCX)
Acknowledgments
We would like to acknowledge reviewers Dr. Meredith Faires and Farzeen Foda; Miranda Galati for executing the search and article procurement; Dr. Rebecca Irwin, Dr. Richard Reid-Smith, Dr. Pascal Michel and Dr. John Nash for their guidance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded through the Public Health Agency of Canada (http://www.phac-aspc.gc.ca). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sheppard SK, Dallas JF, Strachan NJ, MacRae M, McCarthy ND, et al. (2009) Campylobacter genotyping to determine the source of human infection. Clin Infect Dis 48: 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mullner P, Spencer SE, Wilson DJ, Jones G, Noble AD, et al. (2009) Assigning the source of human campylobacteriosis in New Zealand: A comparative genetic and epidemiological approach. Infect Genet Evol 9: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 3. Wilson DJ, Gabriel E, Leatherbarrow AJ, Cheesbrough J, Gee S, et al. (2008) Tracing the source of campylobacteriosis. PLoS Genet 4: e1000203 Available: http://www.plosgenetics.org/article/info%3Adoi%2F10.1371%2Fjournal.pgen.1000203. Accessed 22 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas MK, Murray R, Flockhart L, Pintar K, Pollari F, et al. (2013) Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog Dis 10: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Food Safety Agency (2010) Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008, Part A: Campylobacter and Salmonella prevalence estimates. EFSA Journal 2010 8: 1503 100 pp. Available: http://www.efsa.europa.eu/en/efsajournal/doc/1503.pdf. Accessed 22 July 2014. [Google Scholar]
- 6.United States Food and Drug Administration (2013) NARMS Retail Meat Annual Report 2011. Available: http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm334828.htm. 82 pp. Accessed 22 July 2014.
- 7.Public Health Agency of Canada (2011) Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2011 Short Report. Available: http://publications.gc.ca/site/eng/428466/publication.html. 70 pp. Accessed 22 July 2014.
- 8.Arsenault R (2005) Campylobacter and Salmonella positive commercial broiler chicken farms in Ontario and associated risk factors. MSc Thesis, The University of Guelph. Available: http://www.collectionscanada.gc.ca/thesescanada/index-e.html. Accessed 24 June 2014.
- 9. Arsenault J, Letellier A, Quessy S, Boulianne M (2007) Prevalence and risk factors for Salmonella and Campylobacter spp. carcass contamination in broiler chickens slaughtered in Québec, Canada. J Food Prot 70: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 10. Agunos A, Léger D, Avery B, Parmley E, Deckert A, et al. (2013) Ciprofloxacin-resistant Campylobacter spp. in retail chicken in western Canada. Emerg Infect Dis 19: 1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Agriculture Organization/World Health Organization (2009) Risk assessment of Campylobacter spp. in broiler chickens: Interpretative summary. Microbiological Risk Assessment Series No 11. Available: http://www.who.int/foodsafety/publications/micro/MRA11_En.pdf. 52 pp. Accessed 22 July 2014.
- 12. Adkin A, Hartnett E, Jordan L, Newell D, Davison H (2006) Use of a systematic review to assist the development of Campylobacter control strategies in broilers. J Appl Microbiol 100: 306–315. [DOI] [PubMed] [Google Scholar]
- 13.European Food Safety Authority (2011) Scientific opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011; 9: :2105 2013. 141 pp. Available: http://www.efsa.europa.eu/en/efsajournal/doc/2105.pdf. Accessed 22 July 2014. [Google Scholar]
- 14. Stone D, Davis M, Baker K, Besser T, Roopnarine R, et al. (2013) MLST genotypes and antibiotic resistance of Campylobacter spp. isolated from poultry in Grenada. Biomed Res Int 2013: 794643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDermott PF, Bodeis SM, English LL, White DG, Walker RD, et al. (2002) Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J Infect Dis 185: 837–840. [DOI] [PubMed] [Google Scholar]
