Abstract
Aim
To develop prognostic nomograms for predicting outcomes in patients with locally advanced rectal cancers who do not receive preoperative treatment.
Materials and Methods
A total of 883 patients with stage II–III rectal cancers were retrospectively collected from a single institution. Survival analyses were performed to assess each variable for overall survival (OS), local recurrence (LR) and distant metastases (DM). Cox models were performed to develop a predictive model for each endpoint. The performance of model prediction was validated by cross validation and on an independent group of patients.
Results
The 5-year LR, DM and OS rates were 22.3%, 32.7% and 63.8%, respectively. Two prognostic nomograms were successfully developed to predict 5-year OS and DM-free survival rates, with c-index of 0.70 (95% CI = [0.66, 0.73]) and 0.68 (95% CI = [0.64, 0.72]) on the original dataset, and 0.76 (95% CI = [0.67, 0.86]) and 0.73 (95% CI = [0.63, 0.83]) on the validation dataset, respectively. Factors in our models included age, gender, carcinoembryonic antigen value, tumor location, T stage, N stage, metastatic lymph nodes ratio, adjuvant chemotherapy and chemoradiotherapy. Predicted by our nomogram, substantial variability in terms of 5-year OS and DM-free survival was observed within each TNM stage category.
Conclusions
The prognostic nomograms integrated demographic and clinicopathological factors to account for tumor and patient heterogeneity, and thereby provided a more individualized outcome prognostication. Our individualized prediction nomograms could help patients with preoperatively under-staged rectal cancer about their postoperative treatment strategies and follow-up protocols.
Background
Colorectal cancer is the most commonly diagnosed gastrointestinal malignancy in the world. As most of patients with rectal cancer present with locally advanced disease at diagnosis, neoajuvant chemoradiation is the standard recommendation to improve patients’ outcomes including quality of life. Compared to colon cancer, treatment is more heterogeneous in rectal cancer. In real clinical practice, approximately 20–50% of patients with stage II–III rectal cancer in North America receive definitive surgery prior to adjuvant treatment [1], [2], and the proportion is even higher in Asia [3]. The reasons for not giving neoadjuvant therapy may be multifarious. Although neoadjuvant chemoradiotherapy (CRT) has been confirmed to improve local control for locally advanced rectal cancer, its efficacy in preventing distant metastases and improving OS remains controversial [4]. Because preoperative CRT is associated with increased complications compared to surgery alone, we sought to characterize patients with locally advanced rectal cancer who were adequately treated with surgery followed by adjuvant chemotherapy[5]–[7].
Currently, the TNM stage system from the American Joint Commission on Cancer (AJCC) and the International Union Against Cancer [8], [9] is the most reliable prognostic system for all stages of rectal cancer patients with or without preoperative treatment [10], [11]. However, TNM staging does not integrate demographic features like age, or other pathological features like histopathology, perineural invasion, or tumor location, into a patient’s outcome prediction. More individualized outcome prediction models could help physicians advise patients about personalized treatment strategies and follow-up protocols.
Developing a nomogram for prognosis or treatment prediction has been considered helpful in individualized medicine and successful applications have been utilized in many malignancies[12]–[15]. This statistically based tool provides a predicted probability of a specific outcome, using a combined set of proven or potential prognostic factors. Recently, a nomogram was developed to predict outcomes of locally advanced rectal cancers with preoperative radiotherapy or CRT [16]. However, due to changes in pathological features after preoperative treatment, this nomogram only applies to patients who receive preoperative treatment. Our study was designed to develop prognostic nomograms for patients with locally advanced rectal cancer who did not receive preoperative treatment.
Materials and Methods
Ethics
A retrospective study was conducted at the Fudan University Shanghai Cancer Center. This study was approved by the Fudan University Shanghai Cancer Center Institutional Ethics Committee. According to hospital routine, patients are asked to provide a written informed consent after their admission that their clinical and outcome information will be used in future scientific studies. Patients’ records and follow-up information were anonymized and de-identified prior to analysis. The institutional Ethics Committee approved the exception of informed consent if informed consent could not be obtained due to patients’ death or lost of follow-up in our institutional database.
Patient Population
All patients with AJCC stage II–III (restaged according to 7th Edition) [8] rectal cancers were collected from the institutional colorectal cancer database. The statistical analyses were performed for patients operated between 1986 and 2005 (N = 833), whose tumors were located within 15 cm from anal verge. Patients who met one of the following criteria were excluded: (1) received preoperative treatment, (2) synchronous distant metastases, (3) surgery without curative intent, and (4) complete loss of follow-up after surgery.
