Abstract
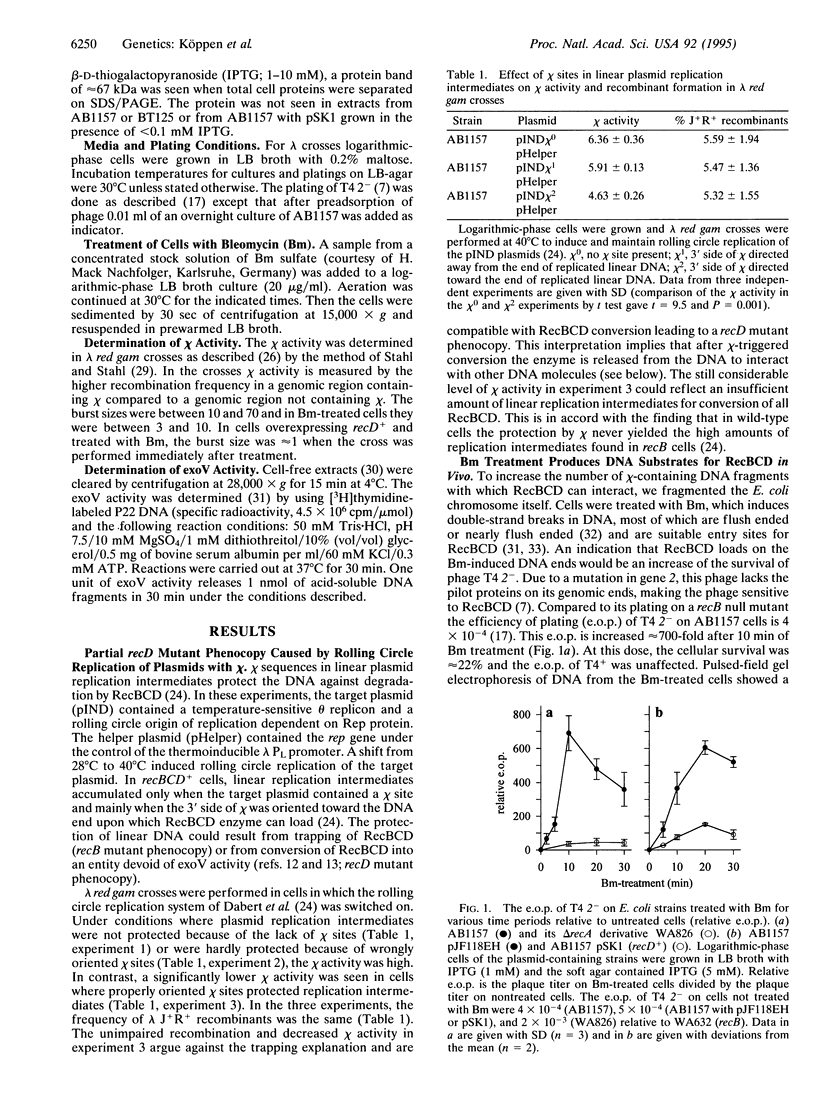
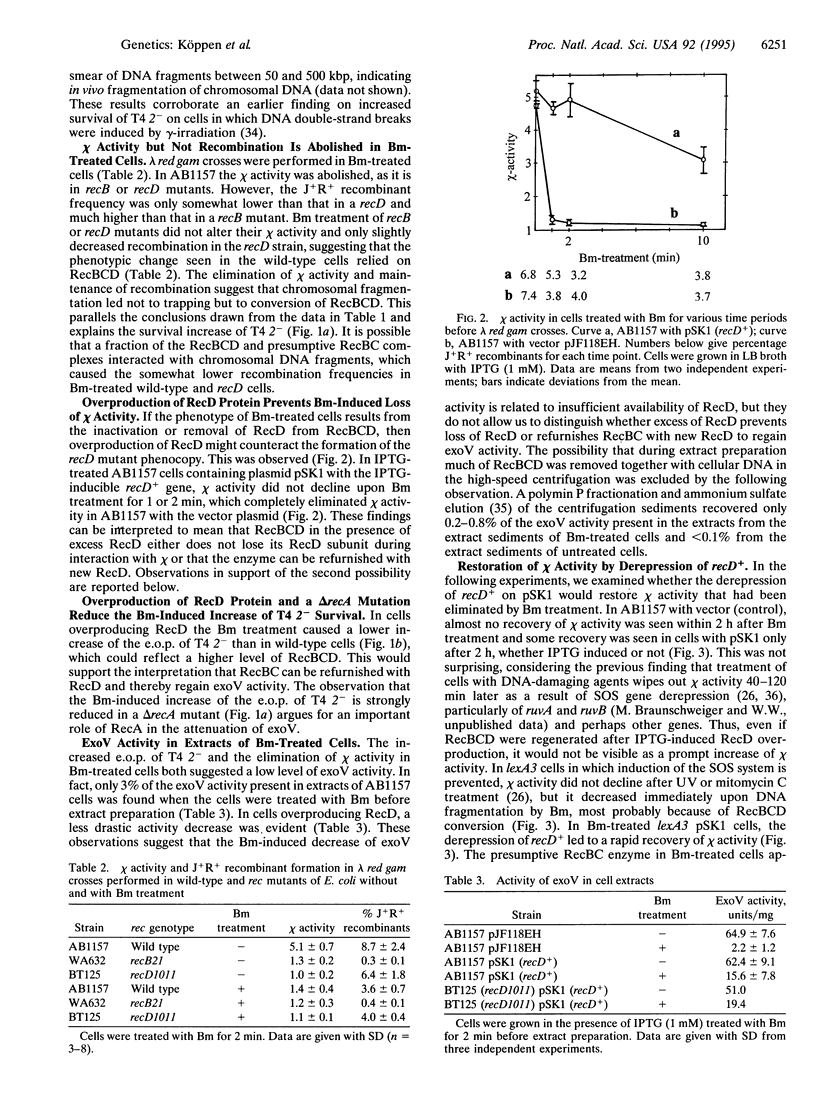
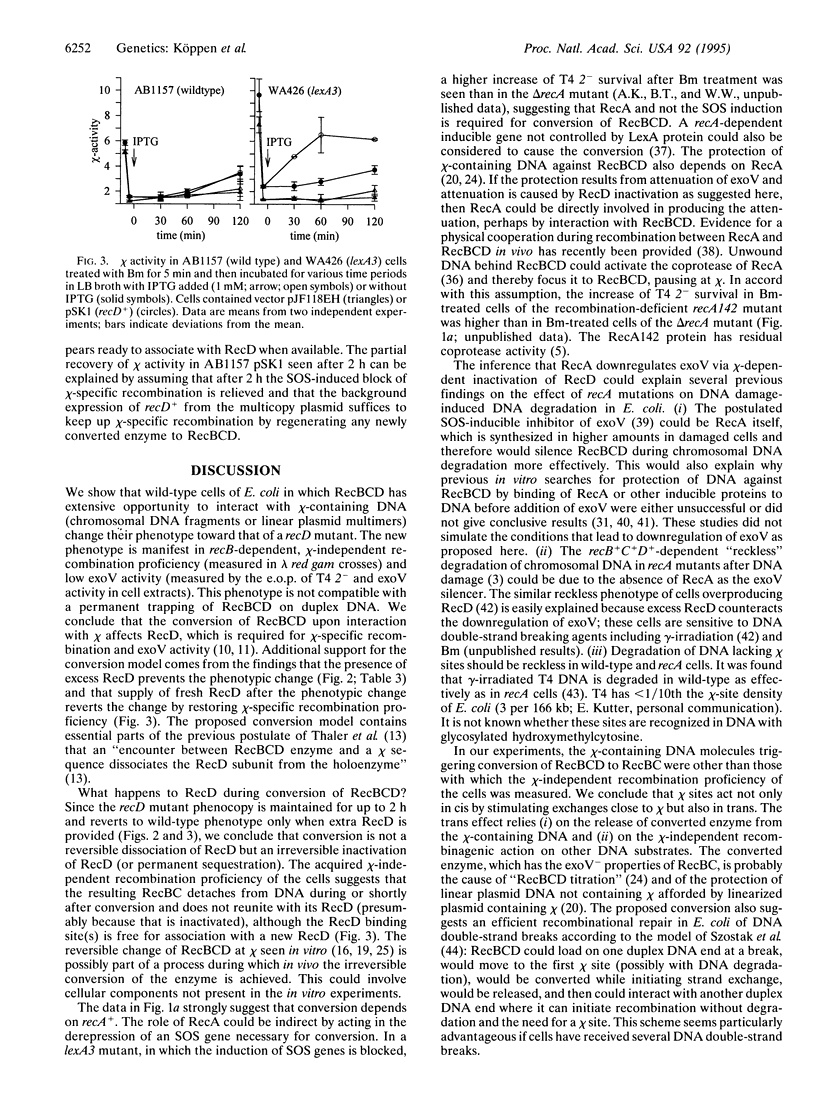
The RecBCD enzyme of Escherichia coli promotes recombination preferentially at chi nucleotide sequences and has in vivo helicase and strong duplex DNA exonuclease (exoV) activities. The enzyme without the RecD subunit, as in a recD null mutant, promotes recombination efficiently but independently of chi and has no nucleolytic activity. Employing phage lambda red gam crosses, phage T4 2- survival measurements, and exoV assays, it is shown that E. coli cells in which RecBCD has extensive opportunity to interact with linear chi-containing DNA (produced by rolling circle replication of a plasmid with chi or by bleomycin-induced fragmentation of the cellular chromosome) acquire the phenotype of a recD mutant and maintain this for approximately 2 h. It is concluded that RecBCD is converted into RecBC during interaction with chi by irreversible inactivation of RecD. After conversion, the enzyme is released and initiates recombination on other DNA molecules in a chi-independent fashion. Overexpression of recD+ (from a plasmid) prevented the phenotypic change and providing RecD after the change restored chi-stimulated recombination. The observed recA+ dependence of the downregulation of exoV could explain the previously noted "reckless" DNA degradation of recA mutants. It is proposed that chi sites are regulatory elements for the RecBCD to RecBC switch and thereby function as cis- and trans-acting stimulators of RecBC-dependent recombination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundsen S. K., Taylor A. F., Chaudhury A. M., Smith G. R. recD: the gene for an essential third subunit of exonuclease V. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5558–5562. doi: 10.1073/pnas.83.15.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brcić-Kostić K., Salaj-Smic E., Marsić N., Kajić S., Stojiljković I., Trgovcević Z. Interaction of RecBCD enzyme with DNA damaged by gamma radiation. Mol Gen Genet. 1991 Aug;228(1-2):136–142. doi: 10.1007/BF00282458. [DOI] [PubMed] [Google Scholar]
- Brcić-Kostić K., Stojiljković I., Salaj-Smic E., Trgovcević Z. Overproduction of the RecD polypeptide sensitizes Escherichia coli cells to gamma-radiation. Mutat Res. 1992 Feb;281(2):123–127. doi: 10.1016/0165-7992(92)90046-k. [DOI] [PubMed] [Google Scholar]
