Abstract
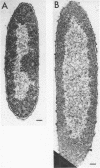
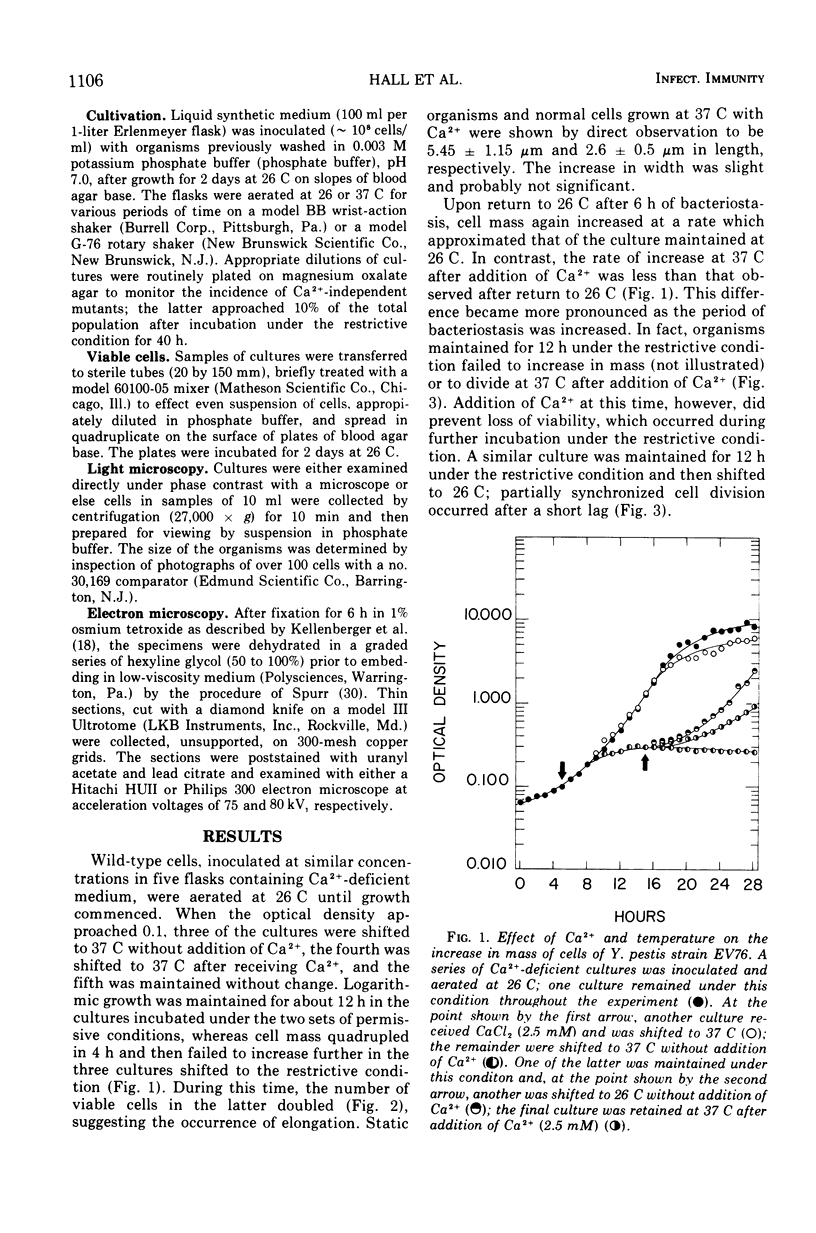
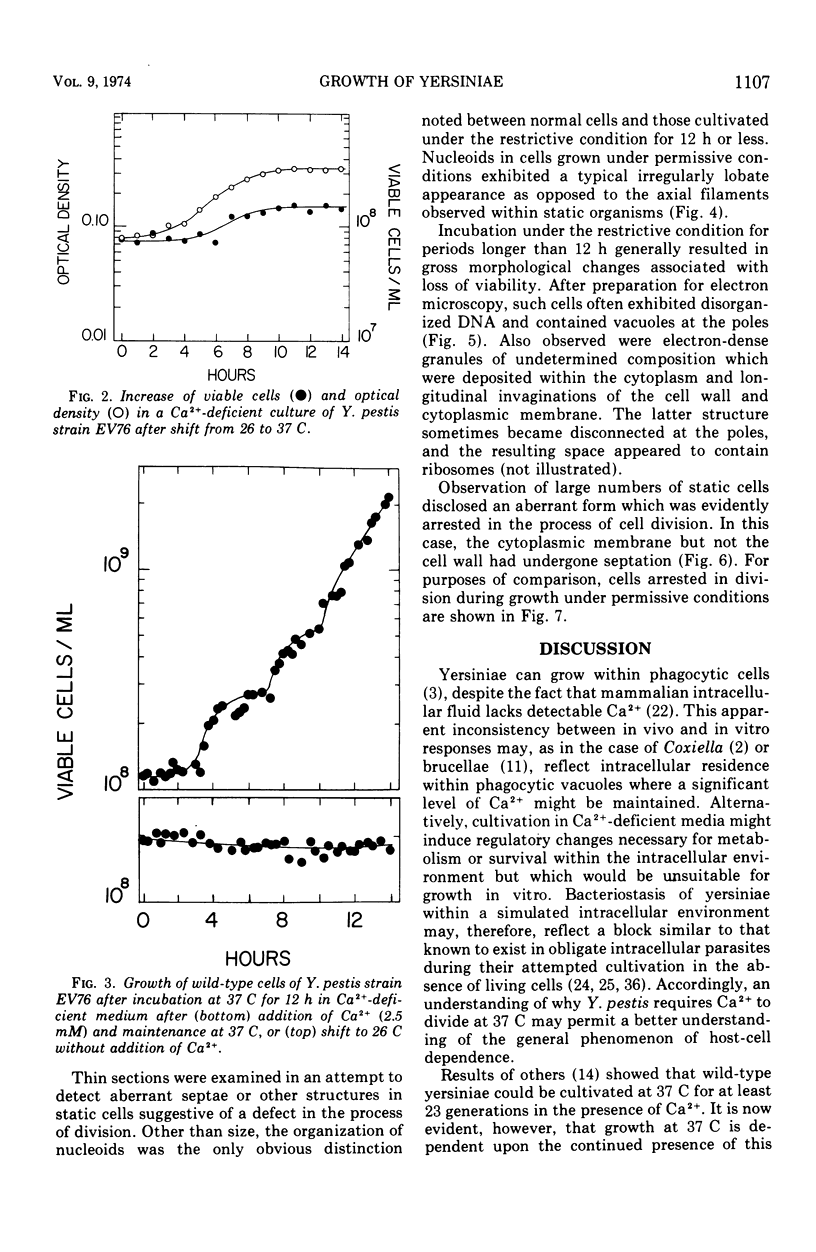
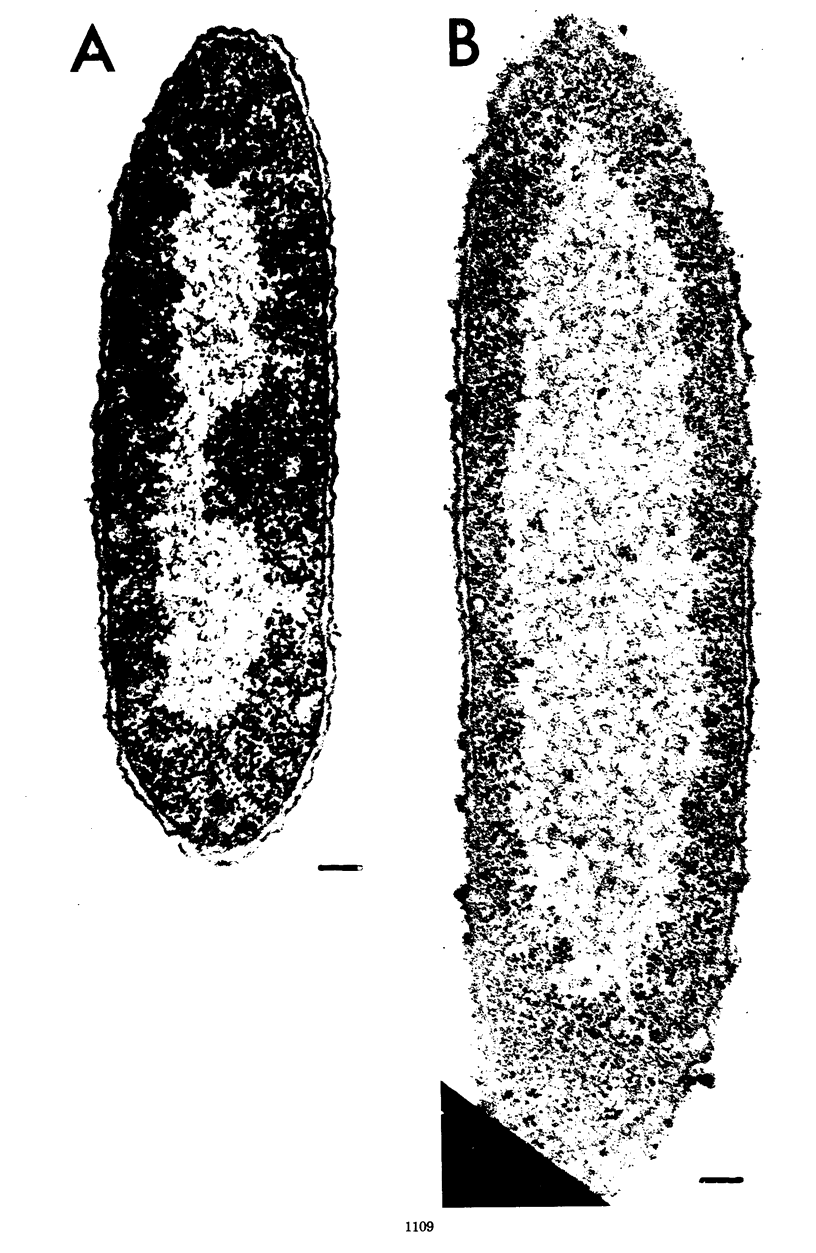
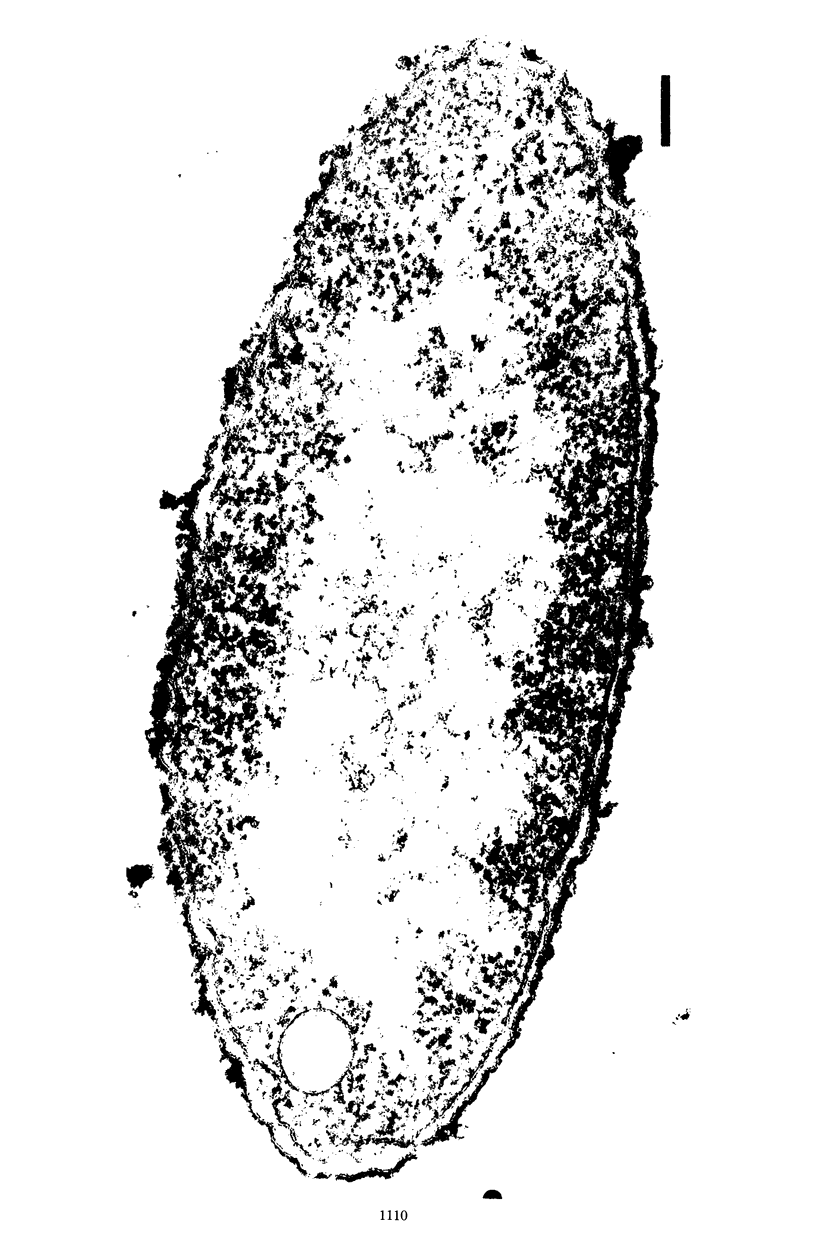
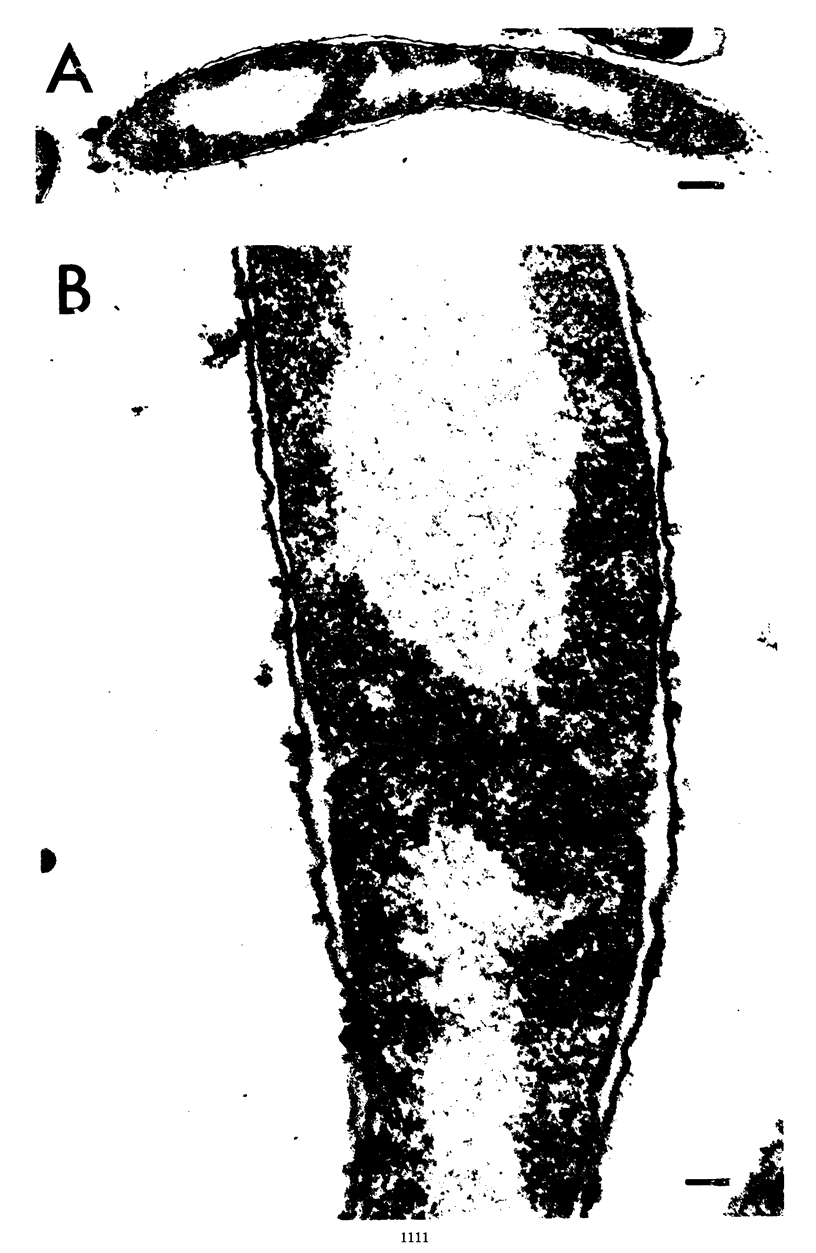
Wild-type cells of Yersinia pestis are known to exhibit a nutritional requirement for physiological levels of Ca2+ (∼2.5 mM) at 37 but not 26 C. Upon shift of Ca2+-deficient cultures from 26 (permissive condition) to 37 C (restrictive condition), bacterial mass quadrupled as the organisms doubled in number and then became elongated to about twice their normal size. As shown in thin sections, the resulting static cells contained axial filaments which differed from the typical irregularly lobate nucleoids of normal yersiniae grown under the permissive condition. Following prolonged cultivation under the restrictive condition (12 h), the organisms