Abstract
Stereoselective synthesis of a cyclopentane nucleus by convergent annulations constitutes a significant challenge for synthetic chemists. Though a number of biologically relevant cyclopentane natural products are known, more often than not, the cyclopentane core is assembled in a stepwise fashion due to lack of efficient annulation strategies. Herein, we report the rhodium-catalyzed reactions of vinyldiazoacetates with (E)-1,3-disubstituted 2-butenols generate cyclopentanes, containing four new stereogenic centers with very high levels of stereoselectivity (99% ee, >97 : 3 dr). The reaction proceeds by a carbene–initiated domino sequence consisting of five distinct steps: rhodium–bound oxonium ylide formation, [2,3]-sigmatropic rearrangement, oxy-Cope rearrangement, enol–keto tautomerization, and finally an intramolecular carbonyl ene reaction. A systematic study is presented detailing how to control chirality transfer in each of the four stereo-defining steps of the cascade, consummating in the development of a highly stereoselective process.
INTRODUCTION
Construction of cyclopentanes constitutes an area of significant interest in the organic community,1–3 as evidenced by numerous campaigns toward landmark natural products, including the prostaglandins,4–16 jatrophanes,17–25 and pactamycin.26,27 Synthetic cyclopentanes are a central motif common to prostaglandin antagonist antiglaucoma agents.28–32 Indeed, the convergent, asymmetric synthesis of a cyclopentane nucleus bearing multiple chiral centers presents a distinct challenge for modern synthetic chemists. A general difficulty for a [3 + 2]-cycloaddition approach is developing a mild protocol to render a transient carbon dipole. One of the classic strategies for chiral cyclopentene synthesis involves stereoselective cyclopropanation of a 1,3-diene, followed by vinylcyclopropane rearrangement.33–36 Contemporary efforts have involved the coupling of a suitably activated olefinic motif with an all carbon (1,3)-dipole bound to a chiral metal center via a formal [3+2]-cycloaddition.37–40 A significant advancement has been the enantioselective cycloaddition of palladated trimethylenemethane with electron-deficient alkenes developed by Trost and co-workers.41–43 Alternatively, Fu44,45 and Zhang46 have disclosed enantioselective variants of the Lu cycloaddition47–53 implementing chiral phosphine catalysts. Though a number of methods are available, the development of complementary strategies for the rapid construction of complex cyclopentanes is nonetheless desirable.
In this article, we describe a convergent, stereoselective synthesis of cyclopentanes with four stereogenic centers from an annulation cascade involving vinyldiazoacetates and chiral allyl alcohols. Moreover, a detailed analysis to account for correlations between starting material architecture and reaction efficacy is described.
RESULTS
1st Generation Synthesis
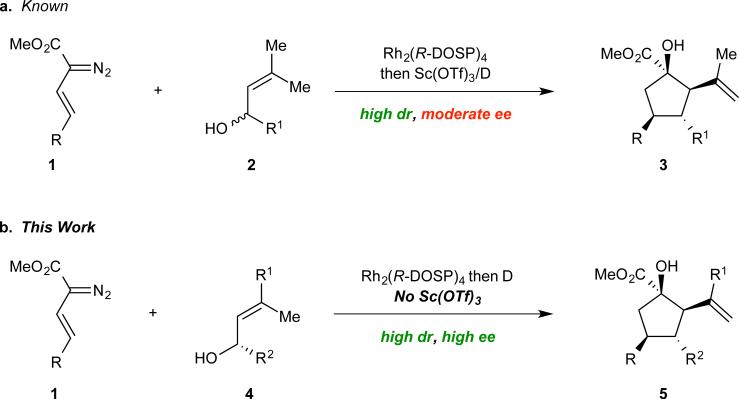
In a 2011 report, we described our initial attempts to synthesize complex cyclopentanes 3 by means of a cascade sequence.54 The strategy involved the coupling of racemic 3,3-dimethyl allyl alcohols (2) and donor/acceptor-substituted Rh(II) carbenes55,56 derived from vinyldiazoacetates 1 in the presence of the dual-catalyst system, Rh2(DOSP)4 and Sc(OTf)3, shown in Figure 1a. Implementing 1 mole % of chiral Rh catalyst enabled a high yielding preparation of a range of cyclopentanes 3, containing four stereogenic centers, as single diastereomers. Though we were pleased with the general efficacy with which a high degree of structural complexity was introduced from readily available building blocks, the reaction was characterized by a significant shortcoming: the enantioselectivity for the synthesis of 3 was variable (68–92% ee). In this paper we describe our diagnosis of the stereochemical limitations in our initial study, and the logical design of a stereocontrolled cyclopentane 5 synthesis, as illustrated in Figure 1b.
Figure 1.
Evolution of a Convergent Cyclopentane Synthesis. (a) One-pot synthesis of a cyclopentane (3) from a vinyldiazoacetate (1) and racemic allyl alcohol (2) in modest enantioselectivity via a rhodium-initiated cascade. (b) A second generation cyclopentane (5) synthesis from analogous starting materials in high stereoselectivity.
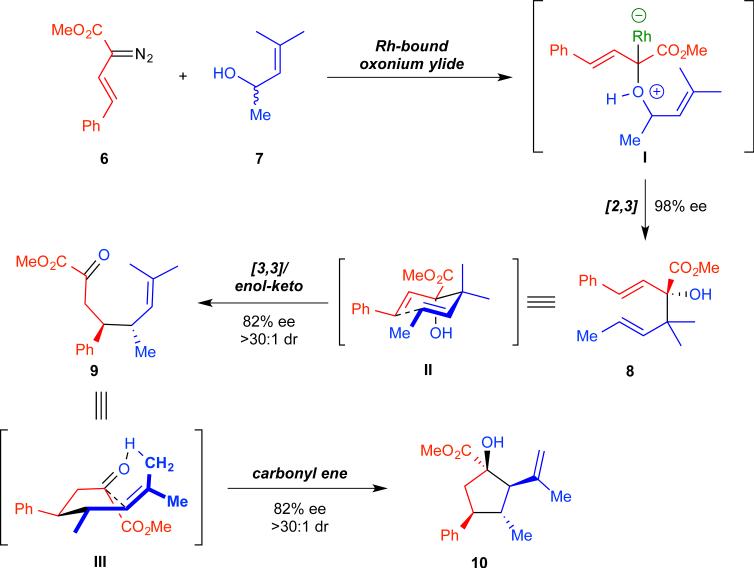
Our previous synthesis of cyclopentanes 3 involved four discrete, stereo-defining reactions, as illustrated for the specific example in Figure 2.52 The steps include: Rh–bound oxonium ylide formation (6 and 7 → I), [2,3]-sigmatropic rearrangement (I → 8),55 oxy-Cope rearrangement (8 → II → 9), and intramolecular carbonyl ene reaction (9 → III → 10). Although the metal–bound ylide intermediate I is neither isolated nor observed in the tandem reaction, its intermediacy is well precedented in the literature.54–60 From previous studies on the tandem ylide formation/[2,3]-sigmatropic rearrangement61 as well as control studies,52 we have analyzed the stereochemically relevant steps in the cascade sequence. We observed excellent enantiocontrol during the rhodium-catalyzed process for generating the 3-hydroxy-1,5-hexadiene 8 (98% ee). Intermediate 8 then underwent a [3,3]-sigmatropic rearrangement through a chair-like transition-state (II). Upon a subsequent enol–keto tautomerization, the product 9, containing two new stereocenters, was furnished. The oxy-Cope rearrangement afforded a single diastereomer of ketoester 9; an observation consistent with chair-like transition states being solely operative.52,61– 64 Isolation and chiral HPLC analysis of 9, however, indicated partial racemization during the course of this step of the cascade sequence. The terminating sequence of the cascade – intramolecular carbonyl ene reaction via III – proceeded smoothly to furnish cyclopentane 10 as a single diastereomer. No further degradation in enantiopurity was observed during the ene reaction. These studies identify the origins of the moderate overall enantioinduction: modest chirality transfer during the oxy-Cope rearrangement.