- 16. Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, et al. (2011) Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl Environ Microbiol 77: 8605–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wales AD, Carrique-Mas JJ, Rankin M, Bell B, Thind BB, et al. (2010) Review of the carriage of zoonotic bacteria by arthropods, with special reference to Salmonella in mites, flies and litter beetles. Zoonoses Public Health 57: 299–314. [DOI] [PubMed] [Google Scholar]
- 18. Cox NA, Richardson LJ, Maurer JJ, Berrang ME, Fedorka-Cray PJ, et al. (2012) Evidence for horizontal and vertical transmission in Campylobacter passage from hen to her progeny. J Food Prot 75: 1896–1902. [DOI] [PubMed] [Google Scholar]
- 19. Sargeant JM, Rajic A, Read S, Ohlsson A (2006) The process of systematic review and its application in agri-food public-health. Prev Vet Med 75: 141–151. [DOI] [PubMed] [Google Scholar]
- 20. Young I, Waddell L, Sanchez J, Wilhelm B, McEwen SA, et al. (2014) The application of knowledge synthesis methods in agri-food public health: Recent advancements, challenges and opportunities. Prev Vet Med 113: 339–355. [DOI] [PubMed] [Google Scholar]
- 21. Katsma WE, De Koeijer AA, Jacobs-Reitsma WF, Mangen MJ, Wagenaar JA (2007) Assessing interventions to reduce the risk of Campylobacter prevalence in broilers. Risk Anal 27: 863–876. [DOI] [PubMed] [Google Scholar]
- 22. Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, et al. (2011) Campylobacter control in poultry by current intervention measures ineffective: Urgent need for intensified fundamental research. Vet Microbiol 152: 219–228. [DOI] [PubMed] [Google Scholar]
- 23. Ganan M, Carrascosa AV, de Pascual-Teresa S, Martinez-Rodriguez AJ (2012) Effect of mannoproteins on the growth, gastrointestinal viability, and adherence to caco-2 cells of lactic acid bacteria. J Food Sci 77: M176–80. [DOI] [PubMed] [Google Scholar]
- 24. Eberle KN, Kiess AS (2012) Phenotypic and genotypic methods for typing Campylobacter jejuni and Campylobacter coli in poultry. Poultry Science 91: 255–264. [DOI] [PubMed] [Google Scholar]
- 25. Javed MA, Cawthraw SA, Baig A, Li J, McNally A, et al. (2012) Cj1136 is required for lipooligosaccharide biosynthesis, hyperinvasion, and chick colonization by Campylobacter jejuni . Infect Immun 80: 2361–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Cochrane Collaboration (2014) Cochrane handbook of systematic reviews and interventions. Available: http://handbook.cochrane.org/. Accessed 24 June 2014.
- 27.The GRADE Working Group (2013) GRADE guidelines - best practices using the GRADE framework. Available: http://www.gradeworkinggroup.org/publications/jce_series.htm. Accessed 24 June 2014.
- 28. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, et al. (2011) GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, et al. (2011) GRADE guidelines: 9. rating up the quality of evidence. J Clin Epidemiol 64: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 30. Schunemann H, Hill S, Guyatt G, Akl EA, Ahmed F (2011) The GRADE approach and Bradford Hill's criteria for causation. J Epidemiol Community Health 65: 392–395. [DOI] [PubMed] [Google Scholar]
- 31. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 32. Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315: 629–634 21 November 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahin O, Kobalka P, Zhang Q (2003) Detection and survival of Campylobacter in chicken eggs. J Appl Microbiol 95: 1070–1079. [DOI] [PubMed] [Google Scholar]
- 34. Idris U, Lu J, Maier M, Sanchez S, Hofacre CL, et al. (2006) Dissemination of fluoroquinolone-resistant Campylobacter spp. within an integrated commercial poultry production system. Appl Environ Microbiol 72: 3441–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shanker S, Lee A, Sorrell TC (1986) Campylobacter jejuni in broilers: The role of vertical transmission. J Hyg (Lond) 96: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandeplas S (2010) Prevalence and sources of Campylobacter spp. contamination in free-range broiler production in the southern part of Belgium. Biotechnol Agron Soc Environ 14: 279–288. [Google Scholar]
- 37. Byrd J, Bailey RH, Wills R, Nisbet D (2007) Recovery of Campylobacter from commercial broiler hatchery trayliners. Poult Sci 86: 26–29. [DOI] [PubMed] [Google Scholar]
