Abstract
Background
Electrophysiological and hemodynamic activity is altered in attention-deficit/hyperactivity disorder (ADHD) during tasks requiring cognitive control. Frontal midline theta oscillations are a cortical correlate of cognitive control influencing behavioral outcomes including reaction times. Reaction time variability (RTV) is consistently increased in ADHD and is known to share genetic effects with the disorder. The etiological relationship between the cognitive control system, RTV, and ADHD is unknown. In a sample of twins selected for ADHD and matched control subjects, we aimed to quantify the strength of the phenotypic, genetic, and environmental relationships between event-related midline theta oscillations, RTV, and ADHD.
Methods
Our sample included 134 participants aged 12 to 15 years: 67 twin pairs (34 monozygotic; 33 dizygotic) with concordance or discordance for ADHD symptomatology assessed at 8, 10, and 12 years of age. Our main outcome measures were frontal midline theta activity, derived from both channel and source decomposed electroencephalographic data, and behavioral performance on a response-choice arrow flanker task known to elicit theta activity.
Results
Variability in stimulus event-related theta phase from frontal midline cortex is strongly related to both RTV and ADHD, both phenotypically and genetically.
Conclusions
This is the first finding to confirm the genetic link between the frontal midline cognitive control system and ADHD and the first to identify a genetically related neurophysiological marker of RTV in ADHD. Variability in the timing of the theta signal in ADHD may be part of a dysfunctional brain network that impairs regulation of task-relevant responses in the disorder.
Keywords: ADHD, biomarker, cognitive control, EEG, genetic, twin study
The ability to quickly adapt thinking and behavior to changing internal states and external circumstances is critical for maintaining efficient, goal-directed behavior. Abnormalities in such cognitive control are associated with numerous psychopathological disorders including attention-deficit/hyperactivity disorder (ADHD). Converging evidence indicates that electrophysiological and hemodynamic measures from medial prefrontal cortex (mPFC) and anterior cingulate cortex are altered in ADHD during action monitoring, conflict detection, error signaling, and reinforcement learning (1–6).
A growing body of work focuses on the relation of frontal midline theta band (5–8 Hz) electroencephalographic (EEG) activity to cognitive control (7). Frontal midline event-related potential (ERP) indices of cognitive control, including those abnormal in ADHD (1,2,6,8,9), have most energy in the theta band (7,10) and can be interpreted as brief bursts or complexes of theta-band activity partially time- and phase-locked to relevant stimulus presentations (11) or motor responses (12). The link between cognitive control and frontal midline theta is further supported by human and monkey reports that theta-band activity in mPFC increases with cognitive control demands, for example, those involved in task rule changes and stimulus feature task conflict (13–16). A number of studies indicate that trial-to-trial tuning of frontocentral theta activity may play a role in optimizing behavioral performance (17–19). Of particular interest to ADHD research, mPFC activation has been shown to influence reaction times (17,20). Monkey studies have also indicated that theta phase dynamics have a general role in response initiation and a specific role in conflict-modulated reactions (15,21).
Reaction time variability (RTV) in repetitive response tasks consistently discriminates between ADHD and control samples in a broad range of tasks and sensory modalities (22–26). Consistent with a relationship between frontal midline brain activity and reaction times, a number of investigations point toward a possible functional relationship between RTV and medial frontal blood oxygen level–dependent activation (27,28), and a functional magnetic resonance imaging report indicated that decreased activation in anterior cingulate, basal ganglia, and thalamus is correlated with the increase in RTV in ADHD (29). Despite the evident role of RTV in ADHD, there is little empirical research into its neurophysiological correlates (30).
Both RTV and cognitive control appear to share an etiological relationship with ADHD. The majority of familial, and genetic, influences on RTV are shared with ADHD (31–34). Similarly, two family studies indicate a shared familial relationship between cognitive control and ADHD (1,2). However, as family designs are unable to discriminate between genetic and environmental influences, the exact etiology of familial overlap between ADHD and cognitive control abnormalities is unknown. Twin studies provide a powerful way to delineate the etiological architecture of cognitive abnormalities associated with ADHD (35,36).
In the present study, we investigated the relative strengths of genetic and environmental relationships between ADHD, RTV, and an EEG marker of cognitive control, event-related frontal midline theta. We measured EEG activity during the well-defined Eriksen arrow flanker task (1,2) in 67 adolescent monozygotic (MZ) and dizygotic (DZ) twin pairs concordant or discordant for high and low ADHD symptom scores. The Eriksen flanker task is known to elicit a strong theta response, particularly in high-conflict trials (21). First, we identified measures that shared phenotypic variance with ADHD symptoms and reaction times and then we applied structural equation modeling to separate this phenotypic covariance into genetic and environmental components (37). In line with previous studies on cognitive control and ADHD (1,2,6), we predicted that the greatest phenotypic relationship between theta measures and ADHD symptoms would be in high-conflict trials. Specifically, we aimed to quantify the strength of the genetic and environmental relationships between stimulus-locked midline theta oscillations and 1) ADHD symptoms and 2) reaction times.
As we are interested in brain activity that may influence reaction times, we measured stimulus-locked theta phase and amplitude in a poststimulus, preresponse window. We measured theta activity both at frontal midline scalp electrodes and also at the source level using independent component analysis (ICA). Independent component analysis is a statistical blind source separation technique that has been found to be useful for separating out independent EEG signals from many cortical and noncortical sources (38–43). Interpretation of ERP indices of cognitive control is limited by the fact that conventional ERPs represent only trial averages, neglecting consideration of the temporally dynamic brain activations that support the adjustments and behaviors across many trials (21,44). The improved signal-to-noise ratio achieved with ICA facilitates identification of single-trial EEG activity that can be more tightly linked to behavior and cognition than scalp channel-based measures (45,46) and may be particularly useful for characterizing trial-to-trial theta-band dynamics in ADHD.
We also compared ADHD and control children on behavior and EEG variables in high-conflict trials (high cognitive control) and low-conflict trials (lower cognitive control), which enabled the examination of increased demand of cognitive control on all measures.
