Abstract
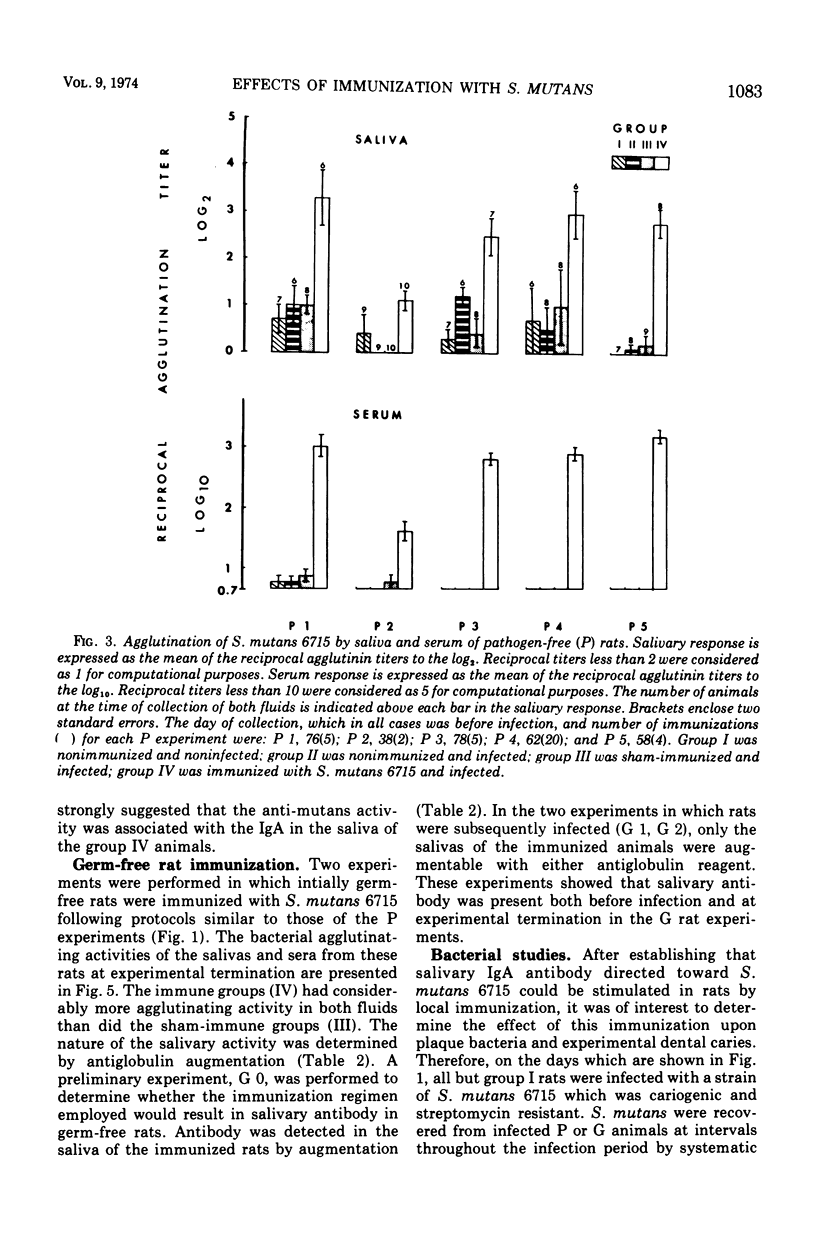
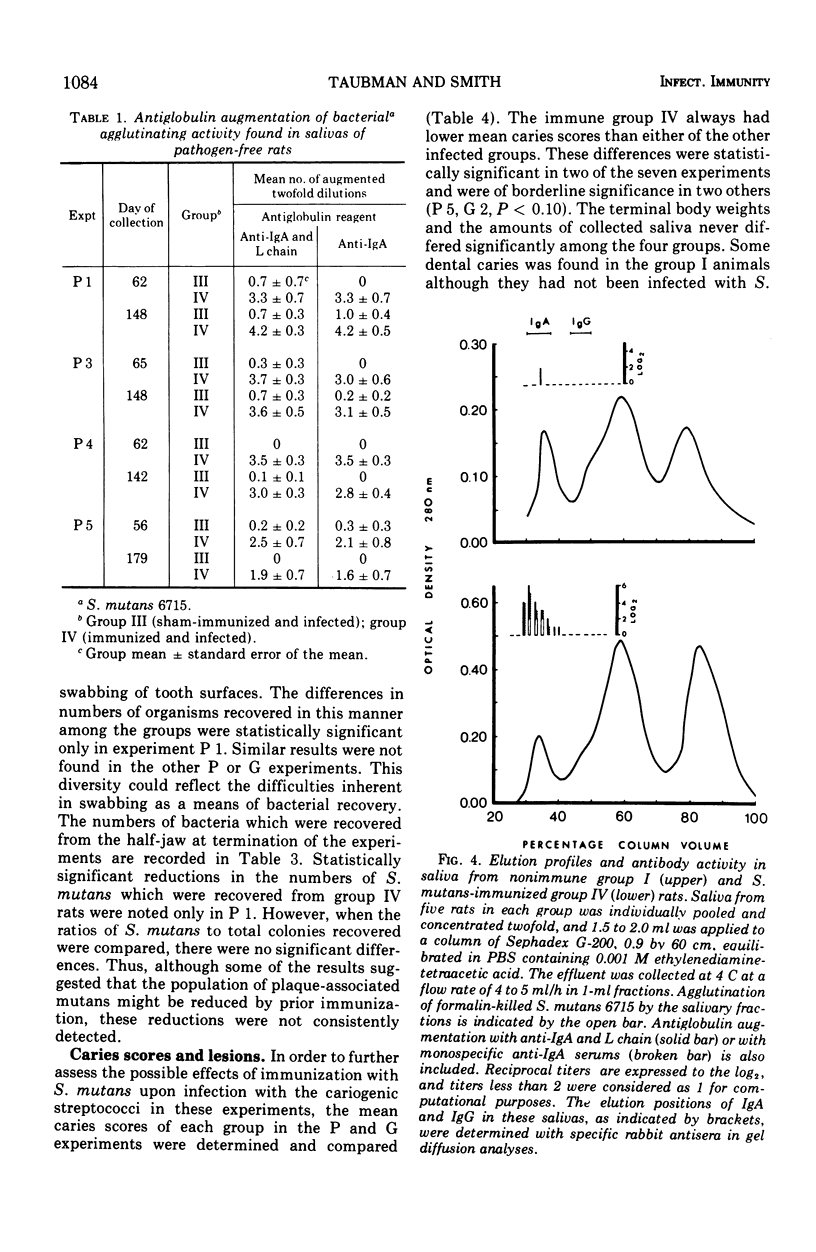
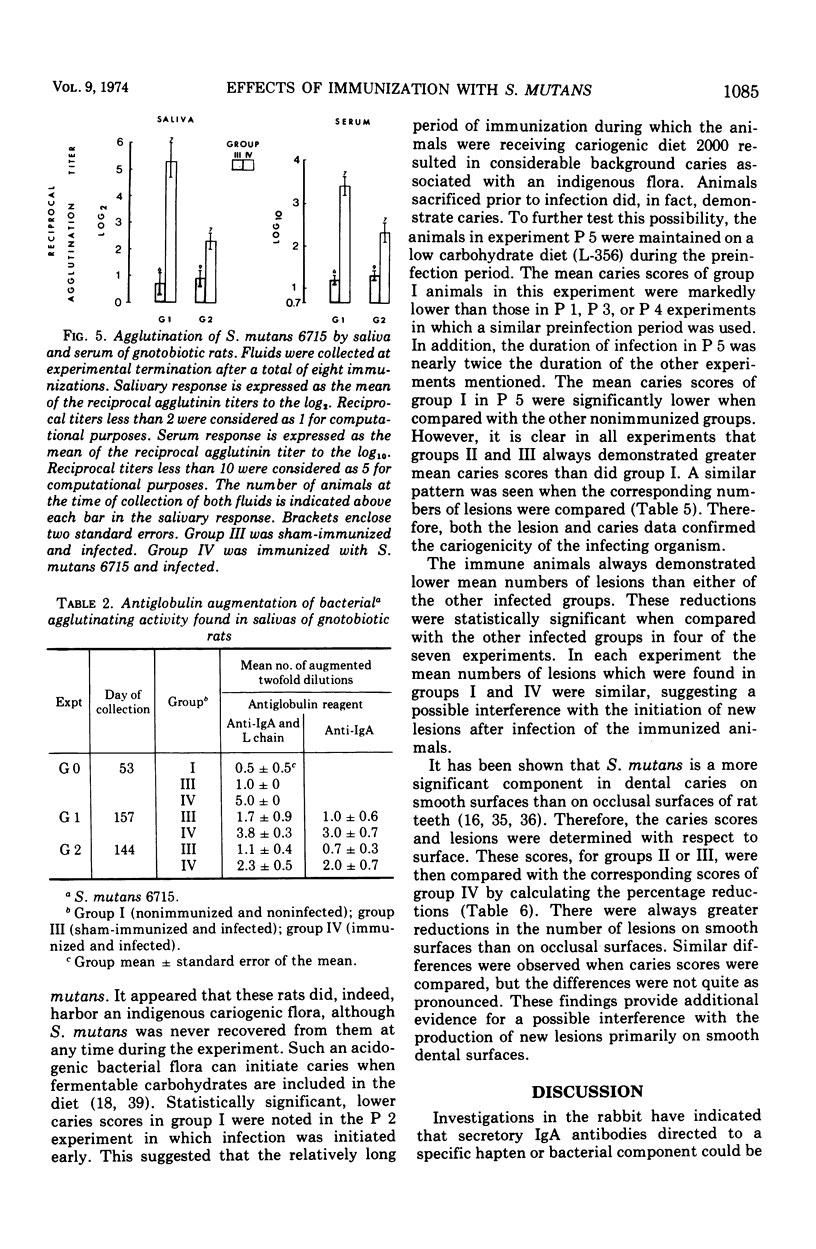
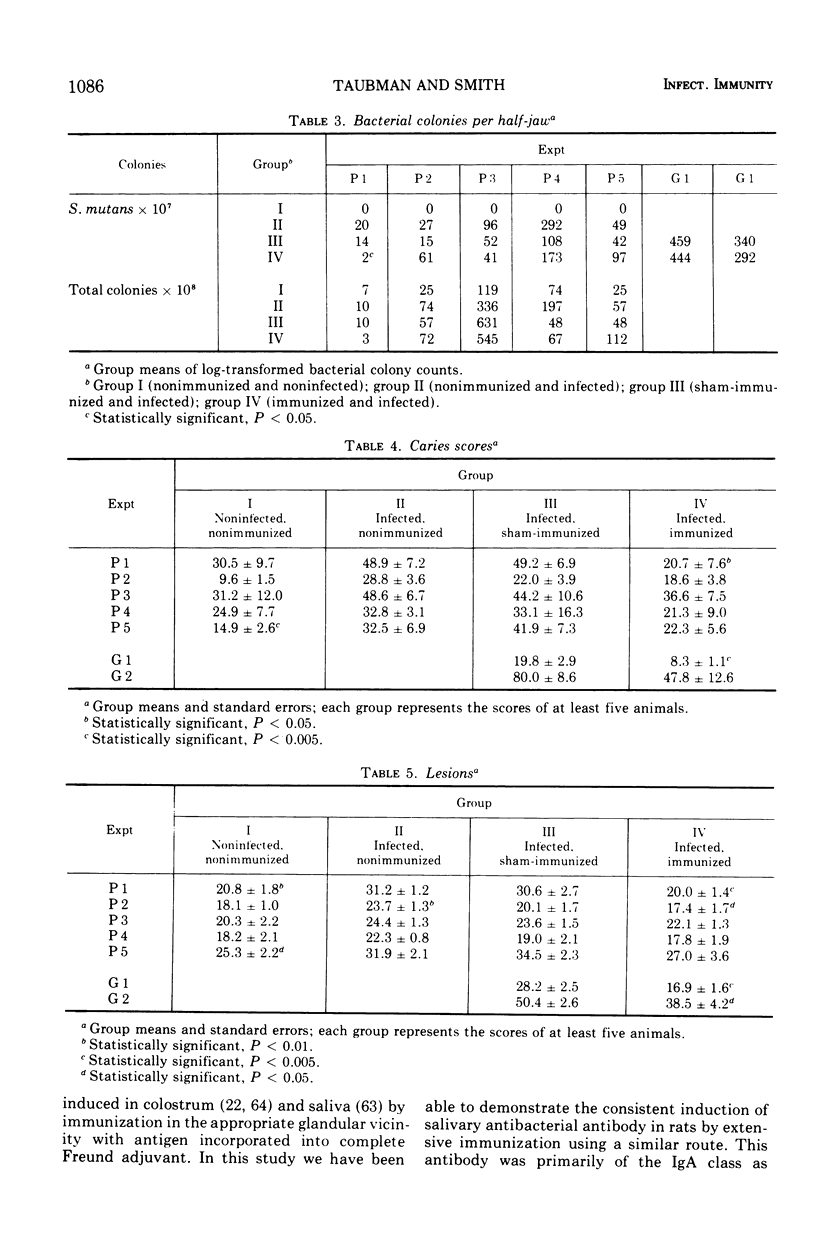
The effect of local immunization with Streptococcus mutans on dental caries in conventional and gnotobiotic rats was studied. Injection of these animals with S. mutans strain 6715 incorporated into complete Freund adjuvant consistently resulted in the presence of antibody in saliva directed to this organism. This antibody was primarily of the immunoglobulin A class as demonstrated by specific antiglobulin augmentation and gel filtration of antibody activity. Serum antibody was also present. Five experiments have been completed in conventional rats and two in gnotobiotic animals. The immunized group of animals always had lower mean caries scores than comparably sham-immunized or nonimmunized control groups. The numbers of lesions were also always lower in the immunized animals, suggesting a possible interference with the formation of new lesions in immunized animals. The reductions in dental caries and lesions were greater on smooth surfaces than on occlusal surfaces. which might be explained as interference with adherence phenomena demonstrated by S. mutans. It is proposed that salivary immunoglobulin A antibody may be viewed as an ecological determinant in the oral cavity by affecting oral microorganisms and possibly their by-products.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altemeier W. A., 3rd, Robbins J. B., Smith R. T. Quantitative studies of the immunoglobulin sequence in the response of the rabbit to a somatic antigen. J Exp Med. 1966 Sep 1;124(3):443–460. doi: 10.1084/jem.124.