An independent group of patients with stage II–III rectal cancer (N = 84) who were operated between January 2006 and June 2007 were selected for validation (Table 1).
Table 1. Characteristics of all patients with locally advanced rectal cancer and outcomes for 833 patients in training group.
| Training Group | Validation Group | |||||||
| Variable | No. (%) | Local Control | Distant Control | Overall Survival | No. (%) | |||
| (n = 833) | 5 Year | P-value | 5 Year | P-value | 5 Year | P-value | (n = 84) | |
| Demographic Variables | ||||||||
| Gender | ||||||||
| Male | 490 (58.8) | 0.770 | 0.423 | 0.637 | 0.006 | 0.608 | 0.008 | 50 (59.5) |
| Female | 343 (41.2) | 0.787 | 0.725 | 0.682 | 34 (40.5) | |||
| Age, years | ||||||||
| < = 49 | 262 (31.4) | 0.738 | 0.354 | 0.649 | 0.191 | 0.609 | 0.097 | 29 (34.5) |
| 50–69 | 432 (51.9) | 0.802 | 0.699 | 0.668 | 50 (59.5) | |||
| > = 70 | 139 (16.7) | 0.772 | 0.637 | 0.604 | 5 (6.0) | |||
| Clinical Variables | ||||||||
| Tumor location | ||||||||
| Low (<5 cm) | 181 (21.7) | 0.656 | <0.001 | 0.638 | 0.221 | 0.563 | 0.011 | 26 (31.0) |
| Mid (5cm, 10 cm) | 570 (68.5) | 0.806 | 0.677 | 0.651 | 54 (64.3) | |||
| High (>10 cm) | 82 (9.8) | 0.846 | 0.719 | 0.715 | 4 (4.8) | |||
| CEA | ||||||||
| < = 5 | 406 (48.7) | 0.817 | 0.005 | 0.696 | 0.073 | 0.660 | 0.062 | 67 (79.8) |
| >5 | 427 (51.3) | 0.740 | 0.650 | 0.616 | 17 (20.2) | |||
| Pathological Variables | ||||||||
| Pathology | ||||||||
| Adenocarcinoma | 683 (82.0) | 0.773 | 0.947 | 0.683 | 0.184 | 0.653 | 0.128 | 79 (94.0) |
| MAC or SRC | 150 (18.0) | 0.792 | 0.625 | 0.574 | 5 (6.0) | |||
| Tumor grade | ||||||||
| Low-intermediate grade | 708 (85.0) | 0.788 | 0.119 | 0.681 | 0.272 | 0.643 | 0.356 | 65 (77.4) |
| High grade | 125 (15.0) | 0.713 | 0.630 | 0.612 | 19 (22.6) | |||
| pT classification | ||||||||
| T1 | 13 (1.6) | 0.587 | 0.003 | 0.539 | 0.002 | 0.539 | 0.001 | 0 (0) |
| T2 | 93 (11.2) | 0.83 | 0.711 | 0.676 | 3 (3.6) | |||
| T3 | 351 (42.1) | 0.836 | 0.756 | 0.725 | 81 (86.4) | |||
| T4 | 376 (45.1) | 0.714 | 0.589 | 0.550 | 0 (0) | |||
| pN classification | ||||||||
| N0 | 324 (38.9) | 0.837 | <0.001 | 0.774 | <0.001 | 0.775 | <0.001 | 14 (16.7) |
| N1a | 142 (17.1) | 0.753 | 0.699 | 0.683 | 13 (15.5) | |||
| N1b | 152 (18.2) | 0.793 | 0.638 | 0.539 | 24 (28.6) | |||
| N2a | 112 (13.4) | 0.734 | 0.581 | 0.548 | 18 (21.4) | |||
| N2b | 103 (12.4) | 0.619 | 0.451 | 0.396 | 15 (17.8) | |||
| Lymphovascular invasion | ||||||||
| Yes | 178 (21.4) | 0.780 | 0.956 | 0.610 | 0.139 | 0.547 | 0.019 | 42 (50.0) |
| No | 655 (78.6) | 0.776 | 0.691 | 0.666 | 42 (50.0) | |||
| Perineural invasion | ||||||||
| Yes | 111 (13.3) | 0.698 | 0.076 | 0.544 | 0.003 | 0.515 | 0.002 | 34 (41.5) |
| No | 722 (86.7) | 0.787 | 0.692 | 0.656 | 50 (58.5) | |||
| No. of metastatic lymph nodes | ||||||||
| = 0 | 324 (38.9) | 0.775 | <0.001 | 0.837 | 0.002 | 0.774 | <0.001 | 14 (16.7) |
| > = 1 | 509 (61.1) | 0.552 | 0.735 | 0.606 | 70 (83.3) | |||
| Treatment Variables | ||||||||
| Surgery type | ||||||||
| AR | 494 (59.3) | 0.832 | <0.001 | 0.689 | 0.087 | 0.660 | 0.012 | 51 (60.7) |
| APR | 339 (40.7) | 0.697 | 0.649 | 0.607 | 33 (39.3) | |||
| Adjuvant treatment | ||||||||
| No treatment | 252 (30.3) | 0.676 | <0.001 | 0.662 | 0.332 | 0.633 | 0.670 | 16 (19.0) |
| CT only | 277 (33.3) | 0.781 | 0.698 | 0.663 | 25 (29.8) | |||