- Chaudhury A. M., Smith G. R. A new class of Escherichia coli recBC mutants: implications for the role of RecBC enzyme in homologous recombination. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7850–7854. doi: 10.1073/pnas.81.24.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Dabert P., Ehrlich S. D., Gruss A. Chi sequence protects against RecBCD degradation of DNA in vivo. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D. A., Churchill J. J., Kowalczykowski S. C. Reversible inactivation of the Escherichia coli RecBCD enzyme by the recombination hotspot chi in vitro: evidence for functional inactivation or loss of the RecD subunit. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2980–2984. doi: 10.1073/pnas.91.8.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D. A., Kowalczykowski S. C. Homologous pairing in vitro stimulated by the recombination hotspot, Chi. Cell. 1991 Jul 26;66(2):361–371. doi: 10.1016/0092-8674(91)90625-9. [DOI] [PubMed] [Google Scholar]
- Dixon D. A., Kowalczykowski S. C. The recombination hotspot chi is a regulatory sequence that acts by attenuating the nuclease activity of the E. coli RecBCD enzyme. Cell. 1993 Apr 9;73(1):87–96. doi: 10.1016/0092-8674(93)90162-j. [DOI] [PubMed] [Google Scholar]
- Dutreix M., Rao B. J., Radding C. M. The effects on strand exchange of 5' versus 3' ends of single-stranded DNA in RecA nucleoprotein filaments. J Mol Biol. 1991 Jun 20;219(4):645–654. doi: 10.1016/0022-2836(91)90661-o. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Jendrisak J. J., Burgess R. R. A new method for the large-scale purification of wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1975 Oct 21;14(21):4639–4645. doi: 10.1021/bi00692a012. [DOI] [PubMed] [Google Scholar]
- Konforti B. B., Davis R. W. ATP hydrolysis and the displaced strand are two factors that determine the polarity of RecA-promoted DNA strand exchange. J Mol Biol. 1992 Sep 5;227(1):38–53. doi: 10.1016/0022-2836(92)90680-i. [DOI] [PubMed] [Google Scholar]
- Korangy F., Julin D. A. Kinetics and processivity of ATP hydrolysis and DNA unwinding by the RecBC enzyme from Escherichia coli. Biochemistry. 1993 May 11;32(18):4873–4880. doi: 10.1021/bi00069a024. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S. C., Dixon D. A., Eggleston A. K., Lauder S. D., Rehrauer W. M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994 Sep;58(3):401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov A., Schabtach E., Stahl F. W. Chi sites in combination with RecA protein increase the survival of linear DNA in Escherichia coli by inactivating exoV activity of RecBCD nuclease. EMBO J. 1994 Jun 15;13(12):2764–2776. doi: 10.1002/j.1460-2075.1994.tb06570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Pollard E. C., Ginoza W., Randall E. P. Involvement of recA and exr genes in the in vivo inhibition of the recBC nuclease. J Bacteriol. 1974 May;118(2):465–470. doi: 10.1128/jb.118.2.465-470.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson C., Boehmer P. E., McDonald F., Chaudhuri S., Hickson I. D., Emmerson P. T. Reconstitution of the activities of the RecBCD holoenzyme of Escherichia coli from the purified subunits. J Biol Chem. 1992 Jul 5;267(19):13564–13572. [PubMed] [Google Scholar]
- Myers R. S., Stahl F. W. Chi and the RecBC D enzyme of Escherichia coli. Annu Rev Genet. 1994;28:49–70. doi: 10.1146/annurev.ge.28.120194.000405. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Goldberg E. B. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol. 1977 Nov;116(4):877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- Pollard E. C., Randall E. P. Studies on the inducible inhibitor of radiation-induced DNA degradation of Escherichia coli. Radiat Res. 1973 Aug;55(2):265–279. [PubMed] [Google Scholar]
- Povirk L. F., Austin M. J. Genotoxicity of bleomycin. Mutat Res. 1991 Mar;257(2):127–143. doi: 10.1016/0165-1110(91)90022-n. [DOI] [PubMed] [Google Scholar]
- Prell A., Wackernagel W. Degradation of linear and circular DNA with gaps by the recBC enzyme of Escherichia coli. Effects of gap length and the presence of cell-free extracts. Eur J Biochem. 1980 Mar;105(1):109–116. doi: 10.1111/j.1432-1033.1980.tb04480.x. [DOI] [PubMed] [Google Scholar]
- Prell A., Wackernagel W. Effect of recA protein on the DNAse activities of the recBC enzyme. J Biol Chem. 1981 Oct 25;256(20):10415–10419. [PubMed] [Google Scholar]
- Rinken R., Thomas B., Wackernagel W. Evidence that recBC-dependent degradation of duplex DNA in Escherichia coli recD mutants involves DNA unwinding. J Bacteriol. 1992 Aug;174(16):5424–5429. doi: 10.1128/jb.174.16.5424-5429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinken R., Wackernagel W. Inhibition of the recBCD-dependent activation of Chi recombinational hot spots in SOS-induced cells of Escherichia coli. J Bacteriol. 