Figure 2.
Mechanism of cyclopentane synthesis. Mechanism of the one-pot domino sequence for the synthesis of a cyclopentane (10) from a diazoacetate (6) and a racemic allyl alcohol (7).
Stereochemical Limitations
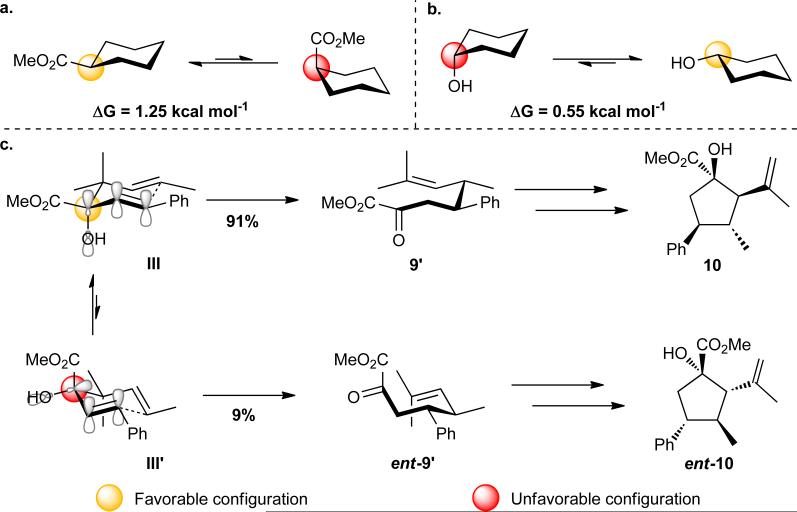
A rationale for the erosion in enantiopurity observed during the course of the oxy-Cope rearrangement is provided schematically in Figure 3. Based upon cyclohexane A–values, the equatorial versus axial positioning of carbomethoxy (Figure 3a) and hydroxy (Figure 3b) substituents would be favored by 1.25 and 0.55 kcal mol−1, respectively.65 Equilibration between two possible chair-like intermediates for the [3,3]-sigmatropic rearrangement, shown in Figure 3c, is more complicated. Both geminal disubstitution66 and pseudoanomeric stabilization of the allyl hydroxy group67 influence the equilibrium between III and III´. Based upon the A–values of the individual substituents and the psuedoanomeric stabilization afforded III, we can rationalize the modest preference for that pathway. Nevertheless, rearrangement via the energetically less favorable intermediate III´ would also be feasible. Upon undergoing the Cope rearrangement, two of the stereogenic centers found in the cyclopentane (10), C(3) and C(4), have been installed. As can be seen from comparison of 9´ and ent-9´, the competing chair-like transition states give rise to enantiomeric intermediates. Thus, enantiomers of the cyclopentane, 10 and ent-10, arise from the competition between III and III´. The 82% ee observed for the cyclopentane 10 synthesis is due to approximately a ten-to-one preference for III over III´.
Figure 3.
Stereoelectronic considerations for an oxy-Cope rearrangement. Cyclohexane A–values for carbomethoxy (a) and hydroxy (b) substituted cyclohexanes. Manifestation of stereoelectronic effects in the competition between two chair-like transition states (III and III´).
Rationale for 2nd Generation Synthesis
Having analyzed the reaction responsible for partial racemization in the cascade sequence, we honed our efforts toward a general solution to the problematic step. On the basis of the hypothesis that competing chair-like transition states during the oxy-Cope rearrangement were the source of poor chirality transfer, we designed new systems that would suppress this equilibration and strongly favor one chair-like transition state over the other.
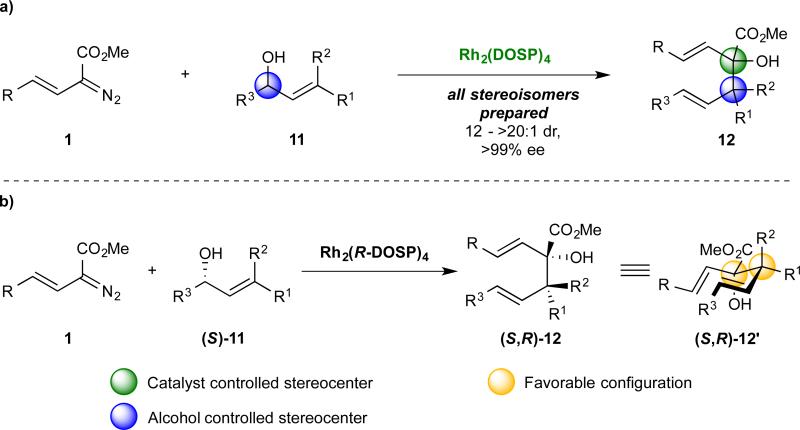
In a recent article, we described the tandem ylide formation/[2,3]-sigmatropic rearrangement of readily available chiral allyl alcohols with donor/acceptor substituted rhodium carbenes.75 For alcohols with variable substitution at C(3) (Figure 4a, 11, R1 ≠ R2), rearrangement products bearing vicinal stereocenters (12) were generated with very high enantiocontrol (>99% ee) and moderately high diastereocontrol (12 – >20 : 1). Specifically, the rhodium catalyst dictated the configuration of the hydroxyester stereocenter (green sphere) while the allyl alcohol controlled the configuration of the second chiral center (blue sphere). Thus, we demonstrated that the proper combination of each enantiomer of Rh2(DOSP)4 and allyl alcohol enabled the synthesis of all four diastereomers of 12. We envisioned that the [2,3]-sigmatropic rearrangement to generate a product with adjacent stereocenters could provide the necessary solution to chirality transfer during the problematic oxy-Cope rearrangement, as shown in Scheme 4b. Specifically, the proper configuration of a 3-hydroxy-1,5-hexadiene (12) would enable a single chair-like intermediate, wherein both chiral centers would orient cooperatively (12′), with the substituents having larger A–values positioned equatorially (R1 > R2). Herein we report the synthesis-based exploration of that hypothesis and the development of a highly stereoselective cyclopentane synthesis.
Figure 4.
Rationale for improved chirality transfer during an oxy-Cope rearrangement. (a) The tandem-ylide formation/[2,3]-sigmatropic rearrangement between a diazoacetate (1) and allyl alcohol (2) to generated a 3-hydroxy-1,5-hexadiene (12) bearing vicinal stereocenters.
