Abstract
Background
Rapid treatment of status epilepticus (SE) is associated with better outcomes. Diazepam and midazolam are commonly used, but the optimal agent and administration route is unclear.
Objectives
To determine by systematic review if non-intravenous midazolam is as effective as diazepam, by any route, in terminating SE seizures in children and adults. Time to seizure cessation and respiratory complications were examined.
Methods
Search of PubMed, Web of Knowledge, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, American College of Physicians Journal Club, Cochrane Central Register of Controlled Trials, the Cumulative Index to Nursing and Allied Health Literature, and International Pharmaceutical Abstracts for studies published January 1, 1950 through July 4, 2009. English language quasi-experimental or randomized controlled trials comparing midazolam and diazepam as first-line treatment for SE, and meeting the Consolidated Standards of Reporting Trials (CONSORT)-based quality measures, were eligible. Two reviewers independently screened studies for inclusion and extracted outcomes data. Administration routes were stratified as non-intravenous (buccal, intranasal, intramuscular, rectal) or intravenous (IV). Fixed-effects models generated pooled statistics.
Results
Six studies with 774 subjects were included. For seizure cessation, midazolam, by any route, was superior to diazepam, by any route, (RR 1.52; 95% CI = 1.27 to 1.82). Non-IV midazolam is as effective as IV diazepam (RR 0.79; 95% CI = 0.19 to 3.36), and buccal midazolam is superior to rectal diazepam in achieving seizure control (RR 1.54; 95% CI = 1.29 to 1.85). Midazolam was administered faster than diazepam (mean difference 2.46 minutes; 95% CI = 1.52 to 3.39 min) and had similar times between drug administration and seizure cessation. Respiratory complications requiring intervention were similar, regardless of administration route (RR 1.49; 95% CI = 0.25 to 8.72).
Conclusions
Non-IV midazolam, compared to non-IV or IV diazepam, is safe and effective in treating status epilepticus. Comparison to lorazepam, evaluation in adults, and prospective confirmation of safety and efficacy is needed.
Keywords: status epilepticus, seizures, benzodiazepines, midazolam, diazepam
INTRODUCTION
Seizures are a common medical emergency, accounting for 1–2% of all emergency department (ED) visits, and status epilepticus (SE) exists in approximately 6% of these encounters.1 However, the optimal agent and route of administration for the treatment of SE remain unclear. Almost one in ten persons will suffer at least one seizure in their lifetime.2 While most seizures are self-limited and short, every year 120,000 to 200,000 people have prolonged convulsions or rapidly recurrent convulsions without interval recovery, and these patients in SE have a true medical emergency.3,4 SE is associated with high morbidity and mortality, and contributes to 55,000 deaths each year in the United States.5–8 Common complications of SE include aspiration, anoxic brain injury, cardiac instability, metabolic and autonomic dysfunction, and direct neuronal damage.9–16
Although clinical outcome in SE is primarily determined by the underlying etiology that caused the seizure, persistent seizure activity is associated with worse outcomes across the spectrum of precipitating conditions.16–19 In otherwise benign epilepsy, refractory SE can still be fatal, or result in neuronal injury and chronic brain damage. In SE resulting from acute trauma or stroke, persistent ictal activity is associated with increased secondary neuronal cell death and worse outcomes.20 Although duration of seizure is associated with higher mortality and worse neurological recovery in survivors in clinical studies, these data do not provide rigorous proof of causality. However, studies of experimental SE in animal models directly demonstrate that neuronal loss increases with duration of seizure, and that kindling effects from persistent seizures are epileptogenic.19,21 Experimental status models also show that the effectiveness of anticonvulsant medications to terminate seizures rapidly decreases as the time between the start of convulsions and drug administration lengthens.22 If seizures are not terminated quickly, escalating doses of benzodiazepines are required to achieve seizure cessation, and seizures eventually become entirely refractory to anticonvulsant therapy.23
Benzodiazepines have been the first line treatment of SE for the last 30 years, but the optimal drug and the best route of administration for seizure control outside of the hospital setting, or without intravenous (IV) access, remains unclear. Lorazepam is a clinical standard for initial treatment of SE in EDs.24,25 While shown to be safe for use by paramedics, lorazepam has a relatively short shelf-life without refrigeration, limiting its practicality in the prehospital setting.26 Furthermore, lorazepam is only effective when given IV, and establishing IV access can be challenging, if not impossible, in convulsing patients.26–28 Diazepam is frequently used for treatment of SE, because it can be delivered either intravenously or rectally.26,27 However, the effectiveness of diazepam in terminating seizures is thought to be inferior to that of other benzodiazepines, especially when given rectally.25 Additionally, diazepam is suspected to cause more complications than other benzodiazepines because of the risk for prolonged sedation and respiratory depression.26,29
Midazolam is rapidly absorbed after intramuscular (IM) injection, does not require refrigeration, and is less expensive than lorazepam.30 Requiring IV access before benzodiazepine administration may unnecessarily delay treatment of SE, placing the patient at risk, even when done in the ED. Non-IV midazolam administration for treatment of SE is an attractive idea, but there are few studies of its efficacy and safety.31 A recent Cochrane Review explored benzodiazepine treatment of pediatric SE, and included many different medication strategies.32 Important secondary outcomes, including time required for administration of medication and time to therapeutic effect, were not described, and the review did not include studies addressing out-of-hospital management of SE.
