Abstract
Background
Oxidative stress in atherosclerosis produces H2O2 and triggers the activation of nuclear factor kappa beta (NF-κB) and increase of inducible nitric oxide synthase (iNOS). The formation of vasa vasorum occurs in atherosclerosis. Vasa vasorum angiogenesis is mediated by VEGFR-1 and upregulated by hypoxia-inducible factor-1α (HIF-1α). The newly formed vasa vasorum are fragile and immature and thus increase plaque instability. It is necessary to control vasa vasorum angiogenesis by using mangosteen pericarp antioxidant. This study aims to demonstrate that mangosteen pericarp ethanolic extract can act as vasa vasorum anti-angiogenesis through H2O2, HIF-1α, NF-κB, and iNOS inhibition in rats given a hypercholesterol diet.
Methods
This was a true experimental laboratory, in vivo posttest with control group design, with 20 Rattus norvegicus Wistar strain rats divided into five groups (normal group, hypercholesterol group, and hypercholesterol groups with certain doses of mangosteen pericarp ethanolic extract: 200, 400, and 800 mg/kg body weight). The parameters of this study were H2O2 measured by using colorimetric analysis, as well as NF-κB, iNOS, and HIF-1α, which were measured by using immunofluorescence double staining and observed with a confocal laser scanning microscope in aortic smooth muscle cell. The angiogenesis of vasa vasorum was quantified from VEGFR-1 level in aortic tissue and confirmed with hematoxylin and eosin staining.
Results
Analysis of variance test and Pearson’s correlation coefficient showed mangosteen pericarp ethanolic extract had a significant effect (P<0.05) in decreasing vasa vasorum angiogenesis through H2O2, HIF-1α, NF-κB, and iNOS inhibition in hypercholesterol-diet-given R. norvegicus Wistar strain.
Conclusion
Mangosteen pericarp ethanolic extract 800 mg/kg body weight is proven to decrease vasa vasorum angiogenesis. Similar studies with other inflammatory parameters are encouraged to clarify the mechanism of vasa vasorum angiogenesis inhibition by mangosteen pericarp ethanolic extract.
Keywords: mangosteen pericarp ethanolic extract, H2O2, HIF-1α, NF-κB, vasa vasorum angiogenesis, hypercholesterol
Introduction
Mortality from coronary heart disease (CHD) caused by atherosclerosis is increased sharply in both industrial countries and developing countries.1 Prevalence of atherosclerotic CHD has been increasing yearly and has been declared by the World Health Organization (WHO) as a global threat.2 It is estimated that 1.9 billion people, or one-third of the world’s population, are affected by this disease.2 In Indonesia, morbidity and mortality from CHD are likely to increase and the mortality rate has reached 50%.2
Risk factors for atherosclerosis include dyslipidemia, free radicals, endothelial dysfunction, and inflammation.3 Increased levels of cholesterol in blood, especially low-density lipoprotein (LDL) cholesterol, is harmful because of the peroxidation process (auto-oxidation) of lipids which are exposed to oxygen. Lipid peroxidation is a chain reaction that continues to produce reactive oxygen species (ROS) such as OH−, RO, and hydrogen peroxide (H2O2)4. H2O2 causes nuclear factor kappa beta (NF-κB) activation. NF-κB is a transcription factor that plays an important role in controlling various biological effects such as inflammation, immune defense mechanism, cell proliferation and differentiation, tumorigenesis, and cell apoptosis.5 NF-κB plays an important role in atherosclerosis because of the chronic inflammatory process that occurs throughout atherosclerosis.6
NF-κB activation products initiate the process of atherosclerosis with endothelial dysfunction, occurrence of platelet adhesion, and the migration and proliferation of smooth muscle cells.7 Inducible nitric oxide synthase (iNOS) expression is increased in macrophages and smooth muscle cells at the early and advanced stages of atherosclerotic lesions. Inflammation plays a crucial role in the development of atherosclerotic lesions affecting coronary arteries. iNOS plays an important role in inflammation through the fast and high production of prostanoid and NO, both of which have proatherosclerotic effects.8
Active metabolism of cells happens in inflammation. The metabolism of active cells enormously increases the demand for oxygen and the reduction of oxygen supply, finally resulting in hypoxia.9 The rise of hypoxia that takes place in vessel walls is also caused by the thickening of arterial walls in atherosclerosis, which disturbs the diffusion of oxygen.10 Hypoxia is strongly associated with angiogenesis and formation of thrombus. The effect of hypoxia is mediated by macrophage, which infiltrate the thrombus. A macrophage is a cell that has the ability to immediately alter not only anaerobic metabolism but also aerobic metabolism (glycolytic turnover). This elevates the oxygen demand.11 Hypoxia promotes the adaptation of cells through the activation of hypoxia-inducible factor-1α (HIF-1α).