1992 Feb;174(4):1172–1178. doi: 10.1128/jb.174.4.1172-1178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. M. Chain-bias of Escherichia coli Rec-mediated lambda patch recombinants is independent of the orientation of lambda cos. Genetics. 1988 Sep;120(1):7–21. doi: 10.1093/genetics/120.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. M., Hastings P. J. The split-end model for homologous recombination at double-strand breaks and at Chi. Biochimie. 1991 Apr;73(4):385–397. doi: 10.1016/0300-9084(91)90105-a. [DOI] [PubMed] [Google Scholar]
- Sargentini N. J., Smith K. C. Quantitation of the involvement of the recA, recB, recC, recF, recJ, recN, lexA, radA, radB, uvrD, and umuC genes in the repair of X-ray-induced DNA double-strand breaks in Escherichia coli. Radiat Res. 1986 Jul;107(1):58–72. [PubMed] [Google Scholar]
- Simmon V. F., Lederberg S. Degradation of bacteriophage lambda deoxyribonucleic acid after restriction by Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):161–169. doi: 10.1128/jb.112.1.161-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Stahl M. M. Recombination pathway specificity of Chi. Genetics. 1977 Aug;86(4):715–725. doi: 10.1093/genetics/86.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Taylor A. F., Smith G. R. Substrate specificity of the DNA unwinding activity of the RecBC enzyme of Escherichia coli. J Mol Biol. 1985 Sep 20;185(2):431–443. doi: 10.1016/0022-2836(85)90414-0. [DOI] [PubMed] [Google Scholar]
- Thaler D. S., Sampson E., Siddiqi I., Rosenberg S. M., Thomason L. C., Stahl F. W., Stahl M. M. Recombination of bacteriophage lambda in recD mutants of Escherichia coli. Genome. 1989;31(1):53–67. doi: 10.1139/g89-013. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and its host Escherichia coli K12: effects on exonuclease V activity. J Mol Biol. 1972 Oct 14;70(3):539–548. doi: 10.1016/0022-2836(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Shibata T., Radding C. M. Escherichia coli recA protein protects single-stranded DNA or gapped duplex DNA from degradation by RecBC DNase. J Biol Chem. 1981 Jul 25;256(14):7573–7582. [PubMed] [Google Scholar]
- de Vries J., Wackernagel W. Recombination and UV resistance of Escherichia coli with the cloned recA and recBCD genes of Serratia marcescens and Proteus mirabilis: evidence for an advantage of intraspecies combination of P. mirabilis RecA protein and RecBCD enzyme. J Gen Microbiol. 1992 Jan;138(1):31–38. doi: 10.1099/00221287-138-1-31. [DOI] [PubMed] [Google Scholar]