Methods and Materials
Sample
The sample was selected from the Twins' Early Development Study, a birth cohort study of all twins born in England and Wales between 1994 and 1996 (47) (Supplement 1). The Neurophysiological Study of Activity and Attention in Twins subset used in this study consisted of 67 male twin pairs in groups of 22 pairs concordant for high levels of ADHD symptoms (corresponding to a clinical diagnosis; MZ: 11; DZ: 11), 8 pairs discordant for ADHD symptoms (MZ: 2; DZ: 6), and 37 control pairs concordant for low levels of ADHD symptoms (MZ: 21; DZ: 16). For further information on selection of twins, see Supplement 1. Demographic characteristics are given in Tye et al. (35). No children were taking medication at the time of the study. The study was approved by King's College London Psychiatry, Nursing and Midwifery Research Ethics Subcommittee. Participating families gave their written informed consent.
Task
The task is identical to the version of the Eriksen arrow flanker paradigm used by us and colleagues in previous studies (2,48,49) and consisted of 10 blocks of 40 trials. Two flankers (black arrowheads above and below the position of a fixation mark) were presented for 100 milliseconds before the central target black arrowhead appeared for an additional 150 milliseconds. Participants had to press a response button with the index finger of the hand (left or right) corresponding to the direction indicated by the target arrow (left or right). On congruent trials, flanker and target arrowheads pointed in the same direction; on incongruent trials, they pointed in opposite directions. Further task details are available in Supplement 1. Performance measures were target reaction time ([MRT]; mean response latency in msec after target onset) and intraindividual variability in reaction time ([RTV]; standard deviation of response latency) in the congruent and incongruent correct trials separately.
IQ
General cognitive ability was assessed at age 14 as part of ongoing Twins' Early Development Study web-based data collection (50). The twins were tested on the Wechsler Intelligence Scale for Children as a Process Instrument vocabulary multiple choice subtests (51) and Raven's standard and advanced progressive matrices (52). Further information on the calculation of IQ is in Supplement 1.
EEG Recording and Processing
Electroencephalographic data were recorded using a 64-channel (BrainAmp DC; Brain Products, GmbH, Munich, Germany) extended 10–20 system montage (reference at FCz). Vertical and horizontal electro-oculogram data were simultaneously recorded from electrodes placed above and below the left eye and at the outer canthi. The EEG and electro-oculogram signals were digitized at a 500-Hz sampling rate. Data processing was performed offline using the EEGLAB toolbox (v11.0.3.1b) (53) for MATLAB (R2012a; The Mathworks, Inc., Natick, Massachusetts). Before processing, the channel signals were re-referenced to average reference. We then applied a 1-Hz high-pass filter. Time points with any channel value larger than 100 μV in absolute value were rejected from the data and excluded from further analysis. We further rejected the .8% of trials in which the button press occurred later than 1200 milliseconds after target presentation.
EEG Analyses
We used adaptive mixture ICA (46,54) to separate the channel data into maximally instantaneous independent component (IC) processes (Supplement 1). We computed equivalent dipole models for each IC scalp topography using a template four-layer adult boundary element method head model implemented in the DIPFIT toolbox for MATLAB (55). Independent components with less than 15% residual variance between estimated dipole projection and IC scalp topography were clustered based on dipole location and spectral and ERP features, yielding (among other clusters) a prominent central midline cluster (Supplement 1). Channel-based measures were calculated at the channel (Cz) where theta activity was maximal, after first removing projections to this channel from ICs accounting for eye movement and blink artifacts. As we were interested in neural activity associated with preparation of the motor response, we focused on theta oscillations in the window between the stimulus and the response.
Calculation of Theta Phase and Amplitude for Both Component and Channel EEG Data
Stimulus-locked theta phase and amplitude were calculated in single trials using a theta frequency of 7 Hz. Both channel- and IC-derived activations were normalized to unit baseline variance by dividing the signal amplitude in each condition by the root mean square power in the baseline interval (−600 to −100 msec, prestimulus). Theta phase and amplitude were computed based on the Fourier coefficients associated with the 7 Hz frequency in the poststimulus time window (further defined in Supplement 1). We computed four statistics related to two measures of central midline theta-band activity: 1) mean phase of the average ERP; 2) single-trial phase variability around this average ERP phase (indexed by interquartile range; i.e., difference 25th–75th percentiles); 3) amplitude mean of the single trial theta amplitudes; and 4) amplitude variability around this mean amplitude (standard deviation). We used interquartile range around the mean phase, as we were specifically interested in the variability of the phase around the well-determined mean ERP phase (Figure 1; Supplement 1).
Figure 1.
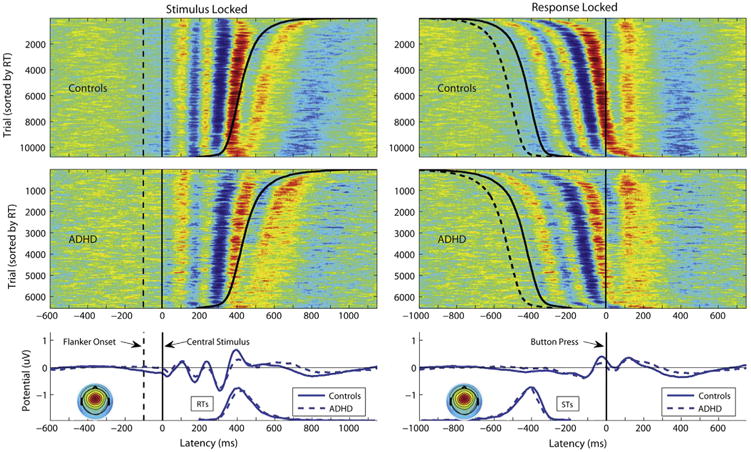
Stimulus-locked and response-locked single-trial and mean event-related potentials (ERPs) in the incongruent-correct condition for the extracted frontocentral independent component cluster. Event-related potential image plots (42) of single trials across control participants and attention-deficit/hyperactivity disorder (ADHD) participants ordered by reaction time (RT) and smoothed vertically with a 100-trial moving average. Color corresponds to relative potential in μV normalized to unit power in the baseline (red > 0, green = 0, blue < 0). Average ERP waveforms of the single trial ERP traces. Scalp maps show the mean interpolated scalp potential projection of the cluster, averaged over all participants. STs, stimulus onset times.