HIF-1α is a transcription factor that regulates glycolysis, angiogenesis, and cell survival.12 HIF-1α in normal oxygen conditions is unstable and cannot be detected due to hydroxylation. In hypoxic conditions, HIF-1α is stable and undergoes translocation from cytoplasm to nucleus and binds to specific promoter hypoxia response element sites that regulate classics genes for responsiveness to hypoxia.11
The possible role of HIF-1α in atherosclerosis is supported by the presence of intraplaque angiogenesis, which is an implication of some HIF-responsive genes in atherosclerosis, such as vascular endothelial growth factor (VEGF), endothelin-1, and matrix-metalloproteinase-2.13–15 VEGF binds to tyrosine kinase receptors and becomes VEGF receptor-1 (VEGFR-1), which is also known as Flt (fms-like tyrosine kinase-1). VEGFR-1 is expressed in early vascular development and postnatal angiogenesis. VEGFR-1 is also expressed in inflammatory cells such as monocytes and macrophages, which coordinate inflammation and become an early marker of pathological angiogenesis associated with atherosclerosis.12 Research conducted by Sluimer et al16 in 2008 suggested that hypoxia is strongly associated with angiogenesis and thrombus formation. Atherosclerosis shows the process of vasa vasorum angiogenesis to be extensive, fragile, and immature, and associated with growth and instability of atherosclerotic plaque.17,18 The vasa vasorum become interesting to discuss for their unique function, because the vasa vasorum are vessels supplying blood to the vessels, or in other words, vessels located inside vessels.19
Collection of evidence related to plaque vasa vasorum angiogenesis and its contribution to the progressivity of atherosclerosis and development of lesion instability demonstrates the importance of controlling angiogenesis through anti-angiogenic agents.20 Because of ROS involvement from the beginning of pathogenicity of atherosclerosis, when the vasa vasorum angiogenesis mechanism is involved, the use of antioxidant as an anti-angiogenic agent is an essential consideration.
Mangosteen pericarp extract (Garcinia mangostana Linn) is one potential antioxidant agent. Bioactive content of the skin of mangosteen has anti-inflammatory, antioxidant, and antihistamine effects, as well as other pharmacological activities. Some of the major compounds in mangosteen skin that are reported are xanthones.21 Mangosteen pericarp extract is proven to inhibit NF-κB activation in rat models with administration of a hypercholesterol diet.22 Mangosteen pericarp extract possess high antioxidant activity that inhibits cellular damage caused by ROS, so NF-κB remains in an inactive state in cytoplasm. Research to prove whether or not mangosteen pericarp ethanolic extract (MPEE) may prevent vasa vasorum angiogenesis through the inhibition of H2O2, HIF-1α, NF-κB, and iNOS expressions in Wistar strain Rattus norvegicus rats with hypercholesterol diet administration has not yet been undertaken. The purpose of this study is to prove the anti-angiogenic vasa vasorum effect of mangosteen pericarp extract through the inhibition of H2O2, HIF-1α, NF-κB, and iNOS in Wistar strain R. norvegicus rats administered a hypercholesterol diet.
Methods
Study group
Twenty male R. norvegicus Wistar strain rats, 8 weeks old, with 150–200 g body weight, were obtained from the Pharmacology Laboratory of Faculty of Medicine, Brawijaya University, Malang, Indonesia. These rats were divided into five groups: negative control group (normal diet group), positive control group (hypercholesterol-diet-given group), and groups with both hypercholesterol diet and administration of treated MPEE at a variety of doses: 200, 400, and 800 mg/kg body weight (BW). The extraction process took place in the Central Laboratory of Pharmacology, Faculty of Medicine, Brawijaya University. Mangosteen pericarp was extracted using ethanol solution and given to the rat models by sonde every day. Hypercholesterol diet in this study was a common food for the rat models with addition of 2% cholesterol, 0.2% cholic acid, and 5% lard, which was given at 30 g daily ad libitum for 3 months, obtained from the Pharmacology Laboratory of Faculty of Medicine, Brawijaya University. The measurement of parameters of this study was conducted at the Biomedical Laboratory and Central Laboratory of Biological Sciences, Brawijaya University after obtaining ethical clearance assessment by the Health Research Ethics Committee with this given number: 054 A/EC/KEPK/02/2013.
Biochemical tests
H2O2 measurement
H2O2 levels were measured in rat plasma using a Colorimetric Hydrogen Peroxide Kit (Assay Design) (Abcam®, Cambridge, UK) and observed at 570 nm with an enzyme-linked immunosorbent assay (ELISA) reader (Life Sciences Advanced Technologies, Inc., St Petersburg, FL, USA).
HIF-1α, NF-κB, and iNOS measurement
HIF-1α, NF-κB, and iNOS were measured by immunofluorescence of aortic tissues that were previously fixed with PHEMO buffer (68 mM PIPES, 25 mM, HEPES, pH 6.9, 15 mM EGTA, 3 mM MgCl2, 10% [v/v] dimethyl sulfoxide containing 3.7% formaldehyde and 0.05% glutaraldehyde) and were processed by imumunofluoresence double labeling with anti-rat antibody HIF-1α using rhodamine secondary antibody and anti-rat antibody NF-κB using fluorescein isothiocyanate secondary antibody (BIOS Inc., Boston, MA, USA). iNOS in smooth muscle cell derived from anti-rat iNOS antibody was colored by fluorescein isothiocyanate (FITC) and α-actin was colored by rhodamine secondary antibody (BIOS Inc.). These three parameters were observed with confocal laser scanning microscopy (Olympus Corporation, Tokyo, Japan) and were quantitatively analyzed using Olympus FluoView software (version 1.7A; Olympus Corporation).