This meta-analysis compares the use non-IV midazolam to that of diazepam in the treatment of seizures. The specific objective was to determine the efficacy, rapidity, and safety of terminating seizures with non-IV midazolam, compared to either IV or non-IV diazepam, as an initial emergency treatment in pediatric and adult patients with SE.
METHODS
Data Sources and Search Strategy
A systematic review of the literature was conducted to identify studies comparing the use of non-IV midazolam to IV or non-IV diazepam in treating SE in pediatric and adult patients. For the purposes of this analysis, seizures lasting longer than 5 minutes are defined as SE, as has been suggested elsewhere.26,33 The following electronic databases were searched: PubMed, Web of Knowledge, Embase, all evidence-based medicine reviews (includes Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, American College of Physicians Journal Club, and Cochrane Central Register of Controlled Trials), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and International Pharmaceutical Abstracts. All studies published or in press between January 1, 1950 and July 4, 2009, were considered. Only reports published in English were included. The majority of articles were retrieved from PubMed and Web of Knowledge using a Boolean search strategy (Appendix 1). In addition to these automated searches, we conducted a hand search of bibliographies of key articles and abstracts presented at several major scientific conferences in 2006 through 2008. These included the annual meetings of the American College of Emergency Physicians, the American Neurological Association, the National Association of EMS Physicians, and the Society for Academic Emergency Medicine. Finally, references of key review articles were hand searched for other relevant articles.
Selection Criteria
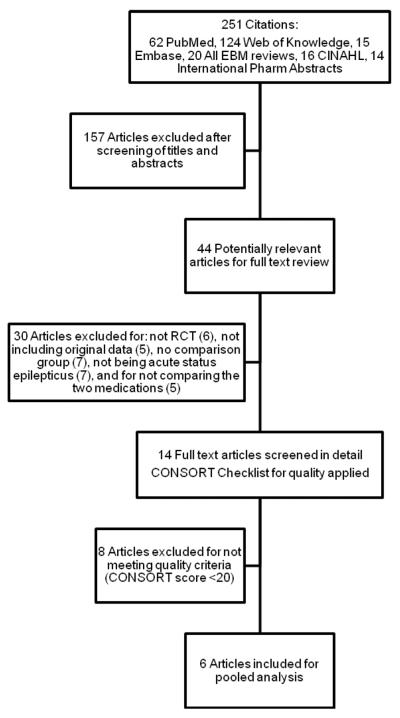
Two reviewers (CS, RS) evaluated each full text article and determined exclusions based on a priori criteria to ensure the comparability of the groups and to allow for pooling of results. These criteria excluded any study that did not compare diazepam to non-IV administration of midazolam as a first line treatment for SE, animal studies, any study design other than randomized controlled or quasi-experimental, and any study that used diazepam or midazolam for sedation or prevention of seizures (Figure 1). Initial disagreements between reviewers regarding study inclusion were resolved by consensus.
Figure 1.
Search strategy for articles reviewed for meta-analysis.
EBM = evidence-based medicine; CINAHL = Cumulative Index to Nursing and Allied Health Literature; RCT = randomized controlled trial; CONSORT = Consolidated Standards of Reporting Trials
Data Extraction and Quality Assessment
Studies that met our preliminary selection criteria were further evaluated by two independent reviewers (CS, JM) using the Consolidated Standards of Reporting Trials (CONSORT) Quality Scale, and the Randomized Controlled Trial (RCT) Checklist.34 The CONSORT Quality Scale has been shown to be useful in determining the methodological quality of randomized clinical trials in a standardized format.34 The 30-point scale assigns points for studies that report key concepts on randomization, allocation concealment, repeatability of observations, etc., and serves as a balance to the quality of writing to judge the strength and validity of findings. An a priori threshold score of at least 20 was established for inclusion. The RCT Checklist serves as a way to abstract data on specific interventions and to further assess key components of study design.