Statistical Analysis
Age and IQ significantly differed between ADHD and control groups (Table 1); these effects were therefore controlled for in the phenotypic analyses and regressed out of the data for the genetic analyses. All measures that had pronounced heterogeneity of variance and skewed distributions were log-transformed to minimize skew using the lnskew0 command in Stata (StataCorp, College Station, Texas). We corrected for nonindependent observations (e.g., genetic relatedness in twin pairs) in all analyses by using the robust clustering command (vce cluster in Stata) to estimate standard errors. The congruency effect was examined in a repeated measures analyses of variance (group, condition). The relationship between RTV and EEG variables was examined in four regression analyses with RTV as the predictor variable (source and scalp for each condition).
Table 1. Summary Statistics and Mean Comparisons Adjusted for Genetic Relatedness for Age, IQ, and ADHD Measures.
| ADHD | Control Subjects | F | p | |
|---|---|---|---|---|
| Age | 13.51 (.78) | 14.01 (.93) | 10.62 | <.001 |
| IQ | 97.13 (8.63) | 103.97 (12.31) | 12.20 | <.0001 |
| Parent Conners Hyperactivity-Impulsivity | .56 (1.11) | .08 (.54) | 12.92 | <.01 |
| Parent Conners Inattention | .86 (1.50) | .06 (.44) | 10.49 | <.01 |
| Teacher Conners Hyperactivity-Impulsivity | .35 (.94) | 0 (0) | 6.93 | .01 |
| Teacher Conners Inattention | 1 (1.94) | .06 (.31) | 11.32 | <.001 |
ADHD, attention-deficit/hyperactivity disorder.
Selection of Variables for Genetic Model-Fitting Analyses
The purpose of the genetic model fitting was to investigate the etiology of the phenotypic relationship between brain function variables and ADHD and RTV. Therefore, selection of variables for the genetic modeling was based on two criteria: 1) significant phenotypic relationship with ADHD, indexed by a case-control difference in the variable (Table 2); and 2) significant phenotypic relationship with RTV (Table 3). The amplitude measures (source and channel amplitude mean [AmM], amplitude variability [AmV]) were most strongly related to ADHD in the incongruent correct condition, whereas the phase measures (source phase variability [PhV] and phase mean [PhM] and channel PhM) were similarly related to ADHD in both conditions (Table 2). A number of EEG measures were related to RTV in the incongruent condition (Table 3), whereas only PhV (source and channel) and PhM (source) were related to RTV in the congruent condition. Therefore, we investigated the etiology of the phenotypic relationship between selected measures and ADHD and RTV where it was strongest, in the incongruent condition.
Table 2. Summary Statistics and Mean Comparisons (Group, Congruency) for Performance and EEG Measures.
| ADHD | Control Subjects | F(p), ADHD | F(p), Congruency | F(p), ADHD × Congruency | ||
|---|---|---|---|---|---|---|
| Performance | MRT | |||||
| Incongruent | 451.10 (59.13) | 433.31 (43.04) | 2.60 (.11) | 15.60 (<.0001)a | .04 (.86) | |
| Congruent | 355.36 (48.44) | 337.41 (39.11) | ||||
| RTV | ||||||
| Incongruent | 93.80 (29.62) | 76.90 (25.11) | 9.61 (.002)a | .001 (.98) | .06 (.81) | |
| Congruent | 92.98 (30.58) | 75.63 (25.06) | ||||
| Errors | ||||||
| Incongruent | 65.37 (25.31) | 64.18 (26.84) | .71 (.62) | 498.60 (<.0001)a | .55 (.74) | |
| Congruent | 11.08 (16.98) | 5.67 (9.44) | ||||
| Theta Source Activation | Phase variability | |||||
| Incongruent | 1.75 (.42) | 1.47 (.37) | 20.62 (<.0001)a | 63.62 (<.0001)a | .002 (.96) | |
| Congruent | 2.04 (.41) | 1.75 (.44) | ||||
| Mean phase | ||||||
| Incongruent | − 1.21 (1.29) | −1.48 (1.23) | 2.33 (.13) | 1.02 (.31) | .000 (.99)a | |
| Congruent | − 1.12 (1.00) | −1.40 (.97) | ||||
| Amplitude variability | ||||||
| Incongruent | 38.65 (9.18) | 46.28 (11.81) | 12.94 (<.0001)a | 191.16 (<.0001)a | 8.70 (.004)a | |
| Congruent | 31.95 (6.93) | 36.32 (9.65) | ||||
| Mean amplitude | ||||||
| Incongruent | 71.66 (18.85) | 88.33 (26.50) | 15.18 (<.0001)a | 223.70 (<.0001)a | 8.13 (.005)a | |
| Congruent | 55.04 (11.59) | 63.87 (17.04) | ||||
| Theta Channel Activation | Phase variability | |||||
| Incongruent | 1.73 (.49) | 1.52 (.48) | 6.39 (.01)a | 69.68 (<.0001)a | .03 (.87) | |
| Congruent | 2.07 (.49) | 1.88 (.54) | ||||
| Mean phase | ||||||
| Incongruent | −.94 (1.48) | − 1.61 (1.01) | 4.66 (.03)a | .187 (.67) | 7.26 (.008)a | |
| Congruent | −1.20 (.98) | −1.23 (1.11) | ||||
| Amplitude variability | ||||||
| Incongruent | 33.17 (6.90) | 35.35 (10.04) | .93 (.34) | 172.59 (<.0001)a | 3.70 (.06) | |
| Congruent | 27.76 (4.76) | 28.08 (7.24) | ||||
| Mean amplitude | ||||||
| Incongruent | 62.26 (15.02) | 68.19 (20.51) | 1.90 (.17) | 203.38 (<.0001)a | 5.53 (.02)a | |
| Congruent | 49.98 (8.55) | 51.07 (13.07) |
ADHD, attention-deficit/hyperactivity disorder; EEG, electroencephalographic; MRT, mean response latency; RTV, reaction time variability.
Significant findings.