Angiogenesis vasa vasorum measurement
Vasa vasorum angiogenesis measurement was done by measuring levels of aortic VEGFR-1 by ELISA (Abcam). Histopathological description of vasa vasorum was observed by hematoxylin and eosin staining and microscope BX 53 (Olympus Corporation) at 600× magnification. The amount of vasa vasorum was identified from the characteristic of aortic lumen which contains erythrocyte.
Statistical analysis
This study used analysis of variance (ANOVA) test to determine the effect of MPEE on the reduction of VEGFR-1, H2O2, HIF-1α, NF-κB, and iNOS in Wistar strain R. norvegicus rats with hypercholesterol administration. Analysis was continued with post hoc test with Duncan method to detect the differences of parameters among treatment groups. SPSS software (v 20; IBM Corporation, Armonk, NY, USA) was used for data analysis.
Results
Levels of VEGFR-1 in the various treatment groups ranged from 10.650 to 18.239 pg/mL. The negative control group had lower levels of VEGFR-1 (10.650–15.440 pg/mL), while the hypercholesterol groups treated with various doses of MPEE had VEGFR-1 levels from 15.728–18.239 pg/mL. The formation of VEGFR-1 is inhibited by the increased doses of MPEE. ANOVA test with 95% confidence interval showed that the addition of MPEE had a significant effect (P=0.04) on the reduction of VEGFR-1 levels. Post hoc Duncan test (pay attention to a, b, c notations in the column) showed a significant difference in VEGFR-1 levels between the hypercholesterol group without MPEE, the normal diet group, and the hypercholesterol group that received MPEE at the 800 mg/kg BW dose.
The counted number of vasa vasorum ranged from one to 23. In the negative control group, the amount of vasa vasorum was lower than in the hypercholesterol group (±2–6), while the hypercholesterol groups with MPEE had an estimated number of vasa vasorum from one to 23. Vasa vasorum, descriptively, are less likely to form with increasing amounts of MPEE. ANOVA test with 95% confidence interval showed that the addition of MPEE played a significant role (P=0.00) in decreasing the amount of vasa vasorum. Post hoc Duncan test showed a significant difference in vasa vasorum number between the hypercholesterol group without MPEE, the normal diet group, and the hypercholesterol group that received MPEE at the 800 mg/kg BW dose.
H2O2 levels in the various treatment groups ranged from 0.132 to 1.968 pg/mL. In the negative control group, the H2O2 level was lower compared to the hypercholesterol groups with various doses of MPEE (0.132–0.237 pg/mL compared to 0.214–1.968 pg/mL). H2O2 is more difficult to form with the increased doses of MPEE. ANOVA test with 95% confidence interval showed that the addition of MPEE played a significant role (P=0.01) in decreasing the levels of H2O2. Post hoc Duncan test showed a significant difference of H2O2 levels between the hypercholesterol group without MPEE, the normal diet group, the hypercholesterol group that received MPEE at the 400 mg/kg BW dose, and the hypercholesterol group that received MPEE at the 800 mg/kg BW dose.
HIF1-α expression in the various treatment groups ranged from 508.20 to 1,555.59 arbitrary unit (au) can be seen in Figure 1. In the negative control group, HIF1-α expression was lower (612.98–1,298.87 au), whereas the hypercholesterol groups given MPEE at various doses had a range of HIF1-α expression from 508.20–1,555.59 au. HIF1-α, descriptively, are less likely to form with higher doses of MPEE. ANOVA test with 95% confidence interval showed that the addition of MPEE played a significant role (P=0.01) in decreasing HIF1-α expression. Post hoc Duncan test showed a significant difference in HIF1-α expressions between the hypercholesterol group without MPEE, the normal diet group, the hypercholesterol group that received MPEE at the 400 mg/kg BW dose, and the hypercholesterol group that received MPEE at the 800 mg/kg BW dose.
Figure 1.
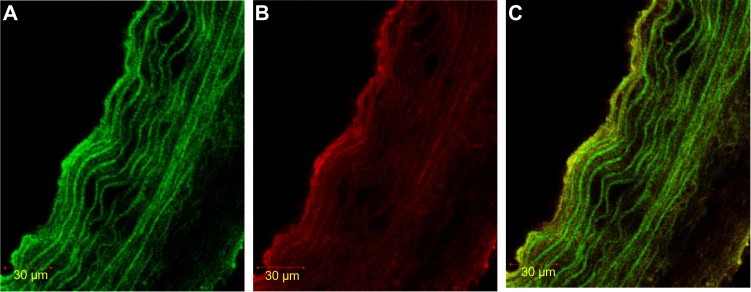
Immunofluorescence staining of HIF-1α in SMC, labeled with α-actin.