- 38. Chuma T, Makino K, Okamoto K, Yugi H (1997) Analysis of distribution of Campylobacter jejuni and Campylobacter coli in broilers by using restriction fragment length polymorphism of flagellin gene. J Vet Med Sci 59: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 39. Pokamunski S, Kass N, Borochovich E, Marantz B, Rogol M (1986) Incidence of Campylobacter spp. in broiler flocks monitored from hatching to slaughter. Avian Pathol 15: 83–92. [DOI] [PubMed] [Google Scholar]
- 40. Bouwknegt M, van de Giessen AW, Dam-Deisz WD, Havelaar AH, Nagelkerke NJ, et al. (2004) Risk factors for the presence of Campylobacter spp. in Dutch broiler flocks. Prev Vet Med 62: 35–49. [DOI] [PubMed] [Google Scholar]
- 41. Pearson AD, Greenwood MH, Feltham RK, Healing TD, Donaldson J, et al. (1996) Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: Intermittent common source, vertical transmission, and amplification by flock propagation. Appl Environ Microbiol 62: 4614–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cox NA, Stern NJ, Hiett KL, Berrang ME (2002) Identification of a new source of Campylobacter contamination in poultry: Transmission from breeder hens to broiler chickens. Avian Dis 46: 535–541. [DOI] [PubMed] [Google Scholar]
- 43. Bull SA, Allen VM, Domingue G, Jorgensen F, Frost JA, et al. (2006) Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl Environ Microbiol 72: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messens W, Herman L, De Zutter L, Heyndrickx M (2009) Multiple typing for the epidemiological study of contamination of broilers with thermotolerant Campylobacter . Vet Microbiol 138: 120–131. [DOI] [PubMed] [Google Scholar]
- 45. Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I, et al. (2003) Routes for Campylobacter contamination of poultry meat: Epidemiological study from hatchery to slaughterhouse. Epidemiol Infect 131: 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnsen G, Kruse H, Hofshagen M (2006) Genetic diversity and description of transmission routes for Campylobacter on broiler farms by amplified-fragment length polymorphism. J Appl Microbiol 101: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 47. Hald B, Skovgard H, Bang DD, Pedersen K, Dybdahl J, et al. (2004) Flies and Campylobacter infection of broiler flocks. Emerg Infect Dis 10: 1490–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ridley AM, Morris VK, Cawthraw SA, Ellis-Iversen J, Harris JA, et al. (2011) Longitudinal molecular epidemiological study of thermophilic Campylobacters on one conventional broiler chicken farm. Appl Environ Microbiol 77: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zweifel C, Scheu KD, Keel M, Renggli F, Stephan R (2008) Occurrence and genotypes of Campylobacter in broiler flocks, other farm animals, and the environment during several rearing periods on selected poultry farms. Int J Food Microbiol 125: 182–187. [DOI] [PubMed] [Google Scholar]
- 50. Ellis-Iversen J, Ridley A, Morris V, Sowa A, Harris J, et al. (2012) Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol Infect 140: 916–924 Available: http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8520559&fileId=S095026881100118X. Accessed 21 July 2014. [DOI] [PubMed] [Google Scholar]
- 51. Colles FM, Dingle KE, Cody AJ, Maiden MC (2008) Comparison of Campylobacter populations in wild geese with those in starlings and free-range poultry on the same farm. Appl Environ Microbiol 74: 3583–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patriarchi A, Fox A, Maunsell B, Fanning S, Bolton D (2011) Molecular characterization and environmental mapping of Campylobacter isolates in a subset of intensive poultry flocks in Ireland. Foodborne Pathog Dis 8: 99–108. [DOI] [PubMed] [Google Scholar]
- 53. Hiett K, Stern N, Fedorka-Cray P, Cox N, Musgrove M, et al. (2002) Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl Environ Microbiol 68: 6220–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nesbit EG, Gibbs P, Dreesen DW, Lee MD (2001) Epidemiologic features of Campylobacter jejuni isolated from poultry broiler houses and surrounding environments as determined by use of molecular strain typing. Am J Vet Res 62: 190–194. [DOI] [PubMed] [Google Scholar]
- 55. Hald B, Wedderkopp A, Madsen M (2000) Thermophilic Campylobacter spp. in Danish broiler production: A cross-sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathol 29: 123–131. [DOI] [PubMed] [Google Scholar]