Table 3. Regression of RTV on EEG Theta Indices.
| Incongruent Correct | Congruent Correct | ||
|---|---|---|---|
|
|
|
||
| RTV | RTV | ||
| Source | PhV | RC = .24, t136 = 2.96a | RC = 23.75, t136 = 4.15b |
| PhM | RC = .10, t136 = 1.79c | RC = 7.43, t136 = 3.31d | |
| AmV | RC = .81, t136 = 2.01c | RC = .04, t136 = .05 | |
| AmM | RC = −.58, t136 = −2.24d | RC = .07, t136 = .15 | |
| Channel | PhV | RC = .29, t136 = 3.59b | RC = 19.30, t136 = 3.80b |
| PhM | RC = .12, t136 = 2.57d | RC = 2.81, t136 = 1.24 | |
| AmV | RC = .01, t136 = 1.73c | RC = .50, t136 = .42 | |
| AmM | RC = −.01, t136 = −1.27 | RC = −.02, t136 = −.03 |
AmM, mean amplitude; AmV, variability in amplitude; EEG, electroencephalographic; PhM, mean phase; PhV, phase variability; RC, regression coefficient; RTV, reaction time variability.
Significant at p = .01 level.
Significant at p = .001 level.
Significant at p ≤ .05 level.
Trend: p = .10.
Due to a significant phenotypic relationship with both ADHD and RTV, the following variables were selected for the genetic model fitting: theta source PhV and AmM and theta channel PhV and PhM (Tables 2 and 3). Neither MRT nor errors were included in the genetic model fitting, as they did not have significant phenotypic associations with ADHD (Table 2).
Preparation of Data for Model Fitting
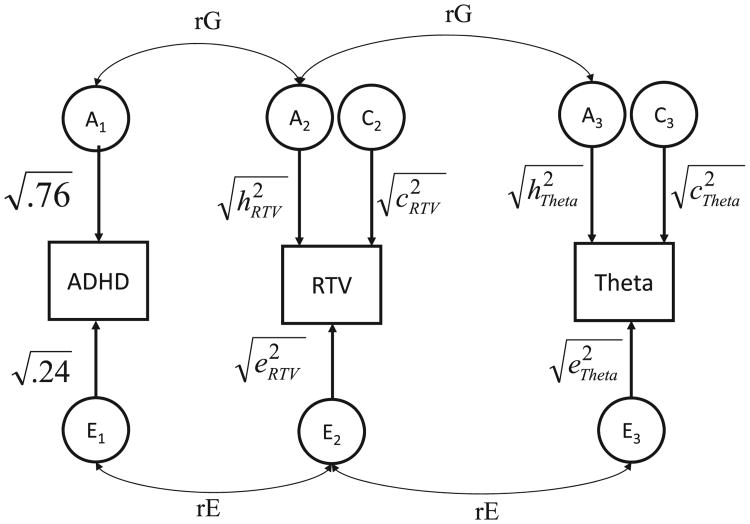
To account for the selected nature of the sample, the group selection variable (ADHD status) was modeled as a threshold, or categorical, variable (yes/no), with its parameters fixed. This inclusion necessitated ordinalizing the age- and IQ-regressed residual scores of the EEG and cognitive variables. Using threshold liability model fitting on the ordered categories in Mx (Richmond, Virginia) (56), polychoric correlations and genetic model parameters were derived using full information maximum likelihood estimation. A limitation of this approach is that the numerical integration increases exponentially with the number of variables, placing a practical limit on the number of variables in the model. Therefore, in each model, we included two variables (RTV and one theta measure) plus the necessary selection variable. The threshold for ADHD status was fixed to give a population prevalence of 5% (Z score set at 1.64); twin correlations and heritability parameters were fixed to population estimates as derived from a meta-analysis (57) (h2 = 76%, c2 = 0%, e2 = 24%, consistent with rMZ = .76 (h2 + c2), rDZ = .38 (.5h2 + c2).
Polychoric Correlations
Correlations were estimated from a constrained phenotypic correlation model to give, for each cognitive and EEG measure, maximum likelihood estimates of the intraindividual cross-trait correlations (i.e., the degree to which two variables covary), the MZ and DZ cross-twin within-trait correlations (e.g., the correlation between one twin's RTV and the co-twin's RTV), and the MZ and DZ cross-twin cross-trait correlations (e.g., the correlation between one twin's RTV and the co-twin's EEG).
Genetic Model Fitting
Based on the biometrical genetic model that MZ twins share 100% and DZ twins share 50% of their genetic influences (but have an equal sharing in terms of environmental factors) (58), the applied genetic multivariate, correlated-factors liability-threshold model (illustrated in Figure 2) uses the MZ:DZ ratio of the cross-twin within-trait correlations to decompose the variation in the cognitive and EEG traits into additive genetic (referred to as A), common environmental (C), and individual-specific environmental (E) influences including measurement error. Furthermore, the MZ:DZ ratio of the cross-trait, cross-twin correlations is used to decompose the covariation between traits (e.g., RTV, theta measure, and ADHD) into genetic (rG), common environmental (rC), and nonshared environmental (rE) correlations. (Because of the lack of C effects on ADHD, there are no rCs with the other traits; Figure 2). Since it is possible for a large rG or rE to explain a very small portion of the observed covariation between two traits, we combine the information from these correlations with the standardized estimates of each trait to establish the genetic (rph-a) and unique environmental (rph-e) contributions to the total phenotypic correlation (rPh) between ADHD and theta measures (35,37).
Figure 2.
Correlated factors solution of the trivariate model for attention-deficit/hyperactivity disorder (ADHD), variability in reaction time (RTV), and electroencephalographic theta measures. Circles represent latent additive genetic (A1, A2, A3), shared environmental (C1, C2, C3), and nonshared environmental (E1, E2, E3) factors. rG and rE represent genetic and environmental correlations between ADHD, RTV, and theta activity. Parameters for ADHD (heritability and unique environmental estimates) are fixed values selected according to meta-analysis (57).