Notes: (A) α-actin with FITC. The color green shows the expression of α-actin (marker SMC). (B) HIF-1α rhodamine. Red shows HIF-1α expression. (C) Double stained α-actin FITC – HIF-1α. Yellow shows double staining of HIF-1α expression in SMC using. rhodamine.
Abbreviations: FITC, fluorescein isothiocyanate; HIF-1α, hypoxia-inducible factor-1α; SMC, smooth muscle cell.
The expression of NF-κB in the different treatment groups ranged from 1,132.32 to 2,146.59 au can be seen in Figure 2. The negative control group had lower expression of NF-κB (1,364.47–2,146.59 au), whereas the expression of NF-κB in groups with hypercholesterol and MPEE administration at various doses ranged from 1,132.32 to 1,982.18 au. NF-κB, descriptively, are less likely to form with increasing doses of MPEE. ANOVA test with 95% confidence interval showed that the higher doses of MPEE had a significant effect (P=0.01) on the reduction of NF-κB expression. Post hoc Duncan test showed a significant difference of NF-κB expression between the hypercholesterol group without MPEE, the normal diet group, the hypercholesterol group that received MPEE at the 400 mg/kg BW dose, and the hypercholesterol group that received MPEE at the 800 mg/kg BW dose.
Figure 2.
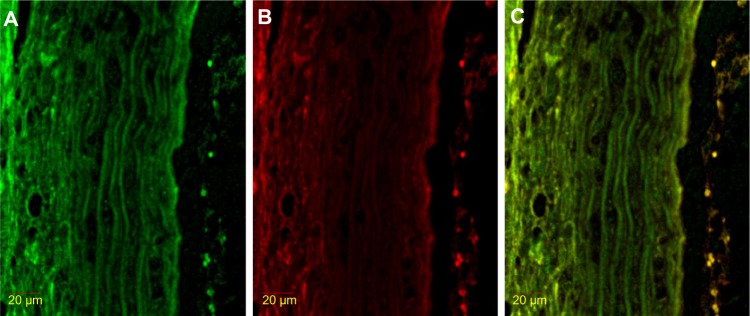
Immunofluorescence result of double stained NF-κB with HIF-1α.
Notes: (A) NF-κB FITC. The color green shows NF-κB expression. (B) HIF-1α rhodamine. Red shows HIF-1α expression. (C) Double staining NF-κB FITC – HIF-1α rhodamine. Yellow shows double staining expression of NF-κB and HIF-1α inside the nucleus.
Abbreviations: FITC, fluorescein isothiocyanate; HIF-1α, hypoxia-inducible factor-1α; NF-κB, nuclear factor kappa beta.
iNOS expression in the various treatment groups ranged from 309.85 to 1,458.66 au can be seen in Figure 3. In the negative control group, iNOS expression was lower (736.25–987.89 au) than that in the hypercholesterol groups with the addition of various doses of MPEE (309.85–1,458.66 au). iNOS, descriptively, are less likely to form with higher doses of MPEE. ANOVA test with 95% confidence interval showed that the addition of MPEE played a significant role (P=0.01) in decreasing iNOS expression. Post hoc Duncan test showed a significant difference in iNOS expressions between the hypercholesterol group without MPEE, the normal diet group, the hypercholesterol group that received MPEE at the 400 mg/kg BW dose, and the hypercholesterol group that received MPEE at the 800 mg/kg BW dose.
Figure 3.
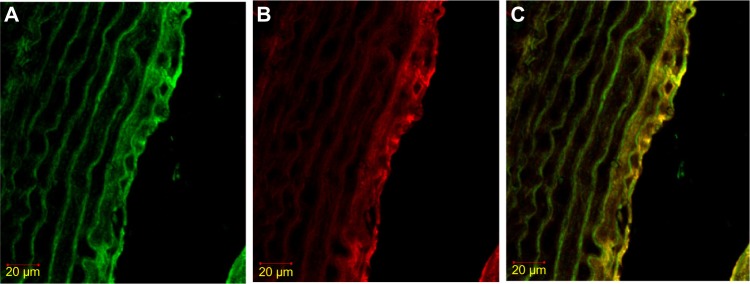
Immunofluorescence staining result of iNOS in SMC, labeled with α-actin.
Notes: (A) α-actin FITC. The color green shows α-actin expression (SMC marker). (B) iNOS rhodamine. Red shows iNOS expression. (C) Double stained α-actin with FITC-and iNOS with rhodamine. Yellow shows double staining expression of iNOS in SMC.
Abbreviations: FITC, fluorescein isothiocyanate; iNOS, inducible nitric oxide synthase; SMC, smooth muscle cell.
Discussion
Vasa vasorum angiogenesis that occurs in Wistar strain R. norvegicus that are given a hypercholesterol diet is closely related to vascular oxidative stress. Oxidative stress is a condition in which there is an imbalance between oxidant and antioxidant. The increase of oxidative stress on arterial walls contributes to atherogenesis. Post hoc Duncan test showed a significant difference in VEGFR-1 levels and number of vasa vasorum between normal and hypercholesterol diet groups. This result of Post Hoc Test reveals that angiogenesis involved in the increased level of H2O2, HIF-1α, NF-κB, and iNOS levels in hypercholesterol groups.
