Abstract
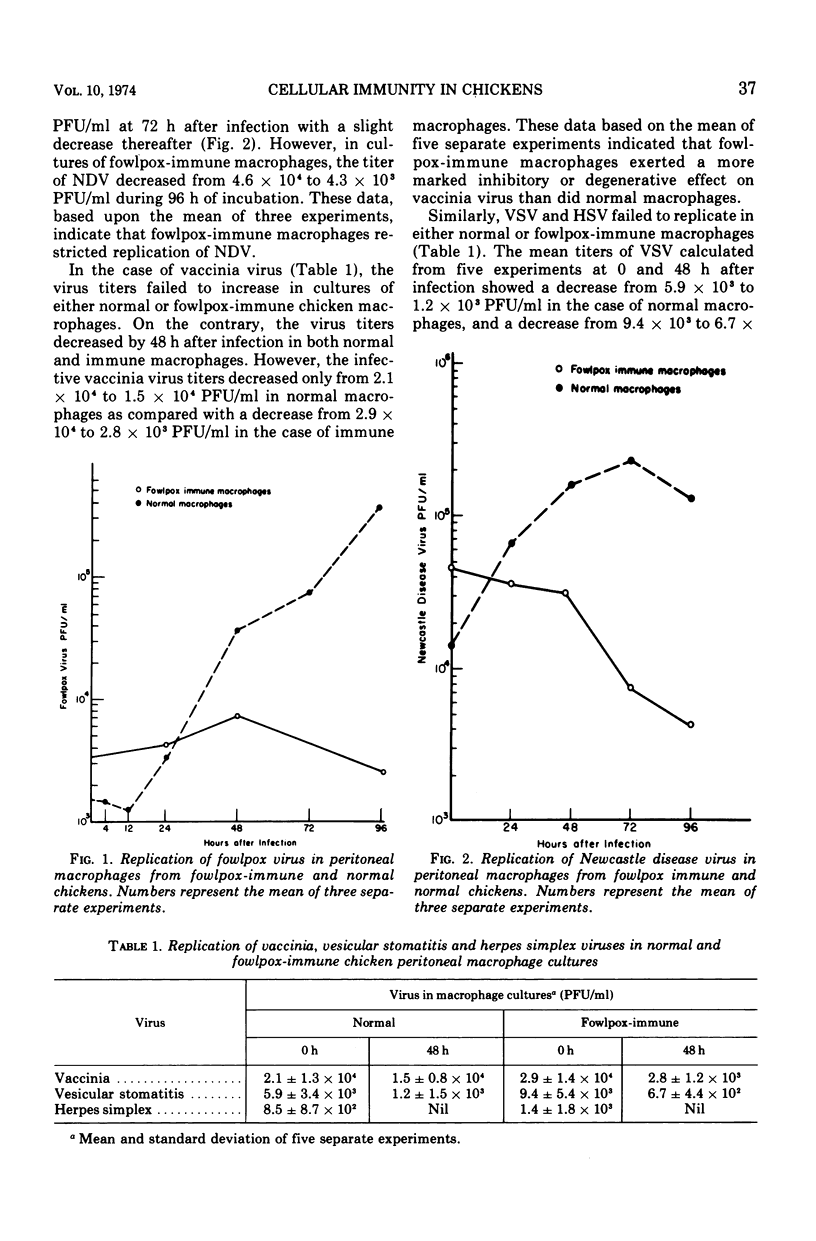
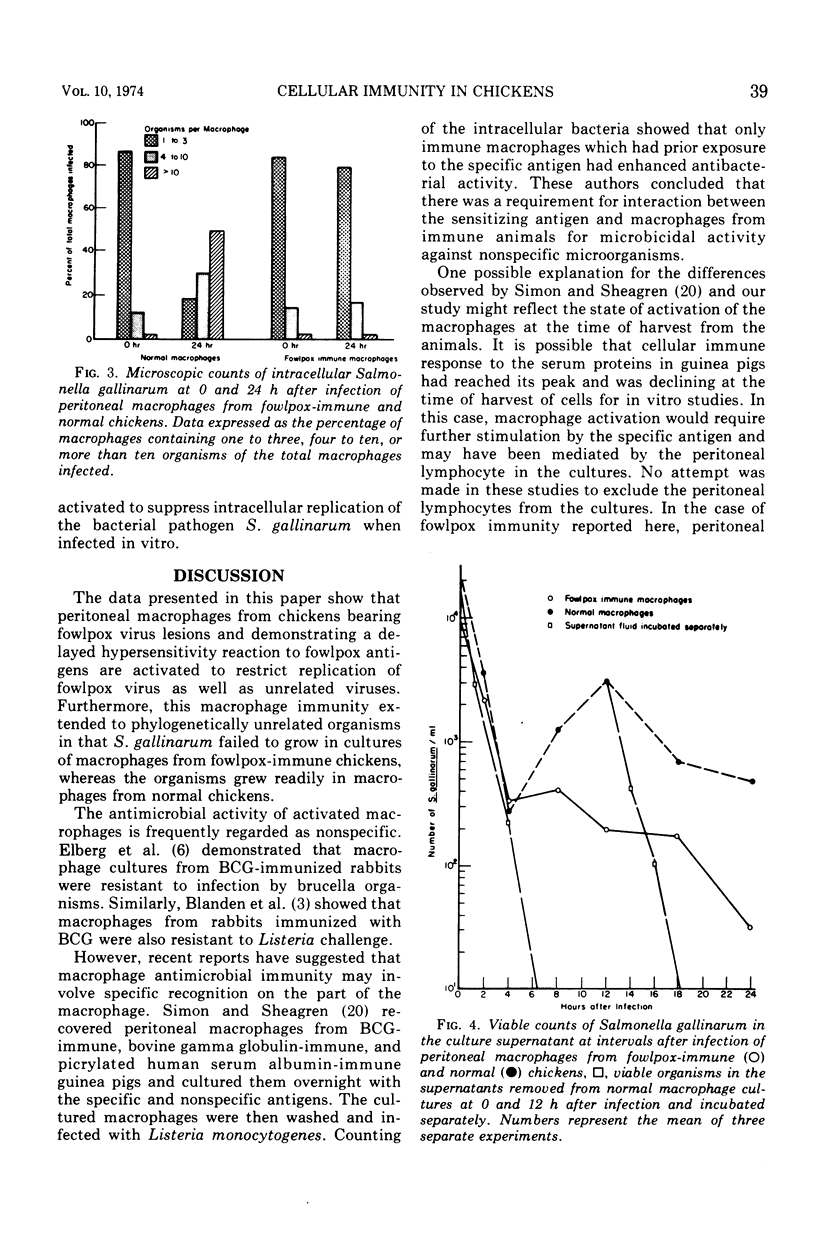
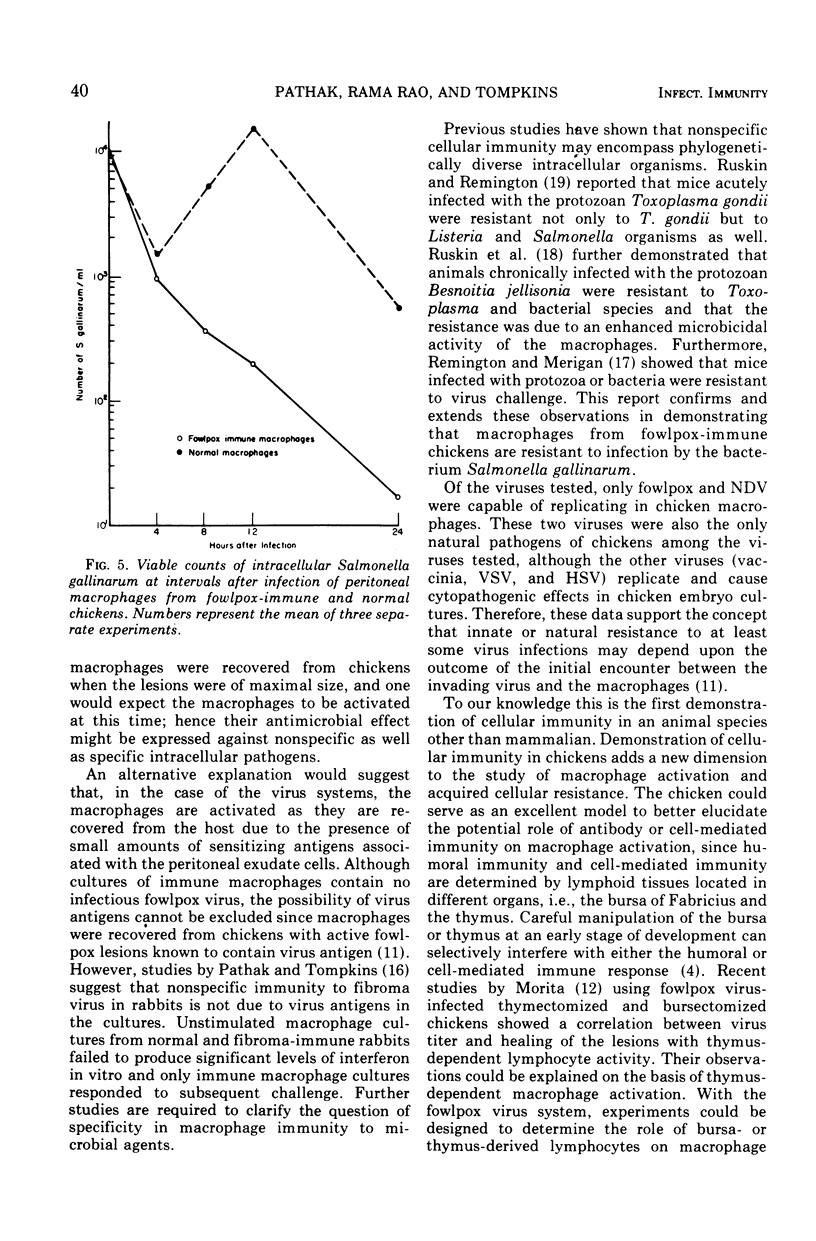
Peritoneal macrophages recovered from chickens 15 to 20 days after inoculation with fowlpox virus and showing a delayed hypersensitivity reaction against fowlpox antigens demonstrated an enhanced antimicrobial effect against fowlpox virus as well as unrelated viruses and bacteria. Inoculation of normal chicken macrophage cultures with fowlpox virus resulted in approximately a 200-fold increase in virus titer by 96 h, whereas the virus showed less than a fourfold increase in macrophage cultures from fowlpox-immune chickens. Similarly, the titer of Newcastle disease virus increased by more than 1 log in cultures of normal macrophages, whereas the titer decreased by approximately 1 log after infection of fowlpox-immune macrophages. Vaccinia, vesicular stomatitis, and herpes simplex viruses, which are not natural pathogens of chickens, failed to replicate in cultures of either normal or fowlpox-immune macrophages. By using Salmonella gallinarum, it was further demonstrated that the antimicrobial activity of fowlpox-immune macrophages encompassed not only nonspecific viruses but also bacteria. Infection of normal