Results
Phenotypic Analyses
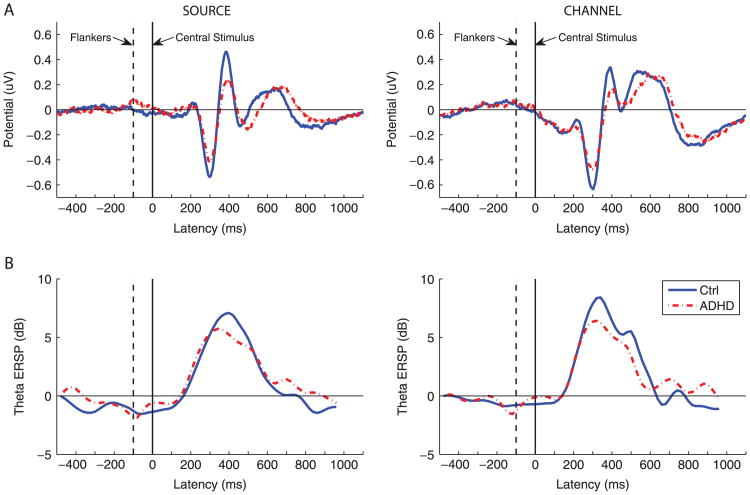
A main effect of ADHD was evident for RTV, source PhV, source AmV, source AmM, channel PhV, and channel AmM (Table 2, Figure 1). A main effect of congruency emerged for most performance and EEG variables: MRT, source PhV, source AmV, source AmM, channel PhV, channel AmV, and channel AmM (Table 2). A significant group by congruency interaction where the ADHD participants did not show a change between the conditions but the control subjects did show a change was evident in the amplitude measures (mean and variability at both source and channel) and mean phase at the channel level (Figure 3; Table 2).
Figure 3.
Interference effects on the mean event-related potential amplitude and mean theta power in attention-deficit/hyperactivity disorder (ADHD) and control (Ctrl) subjects. (A) Event-related potentials for the central midline independent component (left) and Cz channel average (right). The difference in mean potentials between the congruent and the incongruent conditions is plotted. (B) The difference in theta power between the congruent and the incongruent conditions in a 350-millisecond sliding window event-related spectral perturbation (ERSP).
Twin Correlations and Genetic Model Fitting
The larger MZ compared with DZ cross-twin within-trait correlations for RTV and all EEG variables indicated a contribution of genetic factors to individual differences in these variables. Similarly, larger MZ compared with DZ cross-twin cross-trait correlations indicated shared genetic variance between the traits (Table 4). In line with these correlations, the genetic model fitting indicated significant heritability estimates for RTV and for all of the selected theta-band EEG measures: RTV had the highest point estimate (.59). However, the confidence intervals overlapped for all heritability estimates, which indicated no significant differences in heritability between any of the variables in this sample (Table 5). Our analyses did not indicate significant shared environmental influences on any measure other than phase variability in the channel space (Table 5).
Table 4. MZ and DZ Cross-Twin Within-Trait Correlations for all Variables and Cross-Twin Cross-Trait Correlations between RTV/EEG Variables and ADHD and the EEG Variables and RTV.
| Twin 1 | Cross-Twin Within-Trait | Cross-Twin Cross-Trait: With ADHD | Cross-Twin Cross-Trait: With RTV | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Twin 2 | Twin 2 | Twin 2 | ||||
|
|
|
|
||||
| MZ | DZ | MZ | DZ | MZ | DZ | |
| RTV | .58 (.36 to .76)a | .07 (−.37 to .46) | .43 (.21 to .59)a | .33 (.09 to .53)a | ||
| Source PhV | .42 (.21 to .59)a | .15 (.10 to .66)a | .54 (.33 to .69)a | .31 (.09 to .51)a | .43 (.09 to .68)a | −.02 (−.44 to .30) |
| Source AmM | .63 (.53 to .83)a | .44 (.34 to .46)a | −.51 (−.69 to −.30)a | −.32 (−.46 to −.11)a | −.23 (−.53 to .12) | −.06 (−.36 to .37) |
| Channel PhV | .46 (.13 to .48)a | .37 (−.06 to .59) | .25 (.21 to .62)a | .26 (.01 to .64)a | .37 (−.05 to .68) | .16 (−.19 to .47) |
| Channel PhM | .51 (.18 to .59)a | .11 (−.32 to .47) | .19 (.03 to .52)a | .11 (.09 to .32)a | .24 (−.11 to .53) | 0 (−.45 to .45) |
The MZ and DZ cross-twin correlations for ADHD are fixed to population values in all models to account for selection: rMZ = .76 and rDZ = .38 and the threshold to a population prevalence of 5%. All incongruent correct. Twins are randomly assigned to be Twin 1 and Twin 2.
ADHD, attention-deficit/hyperactivity disorder; AmM, mean amplitude; AmV, variability in amplitude; DZ, dizygotic; EEG, electroencephalographic; MZ, monozygotic; PhM, mean phase; PhV, phase variability; RTV, reaction time variability.
Significant estimates.
Table 5. Standardized Estimates (with 95% CI) of Genetic, Common, and Nonshared Environmental Contributions to Variance of RTV and EEG Measures.
| h2 | c2 | e2 | |
|---|---|---|---|
| RTV | .59 (.20 to .77)a | 0 (0 to.48) | .41 (.23 to .49)a |
| Source PhV | .45 (.09 to .68)a | 0 (0 to.59) | .55 (.32 to .95)a |
| Source AmM | .52 (.12 to .76)a | 0 (0 to.38) | .48 (.24 to .88)a |
| Channel PhV | .17 (.15 to .49)a | .27 (.11 to .92)a | .44 (.17 to .89)a |
| Channel PhM | .43 (.41 to .65)a | 0 (0 to.72) | .57 (.52 to .99)a |
h2, c2 and e2: standardized genetic and environmental variance components. For ADHD, these are fixed to h2 = .76 and e2 = .24. All incongruent correct.
ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval; EEG, electroencephalographic; AmM, mean amplitude; AmV, variability in amplitude; PhM, mean phase; PhV, phase variability; RTV, reaction time variability.
Significant estimates.