A certain amount of antioxidant is required to maintain the stability between oxidative stress and antioxidant to minimize cellular damage. A variety of antioxidant substances may prevent or significantly inhibit the oxidation process. Antioxidants are substances that become effective against oxidative damage.23 This study used MPEE that contains antioxidant which plays a role in vasa vasorum anti-angiogenesis.
Based on data shown in this study, only a small amount of vasa vasorum can be found not only in hypercholesterol groups with 200, 400, and 800 mg/kg BW of MPEE, but also in the normal diet group. In the hypercholesterol group without administration of MPEE, many vasa vasorum were formed (Figure 4). ANOVA analysis affirms that MPEE administration can significantly decrease vasa vasorum angiogenesis in hypercholesterol Wistar strain R. norvegicus. Inhibition of VEGFR-1 levels by MPEE is followed by a decrease in the number of formations of vasa vasorum in hypercholesterol Wistar strain R. norvegicus. MPEE contains a variety of antioxidants, some of which are xanthones such as alpha, beta, and gamma mangosteen. The antioxidant component of mangostin from mangosteen pericarp has been proven to play a role in lipid peroxidation by donating hydrogen atoms and effectively inhibiting activity of lipid radicals.24 Data from this study assert that significant differences between VEGFR-1 levels can be explained by the dose of MPEE administered. The negative effect of MPEE doses on VEGFR-1 levels enables higher doses of MPEE to strongly reduce VEGFR-1 levels. Post hoc Duncan test revealed that there was no significant difference in VEGFR-1 levels between the hypercholesterol groups with 200, 400, and 800 mg/kg BW of MPEE and the normal diet group. The same can be seen in the amount of vasa vasorum; thus it can be stated that an 800 mg/kg BW dose of MPEE is the most optimal dose among those used in this study. According to studies that used MPEE in animal models of atherosclerosis, an 800 mg/kg BW dose of MPEE is the best dosage for lowering proatherosclerosis markers such as tumor necrosis factor alpha (TNF-α), NF-κB, NO, SOD (superoxide dismutase), and MDA (malondialdehyde).25,26
Figure 4.
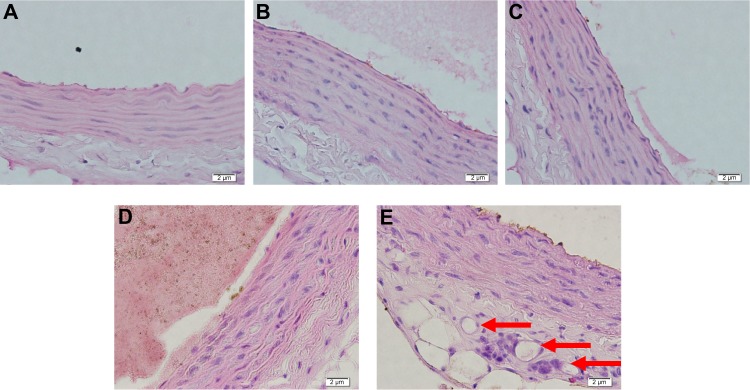
Hematoxylin and eosin staining in aortic tissue of the rat models in various treatment groups.
Notes: (A) MPEE 1: group with administration of 200 mg/kg BW doses of MPEE. (B) MPEE 2: group with administration of 400 mg/kg BW doses of MPEE. (C) MPEE 3: group with administration of 800 mg/kg BW doses of MPEE. (D) Normal diet group. (E) Hypercholesterol group. Red arrows show the newly formed vasa vasorum.
Abbreviations: BW, body weight; MPEE, mangosteen pericarp ethanolic extract.
The inhibitory effect of vasa vasorum angiogenesis by MPEE in this study was verified by the reduction of H2O2, HIF-1α, NF-κB, and iNOS levels. ANOVA test results showed that the administration of MPEE played a significant role in reducing the levels of H2O2, HIF-1α, NF-κB, and iNOS levels in hypercholesterol Wistar strain rats. Mangostin has been reported to effectively protect LDL from lipid peroxidation induced by Cu2+ and peroxyl radicals.24 The structure of phenol in mangosteen skin acts as a conventional free radical scavenger in in vitro systems. The ability of mangosteen as a scavenger against various free radicals has been revealed in various studies; one of the free radicals is 2,2-diphenyl-1-picrylhydrazyl radical. Hydroxyl radical scavenging activity of xanthones isolated from mangosteen rind powder have been affirmed. α-mangostin displayed scavenging activity against hydroxyl radicals.27 The ability of mangosteen as a scavenger against hydroxyl radicals has also been observed.28 Activity of α-mangostin and its synthetic derivatives was also found to prevent the decrease of α-tocopherol consumption induced by LDL oxidation.23 Vascular antioxidant is necessary for intracellular and extracellular protection against radical electrons. In the context of oxidative modification hypothesis, antioxidant protection against LDL in extracellular regions becomes a focus of attention because of potential proatherogenic activity of oxidized LDL (oxLDL) and accumulation of oxLDL, which is considered as a sign of atherosclerosis.30,31
NF-κB was more difficult to form at higher doses of MPEE in this study. In this study, ROS, especially H2O2, triggers activation of NF-κB. MPEE with bioactive antioxidant ability plays a role in lowering levels of H2O2 so that NF-κB activation can be inhibited. Mangosteen rind extract has shown potency as an antioxidant.32 Furthermore, a study of antioxidant activity of mangosteen pericarp extract was conducted using water extracts, 50% and 95% ethanol, and ethyl acetate.33 The method used was the capture of 2,2-diphenyl-1-picrylhydrazyl free radical. Results of this study showed that all extracts had potency as free radical scavengers. This is in line with the results of this study, in which ethanol extract was used.