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Balish E., Yale C. E., Hong R. Serum proteins of gnotobiotic rats. Infect Immun. 1972 Aug;6(2):112–118. doi: 10.1128/iai.6.2.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanti J. A., Buescher E. L., Brandt W. E., Dangerfield H. G., Crozier D. Characterization of human serum and nasal hemagglutinating antibody to Francisella tularensis. J Immunol. 1967 Jan;98(1):171–178. [PubMed] [Google Scholar]
- Bistany T. S., Tomasi T. B., Jr Serum and secretory immunoglobulins of the rat. Immunochemistry. 1970 May;7(5):453–460. doi: 10.1016/0019-2791(70)90227-2. [DOI] [PubMed] [Google Scholar]
- Bowen W. H. A vaccine against dental caries. A pilot experiment in monkeys (Macaca irus). Br Dent J. 1969 Feb 18;126(4):159–160. [PubMed] [Google Scholar]
- Bowen W. H. The induction of rampant dental caries in monkeys (Macaca irus). Caries Res. 1969;3(3):227–237. doi: 10.1159/000259597. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Fjellanger I., Gjeruldsen S. T. Human secretory immunoglobulins. I. Salivary secretions from individuals with normal or low levels of serum immunoglobulins. Scand J Haematol Suppl. 1970;12:3–83. [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Buscho R. F., Perkins J. C., Knopf H. L., Kapikian A. Z., Chanock R. M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J Immunol. 1972 Jan;108(1):169–177. [PubMed] [Google Scholar]
- CHODIRKER W. B., TOMASI T. B., Jr GAMMA-GLOBULINS: QUANTITATIVE RELATIONSHIPS IN HUMAN SERUM AND NONVASCULAR FLUIDS. Science. 1963 Nov 22;142(3595):1080–1081. doi: 10.1126/science.142.3595.1080. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Krasse B. Inhibition of streptococcal dextransucrase by sera of rabbits infected with Streptococcus sanguis. Arch Oral Biol. 1968 Jul;13(7):849–852. doi: 10.1016/0003-9969(68)90109-x. [DOI] [PubMed] [Google Scholar]