The following variables were extracted from the studies: type of study design, definition of SE, types of complications reported, absolute numbers of patients in the diazepam and the midazolam groups that had seizure activity terminated, route of administration, and dosage of drug administered.
Data Analysis
Study inclusion agreement between investigators was evaluated by kappa statistics. Pooled risk ratios were determined using both the Mantel-Haenszel fixed effects, and DerSimonian and Laird random-effects models.35 Data were stratified into two subgroups, one comparing IV diazepam versus non-IV midazolam, and the other comparing non-IV diazepam to non-IV midazolam. Where study data were available, we assessed the mean differences in times between initial assessment and drug administration, and between drug administration and cessation of seizure activity based on route of administration. A fixed-effects model was used to pool times across studies.
Heterogeneity within the group was assessed using Cochran's Q test and I2 statistic, which measures the degree of variation among studies.36 Begg's test and a visual inspection of the funnel plot were conducted to evaluate publication bias. All statistical tests were two-sided. Stata version 10.0 (College Station, TX) and Review Manager 5.0 (RevMan, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) were used to conduct the analyses. A meta-influence analysis was conducted to statistically omit one study at a time to determine the effect on the overall pooled estimate. A sensitivity analysis was performed to assess the effect of removing the most influential study from the pooled subgroup results.
RESULTS
Search and Study Characteristics
The initial literature search yielded 251 references, of which 44 met preliminary selection criteria for inclusion within the meta-analysis (Figure 1). Four authors were contacted to clarify the comparability of groups, to obtain more data, or to clarify definitions of SE. Thirty-eight articles were excluded because trial design was not randomized or controlled (n = 6); data included were not original (n = 5); there was no comparison group (n = 7); acute SE was not described (n = 7); the two drugs chosen for this review were not utilized (n = 5); and the CONSORT score was <20 (n = 8).34 The kappa for inter-rater reliability for inclusion into the study was 0.95.
The characteristics of the six studies included studies containing 774 subjects are shown in Table 1,37–42 and all are RCTs. Although the intent of our analysis was to include all age groups, all of the studies meeting the selection criteria happened to be studies of children and young adults. Five studies included children only; one study included children and adults, however the oldest subject was 22 years old.42 Routes of medication administration included IV and rectal (PR) diazepam and buccal, intranasal (IN), and IM midazolam. Dosing of medications varied slightly among studies: diazepam 0.2–0.3 mg/kg IV or 0.5 mg/kg PR, midazolam 0.2 mg/kg IM and IN, or 0.5 mg/kg buccal. One study used fixed doses of PR diazepam (10 mg) and buccal midazolam (10 mg).42 The determination of seizure cessation was clinically based, and used varying definitions based on time until convulsion stoppage and/or absence of seizure recurrence. Some studies included prolonged simple partial or focal convulsions.37,39,41,42 Despite these clinical and methodological differences, there was no significant statistical heterogeneity in pooled analysis of all included studies (I2 = 0%, Figure 2).
Table 1.
Characteristics of included studies.
| Study, year | n | Age Range |
Diazepam Dose/Route |
Midazolam Dose/Route |
Definition of “Status” |
Definition of Success |
CONSORT Score |
Trial Design | Non Generalized Seizures Included? |
History of Fever (%) |
History of Prior Seizures (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chamberlain, et al. 1997 | 24 | 0 mo. to 18 yr. | 0.3mg/kg IV | 0.2mg/kg IM | seizing >10 minutes | No seizure activity within 5 minutes | 23 | Randomized | Yes (11 partial motor) | 0 | 57 |
| Lahat, et al. 2000 | 52 | 6 mo. to 5 yr. | 0.3mg/kg IV | 0.2mg/kg IN | seizing >10 minutes | No seizure activity within 5 minutes; up to 10 minutes considered delayed success | 24 | Randomized | No | 100 | 77 |
| Mahmoudian, et al. 2004 | 70 | 2 mo. to 15 yr. | 0.2mg/kg IV | 0.2mg/kg IN | seizing at arrival to ED | No seizure activity within 10 minutes | 22 | Randomized | Yes (6 simple partial) | 21 | 39 |
| McIntyre, et al. 2005 | 219 | 6 mo. to 15 yr. | 0.5mg/kg PR | 0.5mg/kg Buccal | seizing at arrival to ED | No seizure activity within 10 minutes and no recurrent seizure within 1 hour | 27 | Randomized | Yes (5 partial included were protocol violations) | 35 | 72 |
| Mpimbaza, et al. 2008 | 330 | 3 mo. to 12 yr. | 0.5mg/kg PR | 0.5mg/kg Buccal | seizing at arrival to ED or >5 minutes | No seizure activity within 10 minutes and no recurrent seizure within 1 hour | 28 | Randomized | Yes (61 focal) | 72 | 4 |
| Scott, et al. 1999 | 79 | 5–22 yr. | 10mg PR | 10mg Buccal | seizing >5 minutes | Seizure cessation within 10 minutes | 26 | Randomized | Yes (17 complex partial, 10 myoclonic) | 0 | 100 |
CONSORT = Consolidated Standards of Reporting Trials
PR = per rectum; IV = intravenous; IN = intranasal; IM = intramuscular; mo = month; yr = year; mg = milligram; kg = kilogram.