DISCUSSION
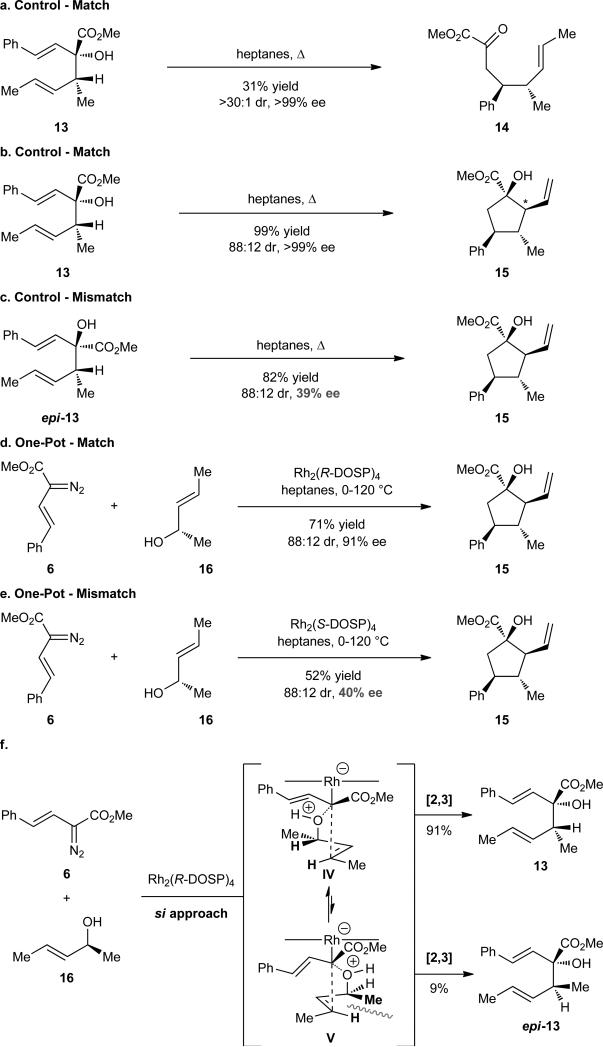
Our investigations into an improved cyclopentane synthesis began with a series of control reactions. First, the diastereo- and enantiomerically pure 3-hydroxy-1,5-hexadiene 1375 was heated in a sealed tube for 12 h. Direct purification by flash chromatography afforded a small amount of the ketoester 14 as a single stereoisomer (Figure 5a). The modest isolated yield of 14 is because the crude reaction mixture contains a mixture of starting material (13), ketoester (14), and cyclopentane (15). Prolonged heating of 13 furnished the desired cyclopentane 15 in excellent yield and with no loss in the level of enantioselectivity, as shown in Figure 5b. The product 15 was, however, formed as an 88 : 12 mixture of diastereomers. Having established that the keto ester 14 is formed as a single diastereomer, it appears that the carbonyl ene reaction to form 15 is no longer highly diastereoselective with these substrates. Comparison with literature precedent22 and our own analyses of the nuclear Overhauser effects (see Supplementary Information/Supplementary Figures 1 and 2) in 15 are indicative of inversion in the configuration at the C(1)- and C(2)-stereocenters in the minor product.
Figure 5.
Control studies relevant to the synthesis of cyclopentane 15. (a) Chirality transfer control for the oxy-Cope rearrangement. Matched (b) and mismatched (c) tandem oxy-Cope rearrangement hetero-ene reactions of stereoisomerically pure 3-hydroxy-1,3-hexadienes (13 and epi-13, respectively). Matched (d) and mismatched (e) one-pot cyclopentane (15) syntheses. (f) Justification for high, but imperfect, diastereocontrol during the rhodium-catalyzed ylide formation/[2,3]-sigmatropic rearrangement.
In order for the Cope rearrangement to proceed with high enantiocontrol, the two chiral centers of the hexadiene must cooperatively reinforce a single chair-like transition state, as illustrated in Schemes 4a and b. In the negative control reaction with the epimeric material epi-13, the two chiral centers are orientated competitively, such that only the carbomethoxy or the methyl group can occupy an equatorial position in the two available chair-like forms (Figure 5c). The reaction with epi-13 demonstrated the detrimental effect on enantiocontrol, as the cyclopentane 15 was obtained with considerable racemization (39% ee) due to competing chair-like transition states. The identical diastereomeric ratio of product cyclopentanes observed for the matched and mismatched reactions (Figure 5b and c) was again consistent with a problematic carbonyl ene reaction. These studies revealed that a second stereocenter could be manipulated for enantiocontrol in the oxy-Cope rearrangement, but generated new problems with regard to the diastereoselectivity of the ene reaction.
The control studies revealed that the rearrangement of the hexadienol 13 to cyclopentanol 15 could be achieved under thermal conditions without the use of scandium triflate as a Lewis acid catalyst. Therefore, modified conditions were developed for the one-pot process. Rh2(R-DOSP)4-catalyzed reaction of vinyldiazoacetate 6 with the allyl alcohol 16 followed by heating of the crude mixture for 24 h afforded the desired product in a gratifying 71% yield (Figure 5d). While the diastereomeric ratio for the one-pot process was identical to that for the control reaction shown in Figure 5b, an unanticipated decrease in the level of enantioselectivity was observed in the matched reaction to form 15. The level of enantioselectivity was 91% ee, which is an improvement over our previous studies,52 but still not ideal. The one-pot mismatched reaction (Figure 5e), conducted by implementing the opposite enantiomer of catalyst [Rh2(S-DOSP)4], resulted in an overall less efficacious synthesis of 15, as expected. The enantioselectivity of the transformation was consistent with that observed during the tandem oxy-Cope/ene study (Figure 5c).
The discrepancy in enantioselectivity for the cyclopentane formation in the Cope/ene sequence (Figure 5b) versus the complete one-pot sequence (Figure 5d) is attributed to a slight diasteromeric “leakage” occurring during the [2,3]-sigmatropic rearrangement (Figure 5f). In our previous study, we rationalized the driving force for diastereoselection in the ylide formation/[2,3]-rearrangement to be minimization of A1,3–strain in the ylide intermediate. While the strain-minimized transition state IV is preferred, for the pentenol 16, the epimeric product (epi-13) is also formed (13 : epi-13 ratio, ~11 : 1), because the strain formed between a synpentane methyl and hydrogen group in transition state V is not severe. According to the control study described in Figure 5c, the minor [2,3]-product epi-13 will contribute to formation of the enantiomer of 15.