generally exhibited apparent degenerative changes, including separation or infolding of the cell wall and cytoplasmic membrane, degeneration of deoxyribonucleic acid, and appearance of vacuoles within the cytoplasm. At this time, the cells were unable to reinitiate cell division at 37 C upon addition of Ca2+ but divided in partial synchrony after return to 26 C. This observation indicated that, at 37 C, continuous exposure to Ca2+ is necessary for yersiniae to maintain normal morphology and the ability to divide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- Blackford V. L. INFLUENCE OF VARIOUS METABOLITES ON GROWTH OF COXIELLA BURNETII IN MONOLAYER CULTURES OF CHICK EMBRYO ENTODERMAL CELLS. J Bacteriol. 1961 May;81(5):747–754. doi: 10.1128/jb.81.5.747-754.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- CHEN T. H., CROCKER T. T., MEYER K. F. Electron microscopic study of the extracellular materials of Pasteurella pestis. J Bacteriol. 1956 Dec;72(6):851–857. doi: 10.1128/jb.72.6.851-857.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias F. F., Okrend H., Dondero N. C. Calcium nutrition of Sphaerotilus growing in a continuous-flow apparatus. Appl Microbiol. 1968 Sep;16(9):1364–1369. doi: 10.1128/am.16.9.1364-1369.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagon R. G. Cell wall-associated inorganic substances from Pseudomonas aeruginosa. Can J Microbiol. 1969 Feb;15(2):235–236. doi: 10.1139/m69-039. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Simmons G. P., Carson K. J. Evidence for the presence of ash and fivalent metals in the cell wall of Pseudomonas aeruginosa. Can J Microbiol. 1965 Dec;11(6):1041–1042. doi: 10.1139/m65-144. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr Membrane adenosine triphosphatase of Escherichia coli: activation by calcium ion and inhibition by monovalent cations. J Bacteriol. 