Figure 2.
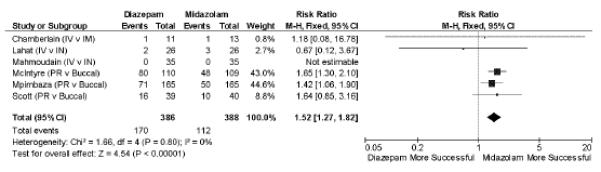
Diazepam versus midazolam in failure to achieve seizure cessation (all routes of administration). M–H = Mantel-Haenszel; IV = intravenous; IM = intramuscular; IN = intranasal; PR = per rectum
Seizure Cessation
Midazolam, by any route, was superior to diazepam, by any route, in achieving seizure cessation in pooled analysis (relative risk [RR] 1.52; 95% confidence interval [CI] = 1.27 to 1.82, n = 6, number needed to treat [NNT] = 7), Figure 2.
Three37–39 studies of 146 subjects compared IM or IN midazolam to IV diazepam. In pooled analysis, there is no apparent difference between non-IV midazolam and IV diazepam in achieving seizure cessation (RR 0.79; 95% CI = 0.19 to 3.26), Figure 3. Statistical heterogeneity of this sub-group of studies was very low (I2 = 0%).
Figure 3.
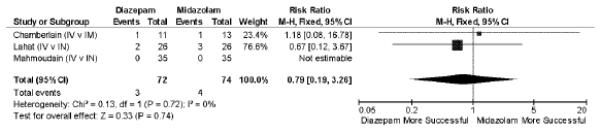
Intravenous diazepam versus non-intravenous midazolam in failure to achieve seizure cessation. M–H = Mantel-Haenszel; IV = intravenous; IM = intramuscular; IN = intranasal
Three40–42 studies of 628 subjects compared rectal diazepam to buccal midazolam. Buccal midazolam is more successful in achieving seizure cessation (RR 1.54; 95% CI = 1.29 to 1.85, I2 = 0%, NNT = 6).
Time to administration and time to seizure cessation
Early treatment of status epilepticus is likely to be most successful and relies on the time intervals of seizure onset to medical contact, medical contact to drug administration, and drug administration to therapeutic effect. These time intervals are separately evaluated when reported by individual studies. No studies reliably report the time from seizure onset to medical contact. Two37,38 studies demonstrate non-IV midazolam was administered 2.46 minutes (95% CI = 1.52 to 3.39) quicker than IV diazepam to seizing patients. Non-IV midazolam and IV diazepam were similar in the time between drug administration and seizure cessation in three37–39 studies (mean difference 0.68 minutes, 95% CI = −0.03 to 1.39).
Respiratory Complications
Respiratory complications were rarely reported. In five38–42 studies of 750 subjects only five instances of respiratory depression requiring intubation or ventilatory support (0.7%) were described, and these all came from a single study of non-IV benzodiazepines.40 There is no apparent difference between the safety of midazolam and diazepam (RR 1.49; 95% CI = 0.25 to 8.72, Figure 4). Causes of respiratory depression were described as multifactorial, but further detail was not provided.
Figure 4.
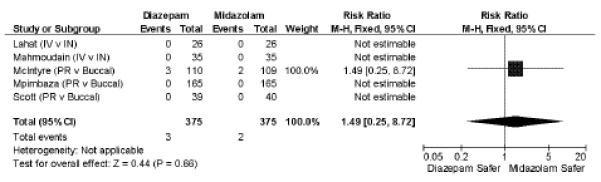
Respiratory complications requiring intervention (assisted ventilations, endotracheal intubation). M–H = Mantel-Haenszel; IV = intravenous; IM = intramuscular; IN = intranasal; PR = per rectum
Sensitivity Analysis
For each outcome, removing individual studies did not affect pooled risk ratios or measurements of statistical heterogeneity. There is also no apparent bias introduced by dose of medication, length of seizure required for inclusion, or inclusion of non-generalized seizures. Outcomes were also analyzed using a random-effects model, with no meaningful effect on the results.