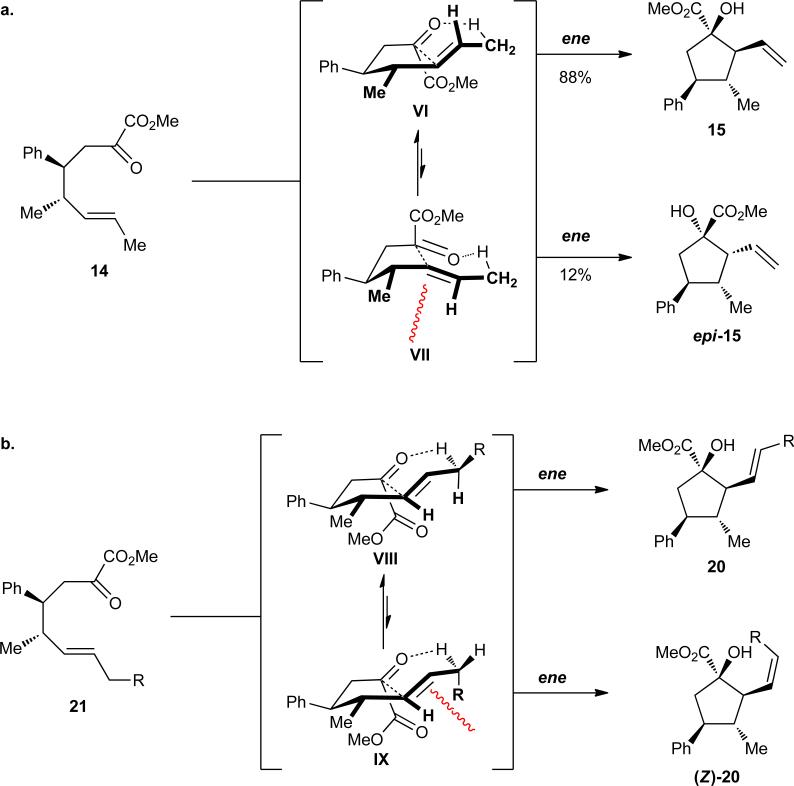
Another challenge with the second-generation substrates was the formation of diastereomers during the ene reactions. The modest level of diastereoselectivity in this step can be rationalized by a transition state analysis. Placing the ene and enophile components of 14 in a syn orientation to allow for requisite orbital overlap renders two feasible transition states (Figure 6a, VI and VII). As with the [2,3]-sigmatropic rearrangement, minimization of A1,3–strain is a governing factor in the carbonyl ene reaction. Since the ene consists of a trans-1,2-disubstituted alkene, the origins of any A1,3–strain are from interaction between the vinylic proton and the pseudoequatorial allylic methyl group. Interestingly, a similar strain interaction occurring during the [2,3]-rearrangement ene reactions produces similar levels of diastereoselectivity. Thus, while diastereomer 15, arising from cyclization via VI is preferred, some of the product epi-15 originating from the slightly more strained transition state VII is also formed.
Figure 6.
Rationale for diastereoselectivities in the hetero-ene reaction. (a) Envelope-like transition states VI and VII for the formation of 15 and epi-15, respectively. (b) Rotational isomers of an ene transition state VIII and IX for the formation of 20 and (Z)-20, respectively.
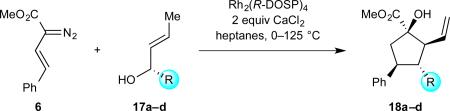
These studies verified both the feasibility of generating cyclopentanes in high levels of enantioselectivity via a one-pot process and the necessity of matching catalyst and alcohol chirality. We decided to explore whether the trend we observed with the allyl alcohol 16 would be extended to other secondary allyl alcohols. The diazoacetate 6 was chosen as the standard carbene precursor as it has been studied extensively in tandem ylide formation/[2,3]-sigmatropic rearrangement chemistry.52,55,68,69 We found that incorporating superstoichiometric quantities of calcium chloride enabled a ten-fold reduction in the loading of dirhodium tetracarboxylate catalyst without any loss in asymmetric induction, and so, it was incorporated as part of the standard reaction conditions. We believe CaCl2 helps minimize competitive coordination of the alcohol to the vacant axial site of the dirhodium tetracarboxylate catalyst; a process which impedes denitrogenative decomposition of the diazoacetate (see Supplementary Information/Supplementary Table 1).
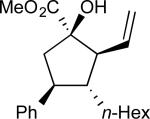
The first series of alcohols (17a–d) examined, like those in our previous cyclopentane synthesis,52 varied in carbinol substitution (Table 1). We envisioned that an increase in steric bulk at the carbinol (compared with 16) could enhance the diastereoselectivity of the [2,3]-sigmatropic rearrangement and, therefore, the overall enantioselectivity of the cyclopentane synthesis. Increasing the length of the alkyl chain (entries 1–2, 17a–b) had no effect on the stereocontrol of the reaction. Introduction of more encumbered secondary carbon or benzylether substituents (entries 3–4, 17c–d, respectively) adjacent to the carbinol provided comparable yields and levels of enantioselectivity with a slight improvement in the levels of diastereoselectivity.
Table 1.
a Variations in Carbinol Substitution.
| |||||
|---|---|---|---|---|---|
| entry | comp'd | product | yield, %b | drc | ee, %d |
| 1 | a |
|
65 | 89:11 | 92 |
| 2 | b |
|
71e | 88:12 | 92 |
| 3 | c |
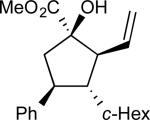
|
70 | 92:8 | 92 |
| 4 | d |

|
55e | 93:7 | 93 |
Reaction conducted with 17 (0.50 mmol, 1.0 equiv), 6 (0.60 mmol, 1.2 equiv) and Rh2(R-DOSP)4 (0.0005 mmol, 0.1 mol %), unless otherwise indicated.
Isolated yield of the major diastereomer.
Determined by 1H NMR analysis of the crude reaction residue.
Determined by HPLC analysis on a chiral stationary phase.
Yield of the reaction with 0.5 mol % Rh2(R-DOSP)4.
We then explored the effect of increasing the bulk of the alcohol C(3) substitituent, which participates in A1,3–interactions during the [2,3]-sigmatropic rearrangement. A modest improvement in enantioselectivity was observed when a C(3)-Me substituent (Scheme 5a, 16) was replaced by an isopropyl moiety (Table 2, entry 1, 19a, R1 = R2 = Me). The diastereoselectivity, however, was similar to that observed for the previous class of allyl alcohols. For alcohols where R1 and R2 were not equivalent, an added element of complexity in the form of olefin geometry was introduced (entries 2–4, 19b–d). The yields and levels of stereoselectivity were fairly consistent throughout the group; however, (E)-selective alkene formation was correlated with the size of R1. Specifically, linear alkyl chains (19b–c) provided comparable ratios of E- and Z-diastereomers (entries 2–3, ca. 3 : 1, E : Z), but a benzyl substituent afforded cyclopentane (E)-20d in greater than 7 : 1 E/Z mixture.
Table 2.
a Variations in Allylic Substitution.

| ||||||
|---|---|---|---|---|---|---|
| entry | comp'd | product | yield, %b | E: Zc | drc | ee, %d |
| 1 | a |
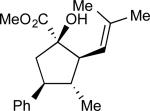
|
71 | – | 91:9 | 95 |
| 2 | b |

|
65 | 2.2:1 | 85:15 | 96 |
| 3 | c |
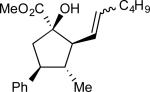
|
67 | 3.4:1 | 90:10 | 94 |
| 4 | d |
|
80e | 7.3:1 | 90:10 | 95 |
Reaction conducted with 19 (0.50 mmol, 1.0 equiv), 6 (0.60 mmol, 1.2 equiv) and Rh2(R-DOSP)4 (0.0005 mmol, 0.1 mol %), unless otherwise indicated.
Isolated yield of the major diastereomer.
Determined by 1H NMR analysis of the crude reaction residue.
Determined by HPLC analysis on a chiral stationary phase.
Yield of the reaction with 0.5 mol % Rh2(R-DOSP)4.