| CRT only | 123 (14.8) | 0.822 | 0.718 | 0.612 | 3 (3.6) | |||
| CRT plus CT | 181 (21.7) | 0.905 | 0.697 | 0.628 | 40 (47.6) | |||
Note: Tumor location was determined the distance from anal verge by preoperative colonoscopy or digital examination.
Abreviations: pT stage, pathological T stage; pN stage, pathological N stage; CEA, carcinoembryonic antigen; MAC, mucinous adenocarcinoma; SRC, signet ring cell carcinoma; AR, anterior resection; APR, abdominoperineal resection; CT, chemotherapy; CRT, chemoradiotherapy.
Follow-up
According to institutional follow-up protocol, all patients were asked to follow-up every 3–6 months after surgery in the first 3 years, and 6–12 months thereafter in the next two years. Follow-up information was recorded in the database. A minimum follow-up of 60 months was required for the patients who are alive in the validation dataset so that their 5-year survival status is known. The primary endpoint is the overall survival (OS) time. Local recurrence (LR) time and distant metastases (DM) time are the secondary endpoints. The LR time was calculated from the time of surgery to the time when cancer recurrence was determined in the pelvis or anastomosis by physical examination, colonoscopy, or imaging studies. The DM time was defined from the time of surgery to the identification of distant recurrence. There were three times of massive follow-up for all off-records patients via mail or telephone in1996, 2002, and 2007.
Statistical Model Creation
Kaplan-Meier plots and log-rank tests were performed for each potential predictive variable for the primary endpoint OS and the secondary endpoints LR and DM. Cox proportional hazards (PH) model was performed to develop the predictive model for OS. All decisions with respect to the grouping of the categorical variables and categorizing the continuous variables were made before modeling. These predictive models were the basis for the nomograms and the estimated probabilities of interest (e.g., 5-year OS) were calculated and presented in the nomograms.
Model Validation
Each nomogram went through two validation procedures: internal validation using the study patients for the model creation and external validation using the independent validation patients. For each outcome variable, the predicted probability from the nomogram was compared with the actual status (e.g., alive or dead 5 years from surgery) for these uncensored observations. In addition, the Harrell’s concordance index (c-index) was calculated for each nomogram [17]. This index calculates the proportion of all usable patient pairs in which the predictions and the outcomes are concordant and has a similar interpretation to that of the AUC. All the above validation analyses were performed for the study patient data and the independent validation data.
All the statistical analyses were performed using R 3.0.1.
Results
Outcomes and survival analyses
Of the 833 patients with locally advanced rectal cancer in training group, 267 patients (32%) experienced local recurrence and/or distant metastases, and 263 patients (31.5%) died of cancer or other reasons up to our last follow-up. Of those alive, median follow-up time was 51 months. The 5-year LR, DM, OS probabilities (estimated using Kaplan-Meier method) for all patients were 22.3%, 32.7% and 63.8%, respectively.