NF-κB is a transcription factor that regulates inflammatory processes. Active metabolism of cells occurs when there is an inflammation, which causes high oxygen demand and the reduction of oxygen supply.9 Elevation of hypoxia in vessel walls is also caused by the thickening of arterial walls that disturbs the diffusion of oxygen.10 HIF-1α activation is a consequence of hypoxia. In this study, hypoxia was induced by inflammatory processes as a result of NF-κB activation, thus contributing to activation of HIF-1α. Hypoxia is strongly associated with angiogenesis and thrombus formation. Effects of hypoxia are mediated by macrophages which infiltrate the thrombus. A macrophage is a cell that has the ability to immediately alter the metabolism in anaerobic and aerobic condition. This causes the increase of oxygen demand.11 When there is hypoxia, cells will adapt to this situation by activating HIF-1α. Furthermore, HIF-1α will stimulate the expression of proangiogenesis genes including VEGFR-1.
Effects of free radicals generated from hypercholesterolemia through the increase of NO from iNOS cause a decrease of antioxidant activity and vulnerability of mitochondria. Antioxidants in MPEE will react with oxidants, thus reducing oxidant capacity to cause damage. Oxygen radicals react with cellular phospholipids and proteins that cause lipid peroxidation and oxidation of thiol groups with membrane changes and dysfunction of various cellular proteins. Supply of antioxidants and capacity of enzymes are significantly reduced following ischemia and reperfusion. Loss of key antioxidant enzymes will decrease supply of myocardial antioxidant. Decreased antioxidant defense cannot provide protection against increase in activity of free radicals and oxidative stress. Based on the results shown in Table 1, higher doses of MPEE will result in lower average levels of iNOS. MPEE at doses of 200 mg/kg BW, 400 mg/kg BW, and 800 mg/kg BW is proven to decrease levels of iNOS from rats that have been treated with a hypercholesterol diet. MPEE doses of 200 mg/kg BW, 400 mg/kg BW, and 800 mg/kg BW can significantly reduce levels of NO and TNF-α in an atherosclerosis group.22
Table 1.
Parameter measurement, ANOVA, and post hoc Duncan test for each treatment group
| Parameters | Treatment groups
|
P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypercholesterol
|
Normal
|
MPEE 200 mg/kg BW
|
MPEE 400 mg/kg BW
|
MPEE 800 mg/kg BW
|
|||||||
| Min–max | Min–max | Min–max | Min–max | Min–max | |||||||
| Angiogenesis | |||||||||||
| • VEGFR-1 (pg/mL) | 16.0±0.4 | 15.7–16.9 | 12.9±1.7 | 10.6–15.4 | 15.9±1.6 | 13.0–17.5 | 15.2±2.2 | 12.2–18.2 | 13.9±1.4 | 12.3–15.7 | 0.04 |
| • Vasa vasorum (amount/HE slide) | 20.5±2.2 | 18.0–23.0 | 3.7±1.6 | 2.0–6.0 | 5.8±1.4 | 4.0–7.0 | 3.5±0.5 | 3.0–4.0 | 2.2±1.4 | 1.0–4.0 | 0.00 |
| H2O2 (pg/mL) | 1.4±0.5 | 0.8–1.9 | 0.2±0.1 | 0.1–0.2 | 1.0±0.3 | 0.5–1.5 | 0.6±0.2 | 0.4–0.9 | 0.4±0.1 | 0.2–0.6 | 0.01 |
| HIF-1α (au) | 1,252.2±85.4 | 1,115.1–1,327.4 | 978.6±243.0 | 612.9–1,298.9 | 1,276.8±280.5 | 823.2–1,555.6 | 858.1±57.6 | 769.2–927.6 | 731.1±269.6 | 508.2–1,189.2 | 0.03 |
| NF-κB (au) | 1,953.2±80.5 | 1,842.9–2,087.2 | 1,655.3±315.1 | 1,364.5–2,146.6 | 1,902.4±58.8 | 1,822.6–1,982.2 | 1,426.2±187.9 | 1,167.9–1,692.6 | 1,343.5±260.7 | 1,132.3–1,767.9 | 0.01 |
| iNOS (au) | 1,276.8±169.0 | 1,006.6–1,458.7 | 849.8±78.3 | 736.2–987.9 | 950.7±252.1 | 654.2–1,321.8 | 660.1±209.3 | 467.9–991.2 | 574.6±198.6 | 309.8–849.0 | 0.00 |
Abbreviations: ANOVA, analysis of variance; au, ; BW, body weight; H2O2, hydrogen peroxide; HE, hematoxylin and eosin; HIF-1α, hypoxia-inducible factor-1α; iNOS, inducible nitric oxide synthase; min–max, minimum to maximum; MPEE, mangosteen pericarp ethanolic extract; NF-κB, nuclear factor kappa beta; SD, standard deviation; VEGFR-1, vascular endothelial growth factor receptor-1.