- 56. Huneau-Salaun A, Denis M, Balaine L, Salvat G (2007) Risk factors for Campylobacter spp. colonization in French free-range broiler-chicken flocks at the end of the indoor rearing period. Prev Vet Med 80: 34–48. [DOI] [PubMed] [Google Scholar]
- 57. Hald B, Sommer HM, Skovgard H (2007) Use of fly screens to reduce Campylobacter spp. introduction in broiler houses. Emerg Infect Dis 13: 1951–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cardinale E, Tall F, Gueye EF, Cisse M, Salvat G (2004) Risk factors for Campylobacter spp. infection in Senegalese broiler-chicken flocks. Prev Vet Med 64: 15–25. [DOI] [PubMed] [Google Scholar]
- 59. Refregier-Petton J, Rose N, Denis M, Salvat G (2001) Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev Vet Med 50: 89–100. [DOI] [PubMed] [Google Scholar]
- 60. Lyngstad TM, Jonsson ME, Hofshagen M, Heier BT (2008) Risk factors associated with the presence of Campylobacter species in Norwegian broiler flocks. Poult Sci 87: 1987–1994. [DOI] [PubMed] [Google Scholar]
- 61. Russa AD, Bouma A, Vernooij JC, Jacobs-Reitsma W, Stegeman JA (2005) No association between partial depopulation and Campylobacter spp. colonization of Dutch broiler flocks. Lett Appl Microbiol 41: 280–285. [DOI] [PubMed] [Google Scholar]
- 62. Allen VM, Weaver H, Ridley AM, Harris JA, Sharma M, et al. (2008) Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. J Food Prot 71: 264–270. [DOI] [PubMed] [Google Scholar]
- 63. Ridley A, Morris V, Gittins J, Cawthraw S, Harris J, et al. (2011) Potential sources of Campylobacter infection on chicken farms: Contamination and control of broiler-harvesting equipment, vehicles and personnel. J Appl Microbiol 111: 233–244. [DOI] [PubMed] [Google Scholar]
- 64. Hansson I, Ederoth M, Andersson L, Vagsholm I, Engvall E (2005) Transmission of Campylobacter spp. to chickens during transport to slaughter. J Appl Microbiol 99: 1149–1157. [DOI] [PubMed] [Google Scholar]
- 65. Hansson I, Vagsholm I, Svensson L, Engvall EO (2007) Correlations between Campylobacter spp. prevalence in the environment and broiler flocks. J Appl Microbiol 103: 640–649. [DOI] [PubMed] [Google Scholar]
- 66. Rasschaert G, Houf K, De Zutter L (2007) External contamination of Campylobacter-free flocks after transport in cleaned and disinfected containers. J Food Prot 70: 40–46. [DOI] [PubMed] [Google Scholar]
- 67. Slader J, Domingue G, Jorgensen F, McAlpine K, Owen RJ, et al. (2002) Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Appl Environ Microbiol 68: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hiett KL, Stern NJ, Fedorka-Cray P, Cox NA, Seal BS (2007) Molecular phylogeny of the flaA short variable region among Campylobacter jejuni isolates collected during an annual evaluation of poultry flocks in the south-eastern United States. Foodborne Pathog Dis 4: 339–347. [DOI] [PubMed] [Google Scholar]
- 69. O'Mahony E, Buckley JF, Bolton D, Whyte P, Fanning S (2011) Molecular epidemiology of Campylobacter isolates from poultry production units in southern Ireland. PLoS One 6: e28490 Available: http://www.plosone.org/article/fetchObject.action?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0028490&representation=PDF. Accessed 21 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Allen VM, Ridley AM, Harris JA, Newell DG, Powell L (2011) Influence of production system on the rate of onset of Campylobacter colonization in chicken flocks reared extensively in the United Kingdom. Br Poult Sci 52: 30–39. [DOI] [PubMed] [Google Scholar]
- 71. Rivoal K, Ragimbeau C, Salvat G, Colin P, Ermel G (2005) Genomic diversity of Campylobacter coli and Campylobacter jejuni isolates recovered from free-range broiler farms and comparison with isolates of various origins. Appl Environ Microbiol 71: 6216–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Damjanova I, Jakab M, Farkas T, Meszaros J, Galantai Z, et al. (2011) From farm to fork follow-up of thermotolerant Campylobacters throughout the broiler production chain and in human cases in a Hungarian county during a ten-months period. Int J Food Microbiol 150: 95–102. [DOI] [PubMed] [Google Scholar]
- 73. Shreeve JE, Toszeghy M, Ridley A, Newell DG (2002) The carry-over of Campylobacter isolates between sequential poultry flocks. Avian Dis 46: 378–385. [DOI] [PubMed] [Google Scholar]