A more broad pooled analysis including the eight studies with CONSORT scores between 15 and 19 yields similar results to all outcomes:27,43–49 overall success (RR 1.50; 95% CI = 1.30 to 1.73, n = 14), IV diazepam vs. non-IV midazolam (RR 0.90; 95% CI = 0.48 to 1.68, n = 5], PR diazepam vs. buccal midazolam (RR 1.51; 95% CI = 1.26 to 1.80, n = 4). Midazolam appears to be associated with fewer respiratory complications in this expanded group (RR 1.74; 95% CI = 1.23 to 2.46, n = 13, I2 = 69%); however, this result is biased by a single study,46 and exclusion resulted in no safety difference between the two medications (RR 1.31; 95% CI = 0.88 to 1.95, n = 12, I2 = 0%).
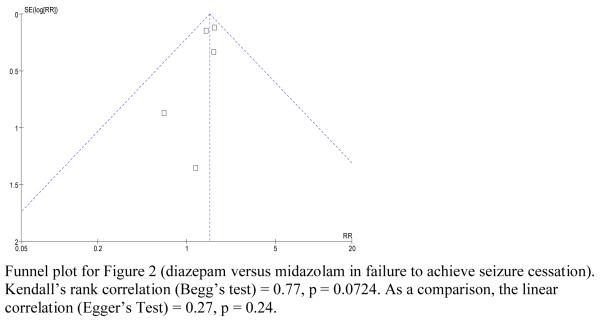
Visual inspection of funnel plots shows no obvious signal of asymmetry, which suggests lack of significant publication bias; this is limited due to the small numbers of studies included. Begg's test was marginal, but not statistically significant (p = 0.0724).
DISCUSSION
This pooled meta-analysis of all published data from 774 subjects in six37–42 studies supports the use of midazolam by non-IV routes as a favorable alternative to diazepam in the initial treatment of SE. Midazolam, by any route, achieved seizure cessation more often than diazepam, by any route (RR 1.52; 95% CI = 1.27 to 1.82). This finding is even more apparent when comparing non-IV administration of diazepam and midazolam. The superior efficacy of midazolam as compared to diazepam likely reflects more favorable pharmacokinetics. Erratic absorption of rectal diazepam often results in low or delayed plasma peak drug concentrations, whereas IM and IN midazolam have a more consistent and higher bioavailability of 87% and 55%, respectively, with a short time to peak concentration.29
Rapidity of seizure cessation is also a clinically important measure of performance that depends on both speed of administration and the onset of action. Earlier termination of seizure reduces the risk of complications due to convulsions, reduces neuronal injury, and is associated with decreased mortality. Rapid termination may also prevent kindling effects, where seizures become more refractory to treatment, and risk of recurrence increases as the duration of convulsions increases.19 Reliance on the IV route for benzodiazepine administration can be an important obstacle to rapid treatment of SE, because of difficulty or delay in obtaining IV access in a convulsing patient. As a result, our meta-analysis appears to favor non-IV midazolam, as its time to administration was more than 2 minutes faster, and seizure cessation less than 45 seconds slower than that of IV diazepam.
Respiratory depression is an expected and accepted side effect of benzodiazepine medications, and this meta-analysis suggests that midazolam is as safe as diazepam with regard to respiratory complications. Overall, 0.8% (3/375) of pediatric patients in this analysis receiving diazepam experienced complications, which is much less than the 10.3% previously observed with diazepam in a trial of the prehospital treatment of SE in adults.26 Midazolam was associated with risk of respiratory complications in children similar to that found with diazepam (0.5%, 2/375). Respiratory depression in patients treated for SE can be a complication of either continued seizures, or an adverse medication effect. At least in the prehospital setting, failure to treat seizures is associated with much higher rates of respiratory complications. Twenty-two percent of placebo-treated patients in a prehospital trial of SE suffered a respiratory complication related to ongoing seizure.26
Current clinical practice does not reflect the findings that non-IV midazolam is a safe and effective treatment for SE. There are no consensus guidelines addressing prehospital treatment of SE, and many large agencies rely solely on diazepam, or allow only restricted use of midazolam. Published professional guidelines for SE management rely on lorazepam or diazepam, and emphasize IV administration.50–53 Only the Royal College of General Practitioners acknowledges the role of buccal midazolam for use in the prehospital environment, but even in that guideline rectal diazepam is preferred.52 Recent surveys of parents and practitioners show a growing acceptance of IN and buccal midazolam over PR valium, but widespread adoption has not yet occurred.54,55 This analysis, in conjunction with previously published systematic reviews,32,56 may inform the development of future evidence-based guidelines. However, further prospective clinical trials are ultimately needed to confirm the efficacy and safety of non-IV midazolam in the treatment of patients with SE, especially in the adult population.