Both source PhV and AmM exhibited significant genetic correlations with ADHD symptoms, which suggests that about half their genetic influences are shared with ADHD (rG = .51 and −.61). The negative genetic correlation between ADHD and source AmM indicates that the genetic influences that cause lower mean theta amplitude also cause increased ADHD symptoms. A significant amount of genetic influences were also shared between RTV and ADHD (rG = .39), which replicates previous findings (Table 6). Neither channel measure (PhM or PhV) shared significant genetic overlap with ADHD. Variability in reaction time, source PhV, and source AmM all showed a moderate amount of phenotypic overlap with ADHD, with around half of the phenotypic variance shared between these traits and ADHD (Table 6). These estimates were significantly larger than the phenotypic correlation between ADHD and channel PhV (Table 6, first column).
Table 6. Decomposition of the Correlations (rPh) between ADHD and RTV/EEG Measures into Genetic (rph-a) and Environmental (rph-e) Components and the Overlap in Genetic (rG) and Environmental (rE) Factors between Them.
| ADHD | |||||
|---|---|---|---|---|---|
|
| |||||
| rPh | rph-a | rph-e | rG | rE | |
| RTV | .47 (.30 to.51)a | .22 (.02 to.43)a | .24 (.09 to .37)a | .39 (.33 to .79)a | .68 (.22 to .92)a |
| Source PhV | .48 (.38 to .66)a | .30 (−.08 to .37)a | .18 (.02 to.19)a | .51 (.15 to .99)a | .50 (.05 to .85)a |
| Source AmM | −.45 (−.60 to −.27)a | −.38 (−.58 to −.15)a | −.07 (−.58 to −.09) | −.61 (−1 to −24)a | −.20 (−.68 to .31) |
| Channel PhV | .25 (.09 to .25)a | .22 (−.15 to .55) | .02 (−.17 to .29) | .80 (−1 to .82) | .03 (−.35 to .37) |
| Channel PhM | .33 (−.24 to .33) | .15 (−.19 to .17) | .19 (−.71 to .19) | .25 (−.03 to .1) | .52 (−.35 to .66) |
rG, rE: The correlation between genetic and nonshared environmental factors. These are combined with the standardized estimates of the traits (Table 4) to establish the genetic (rph-a) and unique environmental (rph-e) contributions to the total phenotypic correlation (rPh). All incongruent correct.
ADHD, attention-deficit/hyperactivity disorder; EEG, electroencephalographic; AmM, mean amplitude; AmV, variability in amplitude; PhM, mean phase; PhV, phase variability; RTV, reaction time variability.
Significant estimates.
Source PhV showed the largest genetic (.66) and phenotypic correlation (.49) with RTV and also the largest genetic contribution to the phenotypic correlation (.34). Although, these estimates were not significantly different from the genetic and phenotypic RTV-channel PhV correlations (Table 7). Neither source AmM nor channel PhM had a significant genetic association with RTV. The negative phenotypic correlation between RTV and source AmM suggests that phenotypically reduced theta amplitude is associated with increased RTV (Table 7).
Table 7. Decomposition of the Correlations (rPh) between RTV and EEG Measures into Genetic (rph-a) and Environmental (rph-e) Components and the Overlap in Genetic (rG) and Environmental (rE) Factors between Them.
| RTV | |||||
|---|---|---|---|---|---|
|
| |||||
| rPh | rph-a | rph-e | rG | rE | |
| Source PhV | .49 (.27 to.69)a | .34 (.1 to.58)a | .15 (0 to .38) | .66 (.14 to .99)a | .32 (−.10 to.63) |
| Source AmM | −.34 (−.55 to −.13)a | −.18 (−.44 to .14) | −.17 (−.27 to −.01)a | −.31 (−.93 to .37) | −.38 (−.66 to −.01)a |
| Channel PhV | .18 (.17 to .44)a | .20 (.18 to .33)a | −.01 (−.37 to .34) | .54 (.22 to .99)a | −.02 (−.11 to .71) |
| Channel PhM | .34 (.13 to .44)a | .18 (−.27 to .47) | .15 (−.03 to .48) | .40 (−.1 to .99) | .29 (−.06 to .58) |
rG, rE: The correlation between genetic and nonshared environmental factors. These are combined with the standardized estimates of the traits (Table 4) to establish the genetic (rph-a) and unique environmental (rph-e) contributions to the total phenotypic correlation (rPh).
EEG, electroencephalographic; AmM, mean amplitude; AmV, variability in amplitude; PhM, mean phase; PhV, phase variability; RTV, reaction time variability.
Significant estimates.
Discussion
Results from these multivariate twin analyses indicate that frontal midline theta activity following target stimulus onset in a manual choice-response task is strongly related, both phenotypically and genetically, to both ADHD symptoms and RTV. Attention-deficit/hyperactivity disorder was most strongly related to the mean amplitude and to variability (i.e., inconsistency) in its trial-to-trial phase at the source level, with these measures showing moderate to large phenotypic and genetic correlations with the disorder. The strong phenotypic and genetic link between ADHD and abnormal theta dynamics indicates that ADHD is related, in part, to impairments in the capacity to orchestrate, coordinate, and direct basic cognitive processes. This finding is in agreement with previous data indicating familial overlap between ADHD diagnosis and impairments in channel-based ERP features known to be related to cognitive control (1,2). These findings indicate that mean amplitude and phase variability of event-related frontal midline theta-band oscillations may be biological markers or candidate intermediate phenotypes for ADHD.
We additionally found that PhV of frontal midline theta, measured at either the source or channel level, was genetically and phenotypically associated with RTV. We speculate that the variability in the latency of the theta complex in ADHD may reflect dysregulation of signaling to implement and optimize results of task-relevant responding and/or recovery strategies. In support of this, previous evidence indicates that frontal midline theta oscillations may be coupled to bursts of dopaminergic activity in basal ganglia that index perceived signal/goal conflicts, unexpected reward opportunities, or unexpected changes in anticipated outcome of current behavior (16). In such circumstances, the phase of the associated theta band field activity regulates the timing and simultaneity of more detailed, spike-conveyed information that can organize behavioral adjustments required to achieve goals (59–61). Our data indicate that abnormality in such phasic signaling may index a systemic deficiency in ADHD that is related to behavioral impairments (specifically to increased variability in response times) and thus advances the search for pathophysiological markers of the disorder. Our interpretation is consistent with previous findings that indicate that trial-by-trial frontal theta activity power is inversely related to occipital alpha in typically developing children but the relationship is absent in ADHD, which suggests impairment in cognitive control of perceptual systems in posterior cortex (62).