The preference for the formation of the E-isomers of 20 is consistent with the transition state model presented for the ene reaction as illustrated in Scheme 8. Minimization of A1,3–interactions dictates the major reaction pathway. By rotating the methylene carbon of the ene fragment (Figure 6b, VIII vs. IX), the allylic R-group would be positioned either trans or cis to the vinylic proton. The former, less strained intermediate would give rise to the (E)-cyclopentane 20, and the latter to the less preferred (Z)-20.
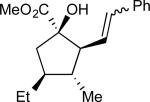
On the basis of the experimental observations and the transition state analysis, it became apparent that a highly stereoselective entry to the cyclopentanes would require effective control of chair transition states in the oxy-Cope rearrangement and maximization of the A1,3–strain control in the [2,3]-sigmatropic rearrangement and the ene reaction. Therefore, we examined a series of alcohols 23 bearing geminal disubstitution at the C(3) position (Table 3). One of the substituents was methyl to maximize chair selectivity in the oxy-Cope rearrangement and to minimize the formation of isomers in the ene reaction. The desired chiral alcohols (23) were readily prepared from commercially available enals or by means of carbometallation68,70 of the requisite alkynes and trapping with acetaldehyde. The results of the cyclopentane formation from 23 are described in Table 3. In each case the reaction proceeds in very high yield (85–89%) and with excellent levels of stereoselectivity. The cyclopentanes 24 were formed in >30 : 1 dr and in 99% ee. The reaction is likely to be applicable to a range of vinyldiazoacetates as the alkyl-substituted vinyldiazoacetate (22, R = Et), was similarly effective in the net transformation. The absolute configuration of cyclopentane 24a was verified by X-ray crystallographic analysis and this assignment was applied to the other products by analogy. Moreover, the absolute configuration was consistent with the assignments in our earlier studies.58
Table 3.
a Variations in Vinylic Substitution.
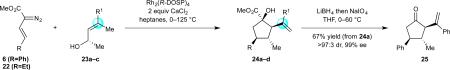
| |||||
|---|---|---|---|---|---|
| entry | comp'd | product | yield, %b | drc | ee, %d |
| 1 | a |
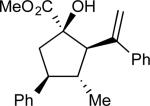
|
87 | >97:3 | 99 |
| 2 | b |
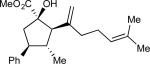
|
85 | >97:3 | 99 |
| 3 | c |
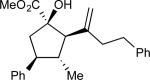
|
86 | >97:3 | 99 |
| 4 | d |
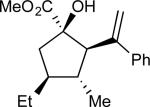
|
89 | >97:3 | 99 |
Reaction conducted with 23 (0.50 mmol, 1.0 equiv), 6 (0.60 mmol, 1.2 equiv) or 22 (1.0 mmol, 2.0 equiv) and Rh2(R-DOSP)4 (0.0005 mmol, 0.1 mol %), unless otherwise indicated.
Isolated yield of the major diastereomer.
Determined by 1H NMR analysis of the crude reaction residue.
Determined by HPLC analysis on a chiral stationary phase.
Although the cyclopentane bearing a quaternary hydroxycarbonyl stereocenter is a common structural motif among numerous polyterpenoid natural products (e.g. jatrophane diterpenoids), we sought to demonstrate the ease with which the products could be converted to the corresponding cyclopentanones. To this end, reduction of the cyclopentane carboxylate to the 1,2-diol was affected by lithium borohydride. Quenching with pH 7.0 buffer followed by direct treatment of the crude reaction mixture with excess sodium periodate furnished the cyclopentanone 25 in good yield with no observable epimerization of the α-stereocenter (Table 3).
A detailed summary and analysis of the processes involved for stereoselective synthesis of (R,R,R,S)-28 from alcohols such as 23 is presented in Figure 7. The four discrete steps involved are the following: oxonium ylide formation (Step 1), [2,3]-sigmatropic rearrangement (Step 2), oxy-Cope rearrangement (Step 3), and carbonyl ene reaction (Step 4).
Figure 7.
Plausible Reaction Pathways in the Cyclopentane Synthesis
The reaction sequence commences with nucleophilic addition of 23 to the rhodium–bound carbene intermediate derived from diazoacetate 6. A high degree of enantiocontrol is exerted by the rhodium catalyst, dictating si approach to the metallocarbene.61,75,76 Thererfore, the analysis shown in Figure 7 is limited to compounds generated from si face attack. Due to the severe A1,3– strain which develops between the allylic methyl substituents in TS2,31, the reaction proceeds selectively through TS2,32, affording the hexadiene diastereomer (S,R)-26. Rendering the reaction intermediate into a chair-like transition state produces TS3,33, where the bulkiest substituents (CO2Me and R) are occupying equatorial positions. Proceeding through an oxy-Cope rearrangement with tandem enol–keto tautomerization provides the α-ketoester (S,S,E)-27. As with the [2,3]-sigmatropic rearrangement, the driving force for diastereoselectivity in the carbonyl ene reaction is minimization of A1,3–interactions. Thus, the transition state where the allylic methyl groups are oriented on opposite faces of the ensuing cyclopentane (TSe-5) is the dominant pathway. The result is the formation of the observed stereoisomer of the cyclopentane (R,R,R,S)-28).
In our previous study where the [2,3]-sigmatropic rearrangement products lacked a second stereocenter (26, R = Me), the 1,5-hexadiene is forged with excellent enantiocontrol. Nonetheless, during the oxy-Cope rearrangement TS3,3-4 becomes a viable, albeit minor, reaction pathway to give rise to (R,R)-27 (R = Me). Since the allylic (ene) portion of the molecule is trisubstitued, it is still able to exploit a high degree of A1,3–strain, resulting in dramatic preference for carbonyl ene reaction through TSe8 rather than TSe7. As a result, the enantiomeric cyclopentane (S,S,S,R)-28 is formed competitively, resulting in moderate enantiomeric excess for the overall synthesis. When the allylic alcohol was not 3,3-disubstituted (e.g. 16, 17, and 19), TS2,31 becomes viable in the 2,3-sigmatropic rearrangement leading to the formation of some of the enantiomeric product (S,S,S,R)-28. Also, TSe2 becomes viable in the ene reaction leading to the formation of some of the diastereomeric product (S,S,R,S)-28
Thus, a domino reaction of rhodium vinylcarbenes and enantiopure allyl alcohols provides a convergent strategy for the synthesis of cyclopentane carboxylates. Employing just 0.1 mole % of chiral catalyst, three new bonds are formed, while installing four contiguous stereocenters in high yield with excellent diastereo- and enantioselectivity. A range of functional groups can be introduced at three of the stereogenic centers by variations in the allyl alcohol and vinyldiazoacetate. The fourth hydroxyester stereocenter is readily manipulated to generate complex cyclopentanones via a one-pot reduction/oxidation procedure. Each discrete step in the reaction sequence involves distinct chirality transfer events, which can be manipulated by appropriate substitution of the alcohol and ensuing intermediates. A detailed understanding of the stereochemical processes involved in the cascade has been developed, allowing for exceptional control over each stereochemical transfer. The low catalyst loadings, readily available starting materials and in-depth understanding of the mechanistic details make this an attractive method for accessing substituted cyclopentanes.