LIMITATIONS
As with all meta-analyses, the primary limitations of this study are those of the source data. The studies included here are relatively small, and contained differences in treatments, routes of medication administration, medication doses, outcome definitions, and inclusion criteria. However, the pooled results demonstrated low statistical heterogeneity, suggesting that comparisons are valid. Given the small numbers of studies included, the visual inspection of the funnel plot and measure of statistical heterogeneity should be interpreted with caution.57–59 A minimal I2 does not guarantee homogeneity, but does provide evidence that there was no observed heterogeneity. Our finding of I2 = 0% is consistent with many previously published Cochrane reviews.36
Adults were virtually unrepresented in the included studies, and extrapolation of these results to the adult population should be done with caution. Future trials specifically targeting adult populations are required to confirm these findings. Studies also used differing definitions of adverse events, which were infrequent. However, this analysis is under-powered to detect differences in complication rates. Although a rigorous search strategy was employed, we did not attempt to identify or analyze non-English language studies. Comparisons with other anticonvulsants are lacking and were not identified or included in this meta-analysis. Despite these limitations, the effects identified here appear robust given the magnitude of the findings and the rather small confidence intervals. Prospective confirmatory investigation, however, is warranted.
CONCLUSIONS
Published data support the efficacy and safety of non-intravenous routes of administration for midazolam, when compared to diazepam administered via any route in treating patients with status epilepticus, in the doses studied. Midazolam has characteristics that may make it an optimal choice for the treatment of seizing patients.
Acknowledgements
This publication was supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant 1UL1RR026314-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Funding: This work was supported in part by NIH grant 5U01NS056975-02.
Appendix A. Boolean Search Strategy
Date of Original Search- Aug. 20th, 2008
Search updated July 4th, 2009
PICO- In pediatric and adult patients with status epilepticus, is the administration of non-intravenous midazolam versus any route of diazepam more effective in ceasing seizures?
Exclusion Criteria:
Any study that does not compare diazepam to midazolam as a first line treatment for status epilepticus
Animal studies.
Studies that are not randomized controlled trials or quasi-experimental studies.
Any study that uses diazepam or midazolam as sedation, or prevention of seizures.
Search Strategy:
PubMed (62) 26 pulled for full text
(“Seizures”[MeSH] OR “status epilepticus” [MeSH) AND (“diazepam”[MeSH Terms] OR “diazepam”[All Fields]) AND (“midazolam”[MeSH Terms] OR “midazolam”[All Fields])
EMBASE (15- all duplicates)
All EBM Reviews (Cochrane, ACP Journal Club) (20 titles- 13 full texts pulled)
CINAHL: (16-all duplicates)
International Pharmaceutical Abstracts: (14- all duplicates)
Web of Knowledge (124) (5 full texts)
Hand Search of Review Article Bibliographies: all duplicates
TOTAL FULL TEXTS REVIEWED= 44
Total INCLUDED=6
Stratified by buccal vs. intranasal vs. intramuscular midazolam and rectal vs. intravenous diazepam
Appendix B: Funnel Plot
Footnotes
Prior Presentations: This work was presented at the 2009 Society for Academic Emergency Medicine annual meeting, New Orleans, LA, and was awarded Best Fellow Presentation.
REFERENCES
- 1.Huff JS, Morris DL, Kothari RU, Gibbs MA. Emergency department management of patients with seizures: a multicenter study. Acad Emerg Med. 2001;8:622–8. doi: 10.1111/j.1553-2712.2001.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 2.Epilepsy Foundation [Accessed Feb 28, 2010];Epilepsy and seizure statistics. Available at: http://www.epilepsyfoundation.org/about/statistics.cfm.