Mangosteen pericarp extract acts as an antioxidant and anti-inflammatory substance that is able to reduce iNOS level and which is potentially proatherosclerotic. Skin of mangosteen contains xanthone, which acts as a powerful free radical scavenger and antioxidant against superoxide anions, hydroxyl, and peroxyl radicals. Xanthone and its derivatives have been shown to have beneficial effects on several cardiovascular diseases, including ischemic heart disease, atherosclerosis, hypertension, and thrombosis.21 Xanthone has a protective effect on the cardiovascular system due to its nature as an antioxidant, anti-inflammatory, and antithrombotic agent that inhibits platelet aggregation. Xanthone compounds were found to inhibit oxLDL.18 Xanthone acts as an antioxidant because of its levels of α-mangostin and γ-mangostin. α-mangostin is a derivative of xanthone that is most abundant in the skin of mangosteen and is known to have potential phytochemical activity. Antioxidant capacity of α-mangostin reached an average of 53.5% in a study with mangosteen phenolic extract.34 It has been confirmed that, as antioxidants, xanthones play a role in inhibiting oxidation of LDL into oxLDL that binds into scavenger receptor-A in macrophages, thus preventing formation of oxLDL.35 When linked to pathogenesis of atherosclerosis, inhibited oxLDL formation will also reduce levels of NO produced by iNOS, because redox-sensitive processes are decreased and ROS production is also inhibited.
MPEE has been found to show radical scavenging activity. α- and γ-mangostin performed antioxidant activity with the use of the ferric thiocyanate method.36 It has also been confirmed that α-mangostins can lower LDL induced by copper oxidation or peroxyl radicals and can also reduce consumption of α-tocopherol induced by oxLDL.36 Ethanol extract of mangosteen pericarp showed significant antioxidant activity in one study, that is proven by inhibition of DPPH radical formation by 50%.37 Furthermore, extracts of G. mangostana significantly reduced ROS process in polymorphonuclear leucocytes.37
It has been shown that iNOS which is stimulated in inflammatory conditions plays a role in pathogenesis of atherosclerosis by the production of NO. Inhibition of NO production has potential therapeutic value in inflammation treatment. It was verified that xanthone, especially that produced by α- and γ-mangostin, evidently blocks the lipopolysaccharides that stimulate NO production.38 It has also been stated that rats induced with a hypercholesterol diet and with higher levels of NO were able to reduce NO after being given MPEE.25 Decline in levels of NO is caused by inhibition of iNOS expression. MPEE has also been confirmed to inhibit other proinflammatory cytokines, such as TNF-α and NF-κB. Results of several studies and theories which are discussed previously are consistent with the finding of our study, that mangosteen pericarp extract is proven capable of inhibiting NO produced by iNOS enzyme. MPEE’s role as an antioxidant and anti-inflammatory agent in this study was determined to lessen vasa vasorum angiogenesis in hypercholesterol rats.
Conclusion
Ethanolic extract of mangosteen pericarp (G. mangostana Linn) can lower vasa vasorum angiogenesis through H2O2, HIF-1α, NF-κB, and iNOS inhibition in Wistar strain R. norvegicus with a hypercholesterol diet. The antioxidant contained in MPEE has been proven to be involved in vasa vasorum anti-angiogenesis processes. Mangosteen pericarp extract contains a variety of xanthone antioxidants, α-, β-, and γ-mangostin. A dose of 800 mg/kg BW of MPEE was effective in vasa vasorum anti-angiogenesis in rats by administration of a hypercholesterol diet. It is recommended that further research explore the benefits of MPEE with other parameters to provide more explanation about the mechanism of MPEE as an agent against vasa vasorum antiangiogenesis in hypercholesterol rat models.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Reynolds LA, Tansey EM, editors. Cholesterol, Atherosclerosis and Coronary Disease in the UK, 1950–2000. Wellcome Witnesses to Twentieth Century Medicine. Vol. 27. London: Wellcome Trust Centre for the History of Medicine at UCL; 2006. [Google Scholar]
- 2.who.int [homepage on the Internet] Cardiovascular disease. World Health Organization; 2014. [Accessed July 3, 2014]. Available from: http://www.who.int/cardiovascular_diseases/en/ [Google Scholar]
- 3.Sargowo D. Atherosclerosis: its manifestation and danger. FK Universitas Brawijaya; 2006. [Google Scholar]
- 4.Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s Illustrated Biochemistry. 25th ed. Jakarta: EGC; 2003. Indonesian. [Google Scholar]
- 5.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Monaco C, Andreakos E, Kiriakidis S, Feldmann M, Paleolog E. T-cell-mediated signalling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2004;3(1):35–42. doi: 10.2174/1568010043483881. [DOI] [PubMed] [Google Scholar]