1969 Nov;100(2):914–922. doi: 10.1128/jb.100.2.914-922.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN B. A., PEARSON G. R., HINES W. D. HOST-PARASITE RELATIONSHIPS IN BRUCELLOSIS. 3. BEHAVIOR OF AVIRULENT BRUCELLA IN TISSUE CULTURE MONOCYTES. J Infect Dis. 1964 Dec;114:441–449. doi: 10.1093/infdis/114.5.441. [DOI] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatourians G. G., Clark D. J., Adler H. I., Hardigree A. A. Cell growth and division in Escherichia coli: a common genetic control involved in cell division and minicell formation. J Bacteriol. 1973 Oct;116(1):226–229. doi: 10.1128/jb.116.1.226-229.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K. Induction of pleomorphy and calcium ion deficiency in Lactobacillus bifidus. J Bacteriol. 1970 Apr;102(1):217–220. doi: 10.1128/jb.102.1.217-220.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K., Suganuma A. Necessity of calcium ion for cell division in Lactobacillus bifidus. J Bacteriol. 1970 Nov;104(2):1010–1013. doi: 10.1128/jb.104.2.1010-1013.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. The contribution of model systems to the understanding of infectious diseases. Perspect Biol Med. 1971 Spring;14(3):486–502. doi: 10.1353/pbm.1971.0024. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Snellen J. E., Raj H. D. Morphogenesis and fine structure of Leucothrix mucor and effects of calcium deficiency. J Bacteriol. 1970 Jan;101(1):240–249. doi: 10.1128/jb.101.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. C., Brubaker R. R. Effect of ca on the synthesis of deoxyribonucleic Acid in virulent and avirulent yersinia. Infect Immun. 1971 Jan;3(1):59–65. doi: 10.1128/iai.3.1.59-65.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]