Though both the channel- and source-based theta PhV measures were similarly related to RTV, source-based PhV was more strongly related to ADHD, both genetically and phenotypically, than channel-based PhV. Source-based AmM was also strongly related to ADHD symptoms, genetically and phenotypically. Our data thus indicate that the improved functional and anatomic separation of the cortical signal sources of EEG data produced by ICA decomposition gives measures that are more etiologically informative for ADHD than channel-based measures. This may be because the channel data are a source signal admixture, so that the effective signal-to-noise ratio of the ICA-separated cortical source activities is much higher than for the scalp channel signals (45).
All of the theta measures that were associated with ADHD and RTV were moderately heritable, agreeing with the large literature that indicates EEG measures are under genetic influence (63,64). Although in this study we investigated preresponse stimulus-locked theta, our finding that the mean source amplitude of the frontal midline theta band is moderately heritable aligns with previous findings indicating moderate heritability of response-locked frontal midline theta activity (error-related negativity) (65).
The significant group by (conflict) condition interactions for the amplitude measures in the phenotypic analyses replicate previous findings showing an attenuation of theta-related brain activity in high-conflict tasks in ADHD (1,2) and agree with studies indicating impaired conflict resolution in ADHD (66). The lack of interaction between group and conflict for RTV and the phase measures indicates that the conflict effect in this study is largely specific to amplitude measures. Cognitive theories of ADHD differ in whether they propose a single underlying cause for the widespread behavioral and cognitive impairments of ADHD, such as a proposed executive function deficit (67) or default mode interference (68), or whether they propose multiple etiological pathways (69). Our findings confirm the importance of executive attention in ADHD pathophysiology but also suggest that theta phase abnormalities in ADHD are independent of demands on the executive attention or cognitive control system, consistent with a model of multiple pathways to the disorder.
One limitation is that the sample size is relatively small for a twin study. Although we were able to identify a number of significant modest to large genetic and phenotypic relationships between ADHD, RTV, and theta measures, we were not able to definitively identify which relationships were significantly stronger because of the overlapping confidence intervals. Nevertheless, the combination of the significant MZ correlations and the significant association between these parameters and ADHD suggest a relationship that has a genetic basis. The limited statistical power restricted the estimation of shared environmental influences (as suggested by some of the twin correlations) and nonadditive genetic influences (particularly suggested by cross-twin cross-trait correlations between RTV and EEG variables). We acknowledge that such influences may have affected the estimates of heritability and genetic overlap between ADHD, RTV, and theta measures in our study; however, the genetic contribution presented is not overestimated. Further analyses in larger twin samples should investigate whether the genetic and phenotypic distinction between these neurophysiological phenotypes in relation to ADHD/RTV is significant.
As we were not able to test causal models with our data, it is possible that the shared genetic variance between theta phase variability and RTV is due to pleiotropic effects or other factors that influence both theta phase variability and RTV. In line with our findings, ADHD is increasingly being conceived as a disorder underpinned by dysfunctions in multiple large-scale brain networks (62,70,71). Future studies should take a systems neuroscience perspective and examine how theta phase and amplitude interact with the task positive and task negative, or default, networks that are implicated in ADHD (35,68,70). These measures should be tested in pharmacologic and longitudinal samples to parse out the causal effects.
In summary, in an analysis combining multivariate twin modeling and computational neuroscience methods, we found evidence that inefficient phasic signaling in the frontal midline cortex is strongly related to RTV and ADHD symptoms, both phenotypically and genetically. This is the first finding that confirms the genetic link between frontal midline EEG activity and ADHD, as previously suggested by family studies (1,2). Our data identify a neurophysiological marker of RTV in ADHD that may be part of a dysfunctional brain network that impairs production of optimal response behavior in the disorder. Our results further indicate that source decomposition of EEG data by ICA can provide more effective measures of cortical theta dynamics than channel-based measures. As source-based measures characterize neural activity more efficiently than scalp-channel measures, ICA may facilitate development of more statistically robust, targeted, noninvasive neuroscience-based phenotypes for genetic analyses in further family and twin based studies, including whole genome and gene-environment relationship studies (72).
Supplementary Material
Acknowledgments
We gratefully acknowledge the participating families and all staff involved in this study, in particular, Charlotte Tye, Chloe Booth, Sarah Lewis, Stuart Newman, Rebecca Pinto, Jonna Kuntsi, Phil Asherson, the Twins' Early Development Study research team, and the Director of Twins' Early Development Study, Robert Plomin.
Dr. McLoughlin was supported for this work by a National Institute for Health Research (United Kingdom) fellowship and a Royal Society (United Kingdom) award. Drs. Palmers and Makeig were supported by the Swartz Foundation.
Footnotes
Dr. Rijsdijk reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.07.020.