- 74. van de Giessen AW, Tilburg JJ, Ritmeester WS, van der Plas J (1998) Reduction of Campylobacter infections in broiler flocks by application of hygiene measures. Epidemiol Infect 121: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Workman SN, Mathison GE, Lavoie MC (2008) An investigation of sources of Campylobacter in a poultry production and packing operation in Barbados. Int J Food Microbiol 121: 106–111. [DOI] [PubMed] [Google Scholar]
- 76. Cokal Y (2011) The presence of Campylobacter jejuni in broiler houses: Results of a longitudinal study. African J Microbiol Res 5: 389–393. [Google Scholar]
- 77. Ogden ID, MacRae M, Johnston M, Strachan NJ, Cody AJ, et al. (2007) Use of multilocus sequence typing to investigate the association between the presence of Campylobacter spp. in broiler drinking water and Campylobacter colonization in broilers. Appl Environ Microbiol 73: 5125–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Snelling WJ, McKenna JP, Lecky DM, Dooley JS (2005) Survival of Campylobacter jejuni in waterborne protozoa. Appl Environ Microbiol 71: 5560–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pearson AD, Greenwood M, Healing TD, Rollins D, Shahamat M, et al. (1993) Colonization of broiler chickens by waterborne Campylobacter jejuni . Appl Environ Microbiol 59: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Skov MN, Spencer AG, Hald B, Petersen L, Nauerby B, et al. (2004) The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks. Avian Dis 48: 9–18. [DOI] [PubMed] [Google Scholar]
- 81. Zadoks RN, Schukken YH (2006) Use of molecular epidemiology in veterinary practice. Vet Clin Food Anim 22: 229–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carrico JA, Pinto FR, Simas C, Nunes S, Sousa NG, et al. (2005) Assessment of band-based similarity coefficients for automatic type and subtype classification of microbial isolates analyzed by pulsed-field gel electrophoresis. J Clin Microbiol 43: 5483–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Borck B, Rosenquist A, Sorensen AIV, Larsen J, Osek J, et al. (2011) Questionnaire survey among broiler producers in six European countries. Camcon Deliverable 1.1.2: 1–48. [Google Scholar]
- 85.United States Environmental Protection Agency (2012) Poultry Production. Available: http://www.epa.gov/oecaagct/ag101/printpoultry.html. Accessed 20 June 2014.
- 86.Proudfoot F, Hamilton R, DeWitt E, Jansen H (1991) Raising chickens and turkey broilers in Canada. Available: http://publications.gc.ca/collections/Collection/A63-1860-1991E.pdf. Accessed 30 June 2014.
- 87.Foreign Affairs and International Trade Canada (2012) Report of the Minister of Foreign Affairs Respecting Operations under the Export and Import Permits Act for the Year 2010. Available: http://www.international.gc.ca/controls-controles/report-rapports/report-rapport-2010.aspx?view=d. Accessed 20 June 2014.
- 88. Callicott KA, Frioriksdottir V, Reiersen J, Lowman R, Bisaillon JR, et al. (2006) Lack of evidence for vertical transmission of Campylobacter spp. in chickens. Appl Environ Microbiol 72: 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pohjanvirta T, Kaukonen E, Ikonen I, Vahanikkila N, Pelkonen S (2013) Extended-spectrum beta-lactamase producing (ESBL) Escherichia coli in Finnish broiler production chain despite low antibiotic usage. Proc XVIIIth WVPA Congress, Nantes, France. 669 p.
- 90.Chicken Farmers of Canada (2013) Chicken Data Booklet. 30 pp. Available: http://www.chickenfarmers.ca/wp-content/uploads/2014/01/Data_Booklet_2013_web.pdf. Accessed 22 July 2014.
- 91. Kazwala RR, Collins JD, Hannan J, Crinion RA, O'Mahony H (1990) Factors responsible for the introduction and spread of Campylobacter jejuni infection in commercial poultry production. Vet Rec 126: 305–306. [PubMed] [Google Scholar]
- 92. Ring M, Zychowska MA, Stephan R (2005) Dynamics of Campylobacter spp. spread investigated in 14 broiler flocks in Switzerland. Avian Dis 49: 390–396. [DOI] [PubMed] [Google Scholar]
- 93.Public Health Agency of Canada (2012) C-EnterNet 2011 Short Report. Available: http://publications.gc.ca/collections/collection_2012/aspc-phac/HP37-8-1-2011-eng.pdf. Accessed 21 July 2014.