- Challacombe S. J., Guggenheim B., Lehner T. Antibodies to an extract of Streptococcus mutans, containing glucosyltransferase activity, related to dental caries in man. Arch Oral Biol. 1973 Jun;18(6):657–668. doi: 10.1016/0003-9969(73)90001-0. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Frostell G., Keyes P. H., Larson R. H. Effect of various sugars and sugar substitutes on dental caries in hamsters and rats. J Nutr. 1967 Sep;93(1):65–76. doi: 10.1093/jn/93.1.65. [DOI] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Fukui Y., Fukui K., Moriyama T. Inhibition of enzymes by human salivary immunoglobulin A. Infect Immun. 1973 Sep;8(3):335–340. doi: 10.1128/iai.8.3.335-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J., Taubman M. A. Secretory gamma-A antibodies induced by local immunization. Nature. 1969 Feb 15;221(5181):679–681. doi: 10.1038/221679a0. [DOI] [PubMed] [Google Scholar]
- Gerbrandy J. L., van Dura E. A. Anamnestic secretory antibody response in respiratory secretions of intranasally immunized mice. J Immunol. 1972 Nov;109(5):1146–1148. [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Hayashi J. A., Shklair I. L., Bahn A. N. Immunization with dextransucrases and glycosidic hydrolases. J Dent Res. 1972 Mar-Apr;51(2):436–442. doi: 10.1177/00220345720510023201. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J. Prevalence of Streptococcus mutans on various tooth surfaces in Negro children. Arch Oral Biol. 1971 Oct;16(10):1237–1240. doi: 10.1016/0003-9969(71)90053-7. [DOI] [PubMed] [Google Scholar]