- 3.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49(5):659–64. [PubMed] [Google Scholar]
- 4.Theodore WH, Porter RJ, Albert P, et al. The secondarily generalized tonic-clonic seizure: a videotape analysis. Neurology. 1994;44(8):1403–7. doi: 10.1212/wnl.44.8.1403. [DOI] [PubMed] [Google Scholar]
- 5.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029–35. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 6.Hauser WA. Status epilepticus: epidemiologic considerations. Neurology. 1990;40(5 Suppl 2):9–13. [PubMed] [Google Scholar]
- 7.Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35(1):27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 8.Claassen J, Lokin JK, Fitzsimmons BF, Mendelsohn FA, Mayer SA. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58(1):139–42. doi: 10.1212/wnl.58.1.139. [DOI] [PubMed] [Google Scholar]
- 9.Meldrum BS, Horton RW. Physiology of status epilepticus in primates. Arch Neurol. 1973;28(1):1–9. doi: 10.1001/archneur.1973.00490190019001. [DOI] [PubMed] [Google Scholar]
- 10.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(5 Suppl 2):13–23. [PubMed] [Google Scholar]
- 11.Aminoff MJ, Simon RP. Status epilepticus. Causes, clinical features and consequences in 98 patients. Am J Med. 1980;69(5):657–66. doi: 10.1016/0002-9343(80)90415-5. [DOI] [PubMed] [Google Scholar]
- 12.Bayne LL, Simon RP. Systemic and pulmonary vascular pressures during generalized seizures in sheep. Ann Neurol. 1981;10(6):566–9. doi: 10.1002/ana.410100613. [DOI] [PubMed] [Google Scholar]
- 13.White PT, Grant P, Mosier J, Craig A. Changes in cerebral dynamics associated with seizures. Neurology. 1961;11((4)Pt 1):354–61. doi: 10.1212/wnl.11.4.354. [DOI] [PubMed] [Google Scholar]
- 14.Boggs JG, Painter JA, DeLorenzo RJ. Analysis of electrocardiographic changes in status epilepticus. Epilepsy Res. 1993;14(1):87–94. doi: 10.1016/0920-1211(93)90077-k. [DOI] [PubMed] [Google Scholar]
- 15.Boggs JG, Marmarou A, Agnew JP, et al. Hemodynamic monitoring prior to and at the time of death in status epilepticus. Epilepsy Res. 1998;31(3):199–209. doi: 10.1016/s0920-1211(98)00031-x. [DOI] [PubMed] [Google Scholar]
- 16.Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3(10):608–17. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- 17.Maegaki Y, Kurozawa Y, Hanaki K, Ohno K. Risk factors for fatality and neurological sequelae after status epilepticus in children. Neuropediatrics. 2005;36(3):186–92. doi: 10.1055/s-2005-865611. [DOI] [PubMed] [Google Scholar]
- 18.Waterhouse EJ, Garnett LK, Towne AR, et al. Prospective population-based study of intermittent and continuous convulsive status epilepticus in Richmond, Virginia. Epilepsia. 1999;40(6):752–8. doi: 10.1111/j.1528-1157.1999.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73(1):1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Towne AR. Epidemiology and outcomes of status epilepticus in the elderly. Int Rev Neurobiol. 2007;81:111–27. doi: 10.1016/S0074-7742(06)81007-X. [DOI] [PubMed] [Google Scholar]
- 21.Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59(9 Suppl 5):S3–6. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- 22.Goodkin HP, Liu X, Holmes GL. Diazepam terminates brief but not prolonged seizures in young, naive rats. Epilepsia. 2003;44(8):1109–12. doi: 10.1046/j.1528-1157.2003.62402.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res. 2002;50(3):301–12. doi: 10.1016/s0920-1211(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 24.Henry JC, Holloway R. Review: Lorazepam provides the best control for status epilepticus [review] ACP J Club. 2006;144(2):35. [PubMed] [Google Scholar]
- 25.Prasad K, Al-Roomi K, Krishnan PR, Sequeira R. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2005;4:CD003723. doi: 10.1002/14651858.CD003723.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345(9):631–7. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 27.Rainbow J, Browne GJ, Lam LT. Controlling seizures in the prehospital setting: diazepam or midazolam? J Paediatr Child Health. 2002;38(6):582–6. doi: 10.1046/j.1440-1754.2002.00046.x. [DOI] [PubMed] [Google Scholar]
- 28.Vilke GM, Sharieff GQ, Marino A, Gerhart AE, Chan TC. Midazolam for the treatment of out-of-hospital pediatric seizures. Prehosp Emerg Care. 2002;6(2):215–7. doi: 10.1080/10903120290938571. [DOI] [PubMed] [Google Scholar]
- 29.Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Focus on delivery routes. Clin Pharmacokinet. 1999;36(6):409–24. doi: 10.2165/00003088-199936060-00003. [DOI] [PubMed] [Google Scholar]