References
- 1.Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M, et al. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: Evidence for an endophenotype. Biol Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J. Performance monitoring is altered in adult ADHD: A familial event-related potential investigation. Neuropsychologia. 2009;47:3134–3142. doi: 10.1016/j.neuropsychologia.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. Am J Psychiatry. 1999;158:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 4.Wiersema JR, van der Meere JJ, Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. J Neural Transm. 2005;112:1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- 5.Groom MJ, Cahill JD, Bates AT, Jackson GM, Calton TG, Liddle PF, Hollis C. Electrophysiological indices of abnormal error-processing in adolescents with attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2010;51:66–76. doi: 10.1111/j.1469-7610.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 6.van Meel CS, Oosterlaan J, Heslenfeld DJ, Sergeant JA. Telling good from bad news: ADHD differentially affects processing of positive and negative feedback during guessing. Neuropsychologia. 2005;43:1946–1954. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 8.van Meel CS, Heslenfeld DJ, Oosterlaan J, Luman M, Sergeant JA. ERPs associated with monitoring and evaluation of monetary reward and punishment in children with ADHD. J Child Psychol Psychiatry. 2011;52:942–953. doi: 10.1111/j.1469-7610.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 9.van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Res. 2007;151:211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makeig S, Delorme A, Westerfield M, Jung TP, Townsend J, Courchesne E, Sejnowski TJ. Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol. 2004;2:e176. doi: 10.1371/journal.pbio.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto T, Shimazu H, Isomura Y, Sasaki K. Theta oscillations in primate prefrontal and anterior cingulate cortices in forewarned reaction time tasks. J Neurophysiol. 2010;103:827–843. doi: 10.1152/jn.00358.2009. [DOI] [PubMed] [Google Scholar]
- 15.Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavanagh JF, Figueroa CM, Cohen MX, Frank MJ. Frontal theta reflects uncertainty and unexpectedness during exploration and exploitation. Cereb Cortex. 2012;22:2575–2586. doi: 10.1093/cercor/bhr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol. 2000;83:1701–1709. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front Psychol. 2011;2:30. doi: 10.3389/fpsyg.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- 24.Kuntsi J, Wood AC, van der MJ, Asherson P. Why cognitive performance in ADHD may not reveal true potential: Findings from a large population-based sample. J Int Neuropsychol Soc. 2009;15:570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamo N, Di MA, Esu L, Petkova E, Johnson K, Kelly S, et al. Increased response-time variability across different cognitive tasks in children with ADHD. J Atten Disord. 2012 doi: 10.1177/1087054712439419. published online ahead of print April 16. [DOI] [PubMed] [Google Scholar]
- 26.Saville CW, Shikhare S, Iyengar S, Daley D, Intriligator J, Boehm SG, et al. Is reaction time variability consistent across sensory modalities? Insights from latent variable analysis of single-trial P3b latencies. Biol Psychol. 2012;91:275–282. doi: 10.1016/j.biopsycho.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS One. 2009;4:e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry. 2007;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Tamm L, Narad ME, Antonini TN, O'Brien KM, Hawk LW, Jr, Epstein JN. Reaction time variability in ADHD: A review. Neurotherapeutics. 2012;9:500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuntsi J, Stevenson J. Psychological mechanisms in hyperactivity: II. The role of genetic factors. J Child Psychol Psychiatry. 2001;42:211–219. [PubMed] [Google Scholar]
- 33.Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67:1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. J Child Psychol Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tye C, Rijsdijk F, Greven CU, Kuntsi J, Asherson P, McLoughlin G. Shared genetic influences on ADHD symptoms and very low-frequency EEG activity: A twin study. J Child Psychol Psychiatry. 2012;53:706–715. doi: 10.1111/j.1469-7610.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuntsi J, Rogers H, Swinard G, Borger N, van der MJ, Rijsdijk F, Asherson P. Reaction time, inhibition, working memory and ‘delay aversion’ performance: Genetic influences and their interpretation. Psychol Med. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toulopoulou T, Picchioni M, Rijsdijk F, Hua-Hall M, Ettinger U, Sham P, Murray R. Substantial genetic overlap between neurocognition and schizophrenia: Genetic modeling in twin samples. Arch Gen Psychiatry. 2007;64:1348–1355. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- 38.Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc Natl Acad Sci U S A. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makeig S, Muller MM, Rockstroh B. Effects of voluntary movements on early auditory brain responses. Exp Brain Res. 1996;110:487–492. doi: 10.1007/BF00229149. [DOI] [PubMed] [Google Scholar]
- 40.Makeig S, Westerfield M, Jung TP, Covington J, Townsend J, Sejnowski TJ, Courchesne E. Functionally independent components of the late positive event-related potential during visual spatial attention. J Neurosci. 1999;19:2665–2680. doi: 10.1523/JNEUROSCI.19-07-02665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 42.Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- 43.Luck SJK, Kappenman ES. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; 2012. [Google Scholar]
- 44.Debener S, Ullsperger M, Siegel M, Engel AK. Towards single-trial analysis in cognitive brain research. Trends Cogn Sci. 2007;11:502–503. doi: 10.1016/j.tics.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Delorme A, Palmer J, Onton J, Oostenveld R, Makeig S. Independent EEG sources are dipolar. PLoS One. 2012;7:e30135. doi: 10.1371/journal.pone.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): A multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res. 2002;5:444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 48.Albrecht B, Banaschewski T, Brandeis D, Heinrich H, Rothenberger A. Response inhibition deficits in externalizing child psychiatric disorders: An ERP-study with the Stop-task. Behav Brain Funct. 2005;1:22. doi: 10.1186/1744-9081-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eriksen CW, Schultz DW. Information processing in visual search: A continuous flow conception and experimental results. Percept Psychophys. 1979;25:249–263. doi: 10.3758/bf03198804. [DOI] [PubMed] [Google Scholar]
- 50.Haworth CM, Harlaar N, Kovas Y, Davis OS, Oliver BR, Hayiou-Thomas ME, et al. Internet cognitive testing of large samples needed in genetic research. Twin Res Hum Genet. 2007;10:554–563. doi: 10.1375/twin.10.4.554. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler D. Wechsler Intelligence Scale for Children—Third Edition UK. London: The Psychological Corporation; 1992. [Google Scholar]
- 52.Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- 53.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Palmer JA, Kreutz-Delgado K, Makeig S. Super-Gaussian mixture source model for ICA. Lect Notes Comput Sci. 2006;3889:854–861. [Google Scholar]
- 55.Oostenveld R, Oostendorp TF. Validating the boundary element method for forward and inverse EEG computations in the presence of a hole in the skull. Hum Brain Mapp. 2002;17:179–192. doi: 10.1002/hbm.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neale MC. Mx: Statistical Modelling. Richmond, VA: Department of Psychiatry, Medical College of Virginia; 1997. [Google Scholar]
- 57.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 58.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 5th. New York: Worth Publishers; 2008. [Google Scholar]
- 59.Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front Hum Neurosci. 2010;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 62.Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, Berry AS, Corbett BA. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 63.van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- 64.Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 65.Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 66.Johnson KA, Robertson IH, Barry E, Mulligan A, Daibhis A, Daly M, et al. Impaired conflict resolution and alerting in children with ADHD: Evidence from the Attention Network Task (ANT) J Child Psychol Psychiatry. 2008;49:1339–1347. doi: 10.1111/j.1469-7610.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 67.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 68.Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJ. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 69.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 70.Castellanos FX, Proal E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.