- 7.Andreakos E, Smith C, Kiriakidis S, et al. Heterogeneous requirement of IkappaB kinase 2 for inflammatory cytokine and matrix metalloproteinase production in rheumatoid arthritis: implications for therapy. Arthritis Rheum. 2003;48:1901–1912. doi: 10.1002/art.11044. [DOI] [PubMed] [Google Scholar]
- 8.Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15(4):265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 10.Torres Filho IP, Leunig M, Yuan F, Intaglietta M, Jain RK. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in scid mice. Proc Natl Acad Sci U S A. 1994;91:2081–2085. doi: 10.1073/pnas.91.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayr, Sidibe A, Zampetaki A. The paradox of hypoxic and oxidative stress in atherosclerosis. J Am Coll Cardiol. 2008;51(13):1266–1267. doi: 10.1016/j.jacc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 13.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 14.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 15.Pasterkamp G, Schoneveld AH, Hijnen DJ, et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000;150:245–253. doi: 10.1016/s0021-9150(99)00371-8. [DOI] [PubMed] [Google Scholar]
- 16.Sluimer JC, Gasc JM, van Wanroij JL, et al. Hypoxia, hypoxiainducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51(13):1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 18.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 19.Sanada JI, Matsui O, Yoshikawa J, Matsuoka T. An experimental study of endovascular stenting with special reference to the effects on the aortic vasa vasorum. Cardiovasc Intervent Radiol. 1998;21:45–49. doi: 10.1007/s002709900210. [DOI] [PubMed] [Google Scholar]
- 20.Slevin M, Krupinski J, Badimon L. Controlling the angiogenic switch in developing atherosclerotic plaques: possible targets for therapeutic intervention. J Angiogenes Res. 2009;1:4. doi: 10.1186/2040-2384-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargowo D, Setiawan M, Hafisalevi MD. Effect of mangosteen pericarp extract as antioxidant rats models of atherosclerosis. Jurnal Kardiologi. 2011;33:71. [Google Scholar]
- 22.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th ed. Oxford: Oxford University Press; 1999. pp. 51–77. [Google Scholar]
- 23.Williams P, Ongsakul M, Proudfoot J, Croft K, Beilin L. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Radic Res. 1995;23(2):175–184. doi: 10.3109/10715769509064030. [DOI] [PubMed] [Google Scholar]
- 24.Adiputro DL, Khotimah H, Widodo MA, Romdoni R, Sargowo D. Effects of ethanolic extracts of Garcinia mangostana fruit pericarp on oxidant-antioxidant status and foam cells in atherosclerotic rats. Oxid Antioxid Med Sci. 2013;2:61–64. [Google Scholar]
- 25.Sargowo D, Setiawan M. Effect of extract from pericarp of mangosteen as antioxidant rats models of atherosclerosis. Jurnal Kardiologi. 2012;33:71. [Google Scholar]
- 26.Chin YW, Jung HA, Chai H, Keller WJ, Kinghorn AD. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen) Phytochemistry. 2008;69:754–758. doi: 10.1016/j.phytochem.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Garcia VV, Magpantay TO, Escobin LD. Antioxidant potential of selected Philippine vegetables and fruits. The Philippine Agricultural Scientist. 2005;88:78–83. [Google Scholar]
- 28.Mahabusarakam W, Proudfoot J, Taylor W, Croft K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radic Res. 2000;33:643–659. doi: 10.1080/10715760000301161. [DOI] [PubMed] [Google Scholar]
- 29.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. 2009;50( Suppl):S376–S381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 2004;90:161–166. doi: 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 32.Weecharangsan W, Opanasopit P, Sukma M, Ngawhirunpat T, Sotanaphun U, Siripong P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn) Med Princ Pract. 2006;15:281–287. doi: 10.1159/000092991. [DOI] [PubMed] [Google Scholar]
- 33.Jiang DJ, Tan GS, Du YH, Xu KP, Li YJ. Protective effects of daviditin a against endothelial damage induced lysophosphatidylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2003;367(6):600–606. doi: 10.1007/s00210-003-0756-x. [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Zhao M, Yang B, Zhao Q, Jiang Y. Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food Chem. 2007;104:176–181. [Google Scholar]
- 35.Soeharto S, Santosaningsih D, Wijaya A. Pengaruh Ekstrak Kulit Buah Manggis (Garcinia mangostana L.) terhadap Penurunan Jumlah Foam cell pada Aorta Tikus (Rattus norvegicus) Model Aterogenik. 2011. [Accessed March 12, 2014]. Available from: old.fk.ub.ac.id/artikel/id/filedownload.kedokteran. Indonesian.
- 36.Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Chomnawang MT, Surassmo S, Nukoolkarn V, Gritsanapan W. Effect Of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007;78:401–408. doi: 10.1016/j.fitote.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46:688–693. doi: 10.1016/j.fct.2007.09.096. [DOI] [PubMed] [Google Scholar]