- 30.Bebin M, Bleck TP. New anticonvulsant drugs. Focus on flunarizine, fosphenytoin, midazolam and stiripentol. Drugs. 1994;48(2):153–71. doi: 10.2165/00003495-199448020-00003. [DOI] [PubMed] [Google Scholar]
- 31.Fountain NB, Adams RE. Midazolam treatment of acute and refractory status epilepticus. Clin Neuropharmacol. 1999;22(5):261–7. [PubMed] [Google Scholar]
- 32.Appleton R, Macleod S, Martland T. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev. 2008;3:CD001905. doi: 10.1002/14651858.CD001905.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40(1):120–2. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 34.Huwiler-Muntener K, Juni P, Junker C, Egger M. Quality of reporting of randomized trials as a measure of methodologic quality. JAMA. 2002;287(21):2801–4. doi: 10.1001/jama.287.21.2801. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13(2):92–4. doi: 10.1097/00006565-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–6. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5(2):253–5. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 40.McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(9481):205–10. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 41.Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121(1):e58–64. doi: 10.1542/peds.2007-0930. [DOI] [PubMed] [Google Scholar]
- 42.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353(9153):623–6. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 43.Baysun S, Aydin OF, Atmaca E, Gurer YK. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr (Phila) 2005;44(9):771–6. doi: 10.1177/000992280504400904. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs. rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34(5):355–9. doi: 10.1016/j.pediatrneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Fisgin T, Gurer Y, Tezic T, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17(2):123–6. doi: 10.1177/088307380201700206. [DOI] [PubMed] [Google Scholar]
- 46.Holsti M, Sill BL, Firth SD, Filloux FM, Joyce SM, Furnival RA. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. 2007;23(3):148–53. doi: 10.1097/PEC.0b013e3180328c92. [DOI] [PubMed] [Google Scholar]
- 47.Shah I, Deshmukh CT. Intramuscular midazolam vs. intravenous diazepam for acute seizures. Indian J Pediatr. 2005;72(8):667–70. doi: 10.1007/BF02724074. [DOI] [PubMed] [Google Scholar]
- 48.Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev. 2008;31(10):744–9. doi: 10.1016/j.braindev.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Warden CR, Frederick C. Midazolam and diazepam for pediatric seizures in the prehospital setting. Prehosp Emerg Care. 2006;10(4):463–7. doi: 10.1080/10903120600885126. [DOI] [PubMed] [Google Scholar]
- 50.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus. Eur J Neurol. 2006;13(5):445–50. doi: 10.1111/j.1468-1331.2006.01397.x. [DOI] [PubMed] [Google Scholar]
- 51.Appleton R, Choonara I, Martland T, Phillips B, Scott R, Whitehouse W. The treatment of convulsive status epilepticus in children. The Status Epilepticus Working Party, Members of the Status Epilepticus Working Party. Arch Dis Child. 2000;83(5):415–9. doi: 10.1136/adc.83.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stokes T, Shaw EJ, Juarez-Garcia A, Camosso-Stefinovic J, Baker R. Clinical Guidelines and Evidence Review for the Epilepsies: diagnosis and management in adults and children in primary and secondary care. Royal College of General Practitioners; London, England: 2008. [Google Scholar]
- 53.Scottish Intercollegiate Guidelines Network . Diagnosis and management of epilepsy in adults: a national clinical guideline. Scottish Intercollegiate Guidelines Network; Edinburgh, Scotland: 2003. [Google Scholar]
- 54.de Haan GJ, van der Geest P, Doelman G, Bertram E, Edelbroek P. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02333.x. online early. [DOI] [PubMed] [Google Scholar]
- 55.Klimach VJ. The community use of rescue medication for prolonged epileptic seizures in children. Seizure. 2009;18(5):343–6. doi: 10.1016/j.seizure.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Sofou K, Kristjansdottir R, Papachatzakis NE, Ahmadzadeh A, Uvebrant P. Management of prolonged seizures and status epilepticus in childhood: a systematic review. J Child Neurol. 2009;24(8):918–26. doi: 10.1177/0883073809332768. [DOI] [PubMed] [Google Scholar]
- 57.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang JL, Liu JL. Misleading funnel plot for detection of bias in meta-analysis. J Clin Epidemiol. 2000;53(5):477–84. doi: 10.1016/s0895-4356(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 59.Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58(9):894–901. doi: 10.1016/j.jclinepi.2005.01.006. [DOI] [PubMed] [Google Scholar]