- 94. Levesque S, Fournier E, Carrier N, Frost E, Arbeit RD, et al. (2013) Campylobacteriosis in urban versus rural areas: A case-case study integrated with molecular typing to validate risk factors and to attribute sources of infection. PLoS One 8: e83731 Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0083731. Accessed 21 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Levesque S, Frost E, Arbeit RD, Michaud S (2008) Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Québec, Canada. J Clin Microbiol 46: 3404–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Young I, Rajic A, Letellier A, Cox B, Leslie M, et al. (2010) Knowledge and attitudes toward food safety and use of good production practices among Canadian broiler chicken producers. J Food Prot 73: 1278–1287. [DOI] [PubMed] [Google Scholar]
- 97. Knudsen KN, Bang DD, Andresen LO, Madsen M (2006) Campylobacter jejuni strains of human and chicken origin are invasive in chickens after oral challenge. Avian Dis 50: 10–14. [DOI] [PubMed] [Google Scholar]
- 98.Canadian Food Inspection Agency (2009) National Avian On-farm Biosecurity Standard. Available: http://www.inspection.gc.ca/DAM/DAM-animals-animaux/STAGING/text-texte/terr_biosec_avian_standard_1375192173847_eng.pdf. Accessed 21 July 2014.
- 99. Arsenault J, Letellier A, Quessy S, Normand V, Boulianne M (2007) Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Québec, Canada. Prev Vet Med 81: 250–264. [DOI] [PubMed] [Google Scholar]
- 100.Chicken Farmers of Canada (2014) Chicken farmers of Canada On-farm Food Safety Assurance Program Manual-Safe, Safer, Safest 96 pp. Available: http://www.saskatchewanchicken.ca/newsletters-more/manuals.html. Accessed 22 July 2014.
- 101. Racicot M, Venne D, Durivage A, Vaillancourt JP (2011) Description of 44 biosecurity errors while entering and exiting poultry barns based on video surveillance in Québec, Canada. Prev Vet Med 100: 193–199. [DOI] [PubMed] [Google Scholar]
- 102. Racicot M, Venne D, Durivage A, Vaillancourt JP (2012) Evaluation of the relationship between personality traits, experience, education and biosecurity compliance on poultry farms in Québec, Canada. Prev Vet Med 103: 201–207. [DOI] [PubMed] [Google Scholar]
- 103. Racicot M, Venne D, Durivage A, Vaillancourt JP (2012) Evaluation of strategies to enhance biosecurity compliance on poultry farms in Québec: Effect of audits and cameras. Prev Vet Med 103: 208–218. [DOI] [PubMed] [Google Scholar]
- 104. Pope C, Wilson J, Taboada EN, Mackinnon J, Felipe Alves CA, et al. (2007) Epidemiology, relative invasive ability, molecular characterization, and competitive performance of Campylobacter jejuni strains in the chicken gut. Appl Environ Microbiol 73: 7959–7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sheppard SK, Colles FM, McCarthy ND, Strachan NJ, Ogden ID, et al. (2011) Niche segregation and genetic structure of Campylobacter jejuni populations from wild and agricultural host species. Mol Ecol 20: 3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gripp E, Hlahla D, Didelot X, Kops F, Maurischat S, et al. (2011) Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics 12: 584 Available: http://www.biomedcentral.com/1471-2164/12/584. Accessed 22 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ogden ID, Dallas JF, MacRae M, Rotariu O, Reay KW, et al. (2009) Campylobacter excreted into the environment by animal sources: Prevalence, concentration shed, and host association. Foodborne Pathog Dis 6: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Taboada EN, Clark C, Sproston EL, Carrillo CD (2013) Current methods for molecular typing of Campylobacter species. J Microbiol Methods 95: 24–31. [DOI] [PubMed] [Google Scholar]
- 109. McCarthy ND, Colles FM, Dingle KE, Bagnall MC, Manning G, et al. (2007) Host-associated genetic import in Campylobacter jejuni . Emerg Infect Dis 13: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Miller WG, Englen MD, Kathariou S, Wesley IV, Wang G, et al. (2006) Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology 152: 245–255. [DOI] [PubMed] [Google Scholar]