- KEYES P. H., JORDAN H. V. PERIODONTAL LESIONS IN THE SYRIAN HAMSTER. III. FINDINGS RELATED TO AN INFECTIOUS AND TRANSMISSIBLE COMPONENT. Arch Oral Biol. 1964 Jul-Aug;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- KEYES P. H. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960 Mar;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- KRASSE B. THE EFFECT OF THE DIET ON THE IMPLANTATION OF CARIES-INDUCING STREPTOCOCCI IN HAMSTERS. Arch Oral Biol. 1965 Mar-Apr;10:215–221. doi: 10.1016/0003-9969(65)90022-1. [DOI] [PubMed] [Google Scholar]
- Krasse B., Edwardsson S., Svensson I., Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967 Feb;12(2):231–236. doi: 10.1016/0003-9969(67)90042-8. [DOI] [PubMed] [Google Scholar]
- König K. G., Guggenheim B. Implantation of antibiotic-resistant bacteria and the production of dental caries in rats. Adv Oral Biol. 1968;3:217–252. doi: 10.1016/b978-1-4832-3119-8.50014-7. [DOI] [PubMed] [Google Scholar]
- König K. G., Larson R. H., Guggenheim B. A strain-specific eating pattern as a factor limiting the transmissibility of caries activity in rats. Arch Oral Biol. 1969 Jan;14(1):91–103. doi: 10.1016/0003-9969(69)90024-7. [DOI] [PubMed] [Google Scholar]
- LARSON R. H., RUBIN M., ZIPKIN I. Frequency of eating as a factor in experimental dental caries. Arch Oral Biol. 1962 Jul-Aug;7:463–468. doi: 10.1016/0003-9969(62)90134-6. [DOI] [PubMed] [Google Scholar]
- Larje O., Larson R. H. Reduction of dental caries in rats by intermittent feeding with sucrose substitutes. Arch Oral Biol. 1970 Sep;15(9):805–816. doi: 10.1016/0003-9969(70)90153-6. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Carbonara A. O., Heremans J. F. Studies on the proteins of human saliva. Biochim Biophys Acta. 1965 Oct 18;107(3):485–500. doi: 10.1016/0304-4165(65)90192-3. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLAND F. J., BLAYNEY J. R., HARRISON R. W., REYNIERS J. A., TREXLER P. C., ERVIN R. F., GORDON H. A., WAGNER M. Experimental caries in germfree rats inoculated with enterococci. J Am Dent Assoc. 1955 Mar;50(3):259–272. doi: 10.14219/jada.archive.1955.0061. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Poliovirus antibody response in serum and nasal secretions following intranasal inoculation with inactivated poliovaccine. J Immunol. 1969 Jan;102(1):15–23. [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. B., Wostmann B. S. Cellular and humoral immune response of germfree mice stimulated with 7S HGG or Salmonella typhimurium. J Immunol. 1966 Aug;97(2):275–286. [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knope H. L., Wenzel R. P., Hornick R. B., Kapikian A. Z., Chanock R. M. Evidence for protective effect of an inactivated rhinovirus vaccine administered by the nasal route. Am J Epidemiol. 1969 Oct;90(4):319–326. doi: 10.1093/oxfordjournals.aje.a121076. [DOI] [PubMed] [Google Scholar]
- Pollard M., Sharon N. Responses of the Peyer's Patches in Germ-Free Mice to Antigenic Stimulation. Infect Immun. 1970 Jul;2(1):96–100. doi: 10.1128/iai.2.1.96-100.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Krichevsky M. I. Acid production from glucose and sucrose by growing cultures of caries-conducive streptococci. J Dent Res. 1972 May-Jun;51(3):734–739. doi: 10.1177/00220345720510030801. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., MACDONALD J. B., SAWYER S. J., AUSKAPS A. M. Quantitative studies of the bacterial flora of the periodontium in rice rats. Arch Oral Biol. 1960 Jul;2:104–110. doi: 10.1016/0003-9969(60)90058-3. [DOI] [PubMed] [Google Scholar]