macrophages with Salmonella resulted in intracellular replication of the organism between 0 and 24 h as determined by microscope examination and viable bacteria counts. In contrast, cultures of fowlpox-immune macrophages failed to show an increase in intracellular organisms and showed a marked decrease in viable bacteria. In conclusion, these studies clearly showed that cellular immunity, previously demonstrated in mammalian species, develops in chickens after infection with fowlpox virus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Increased antibacterial resistance and immunodepression during graft-versus-host reactions in mice. Transplantation. 1969 Jun;7(6):484–497. doi: 10.1097/00007890-196906000-00005. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Cooper M. D., Raymond D. A., Peterson R. D., South M. A., Good R. A. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966 Jan 1;123(1):75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., SCHNEIDER P., FONG J. Cross-immunity between Brucella melitensis and Mycobacterium tuberculosis; intracellular behavior of Brucella melitensis in monocytes from vaccinated animals. J Exp Med. 1957 Oct 1;106(4):545–554. doi: 10.1084/jem.106.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C. Role of humoral and cell-mediated immunity on the recovery of chickens from fowlpox virus infection. J Immunol. 1973 Nov;111(5):1495–1501. [PubMed] [Google Scholar]
- Nelson D. S., Boyden S. V. Macrophage cytophilic antibodies and delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):15–20. doi: 10.1093/oxfordjournals.bmb.a070508. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Resistance to virus challenge in mice infected with protozoa or bacteria. Proc Soc Exp Biol Med. 1969 Sep;131(4):1184–1188. doi: 10.3181/00379727-131-34066. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins W. A., Schultz R. M. Cytotoxic antibody response to tumors induced in adult and newborn rabbits by fibroma virus. Infect Immun. 1972 Oct;6(4):591–599. doi: 10.1128/iai.6.4.591-599.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Nozima T. Delayed hypersensitivity in vaccinia-infected mice. II. Resistance of peritoneal macrophages against vaccinia infection. Acta Virol. 1973 Jan;17(1):41–49. [PubMed] [Google Scholar]
- Warner N. L., Ovary Z., Kantor F. S. Delayed hypersensitivity reactions in normal and bursectomized chickens. Int Arch Allergy Appl Immunol. 1971;40(4-5):719–728. doi: 10.1159/000230454. [DOI] [PubMed] [Google Scholar]