METHODS
General Considerations
All reactions were conducted in oven-dried glassware under an inert atmosphere of dry argon. All chemicals were purchased from either Sigma-Aldrich, TCI America, Acros or Alfa-Aesar, and were used as received. Pentane, hexanes, tetrahydrofuran and diethyl ether were obtained from a Grubbs-type solvent purification system. Heptanes were purchased from Macron Fine Chemicals, and were used as received. Calcium chloride was dried at 200 °C under vacuum for 12 h and stored in a dessicator. 1H NMR spectra were recorded at either 400 MHz on an INOVA-400 spectrometer or at 600 MHz on an INOVA-600 spectrometer. 13C NMR spectra were recorded at 100 MHz on an INOVA-400 spectrometer. NMR spectra were recorded in deuterated chloroform (CDCl3) solutions, with residual chloroform (δ 7.27 ppm for 1H NMR and δ 77.23 ppm for 13C NMR) or tetramethylsilane (δ 0.00 ppm for 1H NMR) taken as the internal standard, and were reported in parts per million (ppm). Abbreviations for signal coupling are as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. Coupling constants were taken from the spectra directly and are uncorrected. IR spectra were collected on a Nicolet iS10 FT-IR spectrometer as neat films. Mass spectra determinations were carried out on a Thermo Finnigan LTQ-FTMS spectrometer with electrospray (ESI) or atmospheric pressure chemical (APCI) ionization. Optical rotations were measured on JASCO P-2000 polarimeter. Gas chromatography (GC) analysis was performed on an Agilent 7890A; column conditions: 30 °C for 1 min, then increasing to 180 °C at a rate of 5 °C/min, then 180 °C for 5 min. Analytical thin layer chromatography (TLC) was performed on silica gel plates using UV light or stained with 10% vanillin/1% sulfuric acid/ethanol solution. Flash column chromatography was performed with silica gel 60 A (230-400 mesh) according to the literature procedure.71 Substrates 2,72 Rh2(SDOSP)4 and Rh2(R-DOSP)4,73 16, 17b–d, 19a and c, 23b,68 17a,74 19b,75 19d,76 and 23a77 were all synthesized according to published procedures. For 1H and 13C NMR data see Supplementary Figures 3–34. For HPLC traces see Supplementary Figures 35–47. For an ORTEP diagram of compound 24a see Supplementary Figure 48.
General Procedure
A 35 mL pressure tube fitted with a rubber septum was evacuated and backfilled with dry Ar (3×). The reaction vessel was charged with Rh2(R-DOSP)4 (0.9 mg, 0.005 mmol, 0.1 mol %) and anhydrous CaCl2 (111 mg, 1.0 mmol, 2.0 equiv) before adding a heptanes solution (1.0 mL) of 16 (43 mg, 0.50 mmol, 1.0 equiv). The suspension was cooled to 0 °C in an ice-bath. A heptanes solution (5 mL) of 6 (121 mg, 0.60 mmol, 1.2 equiv) was added dropwise over 0.5 h accompanied by vigorous stirring. The reaction was further stirred at 0 °C for 2 h, before removing the ice-bath and allowing to warm to ambient temperature. The rubber septum was removed and the vessel was sealed with a Teflon screwcap. The sealed tube was immersed in an oil bath preheated to 125 °C. After 48 h, the reaction was cooled to ambient temperature and the suspension was filtered through a fritted glass funnel, washing the filter cake with pentane (3 x 10 mL), and the filtrate was concentrated in vacuo. Column chromatography (SiO2, pentane/diethyl ether, 10 : 1) afforded a single diastereomer of the pure product 15 (92 mg, 71% yield). For additional procedures see Supplementary Methods.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the National Institutes of Health (GM099142). We thank Dr. John Bacsa (Emory University) for the X-ray crystallographic analysis.
Footnotes
AUTHOR CONRIBUTIONS
B.T.P. designed and performed experiments, analyzed data, and co-wrote the manuscript. H.M.L.D. supervised the project and co-wrote the manuscript.
Accession codes:
The X-ray crystallographic coordinates for structure 24a have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 955223. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Heasley B. Stereocontrolled preparation of fully substituted cyclopentanes: relevance to total synthesis. Eur. J. Org. Chem. 2009:1477–1489. [Google Scholar]
- 2.Lautens M, Klute W, Tam W. Transition metal-mediated cycloaddition reactions. Chem. Rev. 1996;96:49–92. doi: 10.1021/cr950016l. [DOI] [PubMed] [Google Scholar]
- 3.Hudlicky T, Price JD. Anionic approaches to the construction of cyclopentanoids. Chem. Rev. 1989;89:1467–1486. [Google Scholar]
- 4.Arnold LA, Naasz R, Minnaard AJ, Feringa BL. Catalytic enantioselective synthesis of (−)-prostaglandin E1 methyl ester using a tandem 1,4-addition-aldol reaction to a cyclopenten-3,5-dione monoacetal. J. Am. Chem. Soc. 2001;123:5841–5842. doi: 10.1021/ja015900+. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CR, Braun MP. A two-step, three-component synthesis of PGE1: utilization of α-iodo enones in Pd(0)-catalyzed cross-couplings of organoboranes. J. Am. Chem. Soc. 1993;115:11014–11015. [Google Scholar]
- 6.Suzuki M, Yanagisawa A, Noyori R. An extremely short way to prostaglandins. J. Am. Chem. Soc. 1985;107:3348–3349. [Google Scholar]
- 7.Noyori R, Tomino I, Nishizawa M. A highly efficient synthesis of prostaglandin intermediates possessing the 15S configuration. J. Am. Chem. Soc. 1979;101:5843–5844. [Google Scholar]
- 8.Iguchi S, Nakai H, Hayashi M, Yamamoto H. Diisobutylaluminum 2,6-di-tert-butyl-4-methylphenoxide. Novel stereoselective reducing agent for prostaglandin synthesis. J. Org. Chem. 1979;44:1363–1364. [Google Scholar]
- 9.Corey EJ, Ensley HE. Preparation of an optically active prostaglandin intermediate via asymmetric induction. J. Am. Chem. Soc. 1975;97:6908–6909. doi: 10.1021/ja00856a074. [DOI] [PubMed] [Google Scholar]
- 10.Stork G, Isobe M. Simple total synthesis of prostaglandins from 4-cumyloxy-2-cyclopentenone. J. Am. Chem. Soc. 1975;97:6260–6261. doi: 10.1021/ja00854a061. [DOI] [PubMed] [Google Scholar]
- 11.Stork G, Isobe M. General approach to prostaglandins via methylenecyclopentanones. Total synthesis of (±)-prostaglandin F2α. J. Am. Chem. Soc. 1975;97:4745–4746. doi: 10.1021/ja00849a042. [DOI] [PubMed] [Google Scholar]
- 12.Patterson JW, Jr., Fried JH. Synthesis of prostaglandins by conjugate addition and alkylation of a directed enolate ion. 11-Deoxy prostaglandins. J. Org. Chem. 1974;39:2506–2509. doi: 10.1021/jo00931a008. [DOI] [PubMed] [Google Scholar]