- 111. Hiett KL, Cox NA, Stern NJ (2002) Direct polymerase chain reaction detection of Campylobacter spp. in poultry hatchery samples. Avian Dis 46: 219–223. [DOI] [PubMed] [Google Scholar]
- 112. Chuma T, Yamada T, Yano K, Okamoto K, Yugi H (1994) A survey of Campylobacter jejuni in broilers from assignment to slaughter using DNA-DNA hybridization. J Vet Med Sci 56: 697–700. [DOI] [PubMed] [Google Scholar]
- 113. Chuma T, Yano K, Omori H, Okamoto K, Yugi H (1997) Direct detection of Campylobacter jejuni in chicken cecal contents by PCR. J Vet Med Sci 59: 85–87. [DOI] [PubMed] [Google Scholar]
- 114. Kruger NJ, Buhler C, Iwobi AN, Huber I, Ellerbroek L, et al. (2014) “Limits of control”–crucial parameters for a reliable quantification of viable Campylobacter by real-time PCR. PLoS One 9: e88108 Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0088108. Accessed 21 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jokinen CC, Koot JM, Carrillo CD, Gannon VP, Jardine CM, et al. (2012) An enhanced technique combining pre-enrichment and passive filtration increases the isolation efficiency of Campylobacter jejuni and Campylobacter coli from water and animal fecal samples. J Microbiol Methods 91: 506–513. [DOI] [PubMed] [Google Scholar]
- 116. Steele TW, McDermott SN (1984) The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 16: 263–265. [DOI] [PubMed] [Google Scholar]
- 117. Hiett KL, Cox NA, Rothrock MJ Jr (2013) Polymerase chain reaction detection of naturally occurring Campylobacter in commercial broiler chicken embryos. Poult Sci 92: 1134–1137. [DOI] [PubMed] [Google Scholar]
- 118. Ugarte-Ruiz M, Gomez-Barrero S, Porrero MC, Alvarez J, Garcia M, et al. (2012) Evaluation of four protocols for the detection and isolation of thermophilic Campylobacter from different matrices. J Appl Microbiol 113: 200–208. [DOI] [PubMed] [Google Scholar]
- 119. Ugarte-Ruiz M, Wassenaar TM, Gomez-Barrero S, Porrero MC, Navarro-Gonzalez N, et al. (2013) The effect of different isolation protocols on detection and molecular characterization of Campylobacter from poultry. Lett Appl Microbiol 57: 427–435. [DOI] [PubMed] [Google Scholar]
- 120. Williams LK, Sait LC, Cogan TA, Jorgensen F, Grogono-Thomas R, et al. (2012) Enrichment culture can bias the isolation of Campylobacter subtypes. Epidemiol Infect 140: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 121. Lukinmaa S, Nakari UM, Eklund M, Siitonen A (2004) Application of molecular genetic methods in diagnostics and epidemiology of food-borne bacterial pathogens. APMIS 112: 908–929. [DOI] [PubMed] [Google Scholar]
- 122. Zimmer M, Barnhart H, Idris U, Lee MD (2003) Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis 47: 101–107. [DOI] [PubMed] [Google Scholar]
- 123. McCrea BA, Macklin KS, Norton RA, Hess JB, Bilgili SF (2006) A longitudinal study of Salmonella and Campylobacter jejuni isolates from day of hatch through processing by automated ribotyping. J Food Prot 69: 2908–2914. [DOI] [PubMed] [Google Scholar]
- 124. Clark CG, Taboada E, Grant CC, Blakeston C, Pollari F, et al. (2012) Comparison of molecular typing methods useful for detecting clusters of Campylobacter jejuni and C. coli isolates through routine surveillance. J Clin Microbiol 50: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ahmed R, Soule G, Demczuk WH, Clark C, Khakhria R, et al. (2000) Epidemiologic typing of Salmonella enterica serotype Enteritidis in a Canada-wide outbreak of gastroenteritis due to contaminated cheese. J Clin Microbiol 38: 2403–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Study Protocol. Outlines the protocol for relevance and abstract screenings, data characterization, Risk of Bias (RoB), and GRADE, and; data extraction forms.
(DOCX)
List of the 95 references used in the study.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.