- Shaw J. H. An evaluation in rats of the relationship between the frequency of providing food and the caries-producing ability of diets. Arch Oral Biol. 1968 Aug;13(8):1003–1013. doi: 10.1016/0003-9969(68)90015-0. [DOI] [PubMed] [Google Scholar]
- Shivers C. A., James J. M. Specific antibodies produced against antigens of agar-gel precipitates. Immunology. 1967 Dec;13(6):547–554. [PMC free article] [PubMed] [Google Scholar]
- Skelly B. J., Celozzi E., Boeninghaus J. Neonatal thymectomy in gnotobiotic CDF rats. Immunology. 1968 Oct;15(4):497–501. [PMC free article] [PubMed] [Google Scholar]
- Stechschulte D. J., Austen K. F. Immunoglobulins of rat colostrum. J Immunol. 1970 May;104(5):1052–1062. [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Hageage G. J., Jr, Larson R. H. Variable experiences in immunization of rats against Streptococcus mutans-associated dental caries. Arch Oral Biol. 1973 Nov;18(11):1425–1439. doi: 10.1016/0003-9969(73)90117-9. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Genco R. J. Induction and properties of rabbit secretory A antibody directed to group A streptococcal carbohydrate. Immunochemistry. 1971 Dec;8(12):1137–1155. doi: 10.1016/0019-2791(71)90392-2. [DOI] [PubMed] [Google Scholar]
- Tourville D., Bienenstock J., Tomasi T. B., Jr Natural antibodies of human serum, saliva, and urine reactive with Escherichia coli. Proc Soc Exp Biol Med. 1968 Jul;128(3):722–727. doi: 10.3181/00379727-128-33109. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Critchley P. The extracellular polysaccharide produced from sucrose by a cariogenic streptococcus. Arch Oral Biol. 1966 Oct;11(10):1039–1042. doi: 10.1016/0003-9969(66)90204-4. [DOI] [PubMed] [Google Scholar]