- 13.Corey EJ, Becker KB, Varma RK. Efficient generation of the 15S configuration in prostaglandin synthesis. Attractive interactions in stereochemical control of carbonyl reduction. J. Am. Chem. Soc. 1972;94:8616–8618. doi: 10.1021/ja00779a074. [DOI] [PubMed] [Google Scholar]
- 14.Sih CJ, Price P, Sood R, Salomon RG, Peruzzotti G, Casey M. Total synthesis of prostaglandins. II. Prostaglandin E1. J. Am. Chem. Soc. 1972;94:3643–3644. doi: 10.1021/ja00765a073. [DOI] [PubMed] [Google Scholar]
- 15.Corey EJ, Albonico SM, Koelliker U, Schaaf TK, Varma RK. New reagents for stereoselective carbonyl reduction. Improved synthetic route to the primary prostaglandins. J. Am. Chem. Soc. 1971;93:1491–1493. doi: 10.1021/ja00735a033. [DOI] [PubMed] [Google Scholar]
- 16.Corey EJ, Weinshenker NM, Schaaf TK, Huber W. Stereo-controlled synthesis of prostaglandins F2α and E2 (dl). J. Am. Chem. Soc. 1969;91:5675–5677. doi: 10.1021/ja01048a062. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel C, Sterz K, Müller H, Rehbein J, Wiese M, Hiersemann M. Total synthesis of natural and non-natural Δ5,6Δ12,13-jatrophane diterpenes and their evaluation as MDR modulators. J. Org. Chem. 2011;76:512–522. doi: 10.1021/jo1019738. [DOI] [PubMed] [Google Scholar]
- 18.Lentsch C, Rinner U. General synthesis of highly functionalized cyclopentane segments for the preparation of jatrophane diterpenes. Org. Lett. 2009;11:5326–5328. doi: 10.1021/ol902221y. [DOI] [PubMed] [Google Scholar]
- 19.Schnabel C, Hiersemann M. Total synthesis of jatrophane diterpenes from Euphorbia characias. Org. Lett. 2009;11:2555–2558. doi: 10.1021/ol900819u. [DOI] [PubMed] [Google Scholar]
- 20.Helmboldt H, Hiersemann M. Synthetic studies toward jatrophane diterpenes from Euphorbia characias. Enantioselective synthesis of (−)-15-O-acetyl-3-O-propionyl-17-norcharaciol. J. Org. Chem. 2009;74:1698–1708. doi: 10.1021/jo802581g. [DOI] [PubMed] [Google Scholar]
- 21.Shimokawa K, Takamura H, Uemura D. Concise synthesis of a highly functionalized cyclopentane segment: toward the total synthesis of kansusuinine A. Tetrahedron Lett. 2007;48:5623–5625. [Google Scholar]
- 22.Helmboldt H, Köhler D, Hiersemann M. Synthesis of the norjatrophane diterpene (−)-15-acetyl-3-propionyl-17-norcharaciol. Org. Lett. 2006;8:1573–1576. doi: 10.1021/ol060115t. [DOI] [PubMed] [Google Scholar]
- 23.Mulzer J, Giester G, Gilbert M. Toward a total synthesis of macrocyclic jatrophane diterpenes – concise route to a highly functionalized cyclopentane key intermediate. Helv. Chim. Acta. 2005;88:1560–1579. [Google Scholar]
- 24.Gilbert MW, Galkina A, Mulzer J. Toward the total syntheses of pepluanin A and euphosalicin: concise route to a highly oxygenated cyclopentane as a common intermediate. Synlett. 2004:2558–2562. [Google Scholar]
- 25.Helmboldt H, Rehbein J, Hiersemann M. Enantioselective synthesis of the C-14 to C-5 cyclopentane segment of jatrophane diterpenes. Tetrahedron Lett. 2004;45:289–292. [Google Scholar]
- 26.Malinowski JT, Sharpe RJ, Johnson JS. Enantioselective synthesis of pactamycin, a complex antitumor antibiotic. Science. 2013;340:180–182. doi: 10.1126/science.1234756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanessian S, et al. Total synthesis of pactamycin. Angew. Chem. Int. Ed. 2011;50:3497–3500. doi: 10.1002/anie.201008079. [DOI] [PubMed] [Google Scholar]
- 28.Chen M-J, Cheng C-Y, Chen Y-C, Chou C-K, Hsu W-M. Effects of Bimatoprost 0.03% on Ocular Hemodynamics in Normal Tension Glaucoma. J. Ocul. Pharmacol. Ther. 2006;22:188–193. doi: 10.1089/jop.2006.22.188. [DOI] [PubMed] [Google Scholar]
- 29.Patel SS, Spencer C. M. Latanoprost. A review of its pharmacological properties, clinical efficacy and tolerability in the management of primary open-angle glaucoma and ocular hypertension. Drugs Aging. 1996;9:363–378. doi: 10.2165/00002512-199609050-00007. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S, et al. Topical Isopropyl Unoprostone for Retinitis Pigmentosa: Microperimetic Results of the Phase 2 Clinical Study. Ophthalmol. Ther. 2012;1:1–16. doi: 10.1007/s40123-012-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erb C, Lanzl I, Seidova SF, Kimmich F. Preservative-free tafluprost 0.0015% in the treatment of patients with glaucoma and ocular hypertension. Adv. Therapy. 2011;28:575–585. doi: 10.1007/s12325-011-0038-9. [DOI] [PubMed] [Google Scholar]
- 32.Lewis R, et al. Travaprost 0.004% with and without Benzalkonium Chloride: A Comparison of Safety and Efficacy. J. Glaucoma. 2007;16:98–103. doi: 10.1097/01.ijg.0000212274.50229.c6. [DOI] [PubMed] [Google Scholar]
- 33.Hudlicky T, Reed JW. From discovery to application: 50 years of the vinylcyclopropane–cyclopentene rearrangement and its impact on the synthesis of natural products. Angew. Chem. Int. Ed. 2010;49:4864–4876. doi: 10.1002/anie.200906001. [DOI] [PubMed] [Google Scholar]
- 34.Rubin M, Rubina M, Gevorgyan V. Transition metal chemistry of cyclopropenes and cyclopropanes. Chem. Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin JE. Thermal rearrangements of vinylcyclopropanes to cyclopentenes. Chem. Rev. 2003;103:1197–1212. doi: 10.1021/cr010020z. [DOI] [PubMed] [Google Scholar]
- 36.Goldschmidt Z, Crammer B. Vinylcyclopropane rearrangements. Chem. Soc. Rev. 1988;17:229–267. [Google Scholar]
- 37.Trost BM, Morris PJ, Sprague SJ. Palladium-catalyzed diastereo- and enantioselective formal [3 + 2]-cycloadditions of substituted vinylcyclopropanes. J. Am. Chem. Soc. 2012;134:17823–17831. doi: 10.1021/ja309003x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Nanteuil F, Waser J. Catalytic [3+2] annulation of aminocyclopropanes for the enantiospecific synthesis of cyclopentenylamines. Angew. Chem. Int. Ed. 2011;50:12075–12079. doi: 10.1002/anie.201106255. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Montgomery J. Dimerization of cyclopropyl ketones and crossed reactions of cyclopropyl ketones with enones as an entry to five-membered rings. J. Am. Chem. Soc. 2006;128:5348–5349. doi: 10.1021/ja0602187. [DOI] [PubMed] [Google Scholar]
- 40.Trost BM, Lam TM. Development of diamidophosphite ligands and their application to the palladium-catalyzed vinyl-substituted trimethylenemethane asymmetric [3 + 2] cycloaddition. J. Am. Chem. Soc. 2012;134:11319–11321. doi: 10.1021/ja305717r. [DOI] [PubMed] [Google Scholar]
- 41.Trost BM, Silverman SM, Stambuli JP. Development of an asymmetric trimethylenemethane cycloaddition reaction: application in the enantioselective synthesis of highly substituted carbocycles. J. Am. Chem. Soc. 2011;133:19483–19497. doi: 10.1021/ja207550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trost BM, Cramer N, Silverman SM. Enantioselective construction of spirocyclic oxindolic cyclopentanes by palladium-catalyzed trimethylenemethane-[3+2]-cycloaddition. J. Am. Chem. Soc. 2007;129:12396–12397. doi: 10.1021/ja075335w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trost BM, Stambuli JP, Silverman SM, Schwörer U. Palladium-catalyzed asymmetric [3 + 2] trimethylenemethane cycloaddition reactions. J. Am. Chem. Soc. 2006;128:13328–13329. doi: 10.1021/ja0640750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara Y, Fu GC. Application of a new chiral phosphepine to the catalytic asymmetric synthesis of highly functionalized cyclopentenes that bear an array of heteroatom-substituted quaternary stereocenters. J. Am. Chem. Soc. 2011;133:12293–12297. doi: 10.1021/ja2049012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson JE, Fu GC. Synthesis of functionalized cyclopentenes through catalytic asymmetric [3+2] cycloadditions of allenes with enones. Angew. Chem. Int. Ed. 2006;45:1426–1429. doi: 10.1002/anie.200503312. [DOI] [PubMed] [Google Scholar]
- 46.Zhu G, et al. Asymmetric [3 + 2] cycloaddition of 2,3-butadienoates with electron-deficient olefins catalyzed by novel chiral 2,5-dialkyl-7-phenyl-7-phosphabicyclo[2.2.1]heptanes. J. Am. Chem. Soc. 1997;119:3836–3837. [Google Scholar]
- 47.Han X, Wang Y, Zhong F, Lu Y. Enantioselective [3 + 2] cycloaddition of allenes to acrylates catalyzed by dipeptide-derived phosphines: facile creation of functionalized cyclopentenes containing quaternary stereogenic centers. J. Am. Chem. Soc. 2011;133:1726–1729. doi: 10.1021/ja1106282. [DOI] [PubMed] [Google Scholar]
- 48.Xiao H, et al. Asymmetric [3+2] cycloadditions of allenoates and dual activated olefins catalyzed by simple bifunctional N-acyl aminophosphines. Angew. Chem. Int. Ed. 2010;49:4467–4470. doi: 10.1002/anie.201000446. [DOI] [PubMed] [Google Scholar]
- 49.Sampath M, Loh T-P. Highly enantio-, regio- and diastereo-selective one-pot [2 + 3]-cycloaddition reaction via isomerization of 3-butynoates to allenoates. Chem. Sci. 2010;1:739–742. [Google Scholar]
- 50.Voituriez A, Panossian A, Fleury-Brégeot N, Retailleau P, Marinetti A. 2-Phospha[3]ferrocenophanes with planar chirality: synthesis and use in enantioselective organocatalytic [3 + 2] cyclizations. J. Am. Chem. Soc. 2008;130:14030–14031. doi: 10.1021/ja806060a. [DOI] [PubMed] [Google Scholar]
- 51.Cowen BJ, Miller SJ. Enantioselective [3 + 2]-cycloadditions catalyzed by a protected, multifunctional phosphine-containing α-amino acid. J. Am. Chem. Soc. 2007;129:10988–10989. doi: 10.1021/ja0734243. [DOI] [PubMed] [Google Scholar]
- 52.Parr BT, Li Z, Davies HML. Asymmetric synthesis of highly functionalized cyclopentanes by a rhodium- and scandium-catalyzed five-step domino sequence. Chem. Sci. 2011;2:2378–2382. doi: 10.1039/C1SC00434D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies HML, Manning JR. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies HML, Beckwith REJ. Catalytic enantioselective C–H activation by means of metal–carbenoid-induced C–H insertion. Chem. Rev. 2003;103:2861–2904. doi: 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Davies HML. Enantioselective C–C bond formation by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and allylic alcohols. J. Am. Chem. Soc. 2010;132:396–401. doi: 10.1021/ja9075293. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Wang J. Recent studies on the reactions of α-diazocarbonyl compounds. Tetrahedron. 2008;64:6577–6605. [Google Scholar]
- 57.Wee AGH. Rhodium(II)-catalyzed Reaction of diazocompounds in the service of organic synthesis of natural and non-natural products. Curr. Org. Synth. 2006;3:499–555. [Google Scholar]
- 58.Davies HML, Walji AM. Compr. Asymmetric Catal. Suppl. Vol. 2. Springer; Berlin: 1999. p. 539. [Google Scholar]
- 59.Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. Wiley; New York: 1998. [Google Scholar]
- 60.Padwa AP, Hornbuckle SF. Ylide formation from the reaction of carbenes and carbenoids with heteroatom lone pairs. Chem. Rev. 1991;91:263–309. [Google Scholar]
- 61.Paquette LA, Maynard GD. Relevance of oxyanion stereochemistry to chirality transfer in anionic oxy-Cope rearrangements. J. Am. Chem. Soc. 1992;114:5018–5027. [Google Scholar]
- 62.Paquette LA, et al. Boat/chair topographic stereoselection during anionic oxy-Cope rearrangement of 1-alkenyl-2-cyclopentenyl-endo-norbornan-2-ols. J. Am. Chem. Soc. 1990;112:265–277. [Google Scholar]
- 63.Lee E, Shin I-J, Kim T-S. Enantiocontrolled synthesis of quaternary carbon centers via anionic oxy-Cope rearrangement: an efficient synthesis of (+)-dihydromayurone. J. Am. Chem. Soc. 1990;112:260–264. [Google Scholar]
- 64.Evans DA, Baillargeon DJ, Nelson VJ. A general approach to the synthesis of 1,6-dicarbonyl substrates. New applications of base-accelerated oxy-Cope rearrangements. J. Am. Chem. Soc. 1978;100:2242–2244. [Google Scholar]
- 65.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; USA: 2006. [Google Scholar]
- 66.Allinger NL, Tribble MT. Conformational analysis. LXXVIII. The conformation of phenylcyclohexane, and related molecules. Tetrahedron Lett. 1971;12:3259–3262. [Google Scholar]
- 67.Carey FA, Sundberg RJ. Advanced Organic Chemistry. 3rd ed. A. Plenum; New York: 1990. p. 127. [Google Scholar]
- 68.Li Z, Parr BT, Davies HML. Highly stereoselective C–C bond formation by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and chiral allylic alcohols. J. Am. Chem. Soc. 2012;134:10942–10946. doi: 10.1021/ja303023n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Boyarskikh V, Hansen JH, Autschbach J, Musaev DG, Davies HML. Scope and mechanistic analysis of the enantioselective synthesis of allenes by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and propargylic alcohols. J. Am. Chem. Soc. 2012;134:15497–15504. doi: 10.1021/ja3061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Negishi E, Van Horn DE, Yoshida T. Carbometalation reaction of alkynes with organoalene-zirconocene derivatives as a route to stereo-and regiodefined trisubstituted alkenes. J. Am. Chem. Soc. 1985;107:6639–6647. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.