Demographic and clinicopathologic variables that potentially predict OS, LR and DM were collected, including age, gender, tumor location, preoperative carcinoembryonic antigen level (CEA), tumor differentiation, tumor histopathology, number of metastatic lymph nodes, number of total sampled lymph nodes, lymphovascular invasion, perineural invasion, T classification, N classification and adjuvant treatment. For each outcome variable (LR, DM, and OS), univariate analysis identified statistically significant predictors in the demographic features, clinical features, pathological features and treatment modalities. 5-year local control, distant control and overall survival rates were provided for every category of each predictor with p-values obtained from the Log-rank tests (Table 1).
Nomograms
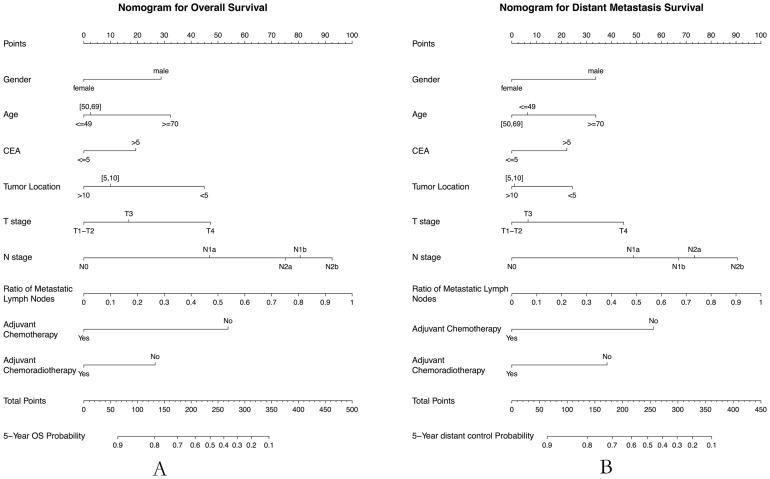
For the development of nomograms, all patients in the main dataset were included (N = 833), and the nomograms were validated using the external dataset (N = 84). Two nomograms for overall survival and distant metastases were successfully developed (Figure 1). The predictors included in the nomograms are gender, age (< = 49, 50–69, > = 70), tumor location (<5 cm, 5 cm-10 cm, >10 cm), adjuvant chemotherapy (No/Yes), adjuvant chemoradiotherapy (No/Yes), T classification (T1–T2, T3, T4), N classification (N0, N1a, N1b, N2a, N2b), CEA (< = 5, >5) and ratio of metastatic lymph nodes. Table 2 presents the hazard ratio (HR) with 95% CI and the pvalue for each predictor, and the c-index for the main dataset and the external dataset respectively. For OS prediction, the c-index was 0.76 in external validation, with a 95% CI of 0.67 to 0.86. Similarly, for DM prediction, the c-index was 0.73 (95% CI, 0.63–0.84). However, the nomogram for local recurrence prediction was not developed because of the poor c-index value in external validation (c-index, 0.6; 95% CI, 0.45–0.75).
Figure 1. Nomograms developed for predicted 5-year overall survival (A) and distant control survival (B).
Each variable value is assigned a score, and the sum of scores is converted to a probability of observed events in the lowest scale.
Table 2. Multivariate analyses of 5-year outcomes: the final predictors for developing the nomograms.
| Variable | Cox PH Regression | Nomogram | |||
| HR | 95% CI | p-value | C-index | 95% CI | |
| Distant Metastases | |||||
| Gender | |||||
| Male vs Female | 1.42 | [1.07,1.88] | 0.014 | ||
| Age (years) | |||||
| 50–69 vs < = 49 | 0.94 | [0.69,1.27] | 0.672 | ||
| > = 70 vs < = 49 | 1.33 | [0.92,1.93] | 0.134 | ||
| Tumor location | |||||
| Mid ([5 cm, 10 cm]) vs Low (<5 cm) | 0.79 | [0.58,1.07] | 0.122 | ||
| High (>10 cm) vs Low (<5 cm) | 0.78 | [0.46,1.31] | 0.344 | ||
| Adjuvant chemotherapy | |||||
| Yes vs No | 0.55 | [0.41,0.74] | <0.0001 | ||
| Adjuvant chemoradiotherapy | |||||
| Yes vs No | 0.67 | [0.50,0,90] | 0.008 | Training Data: 0.68 | [0.64,0.72] |
| pT classification | Validation Data:0.73 | [0.63,0.83] | |||
| T3 vs T1–T2 | 1.07 | [0.66,1.72] | 0.781 | Ten-fold Cross | |
| ‘T4’ vs ‘T1–T2’ | 1.59 | [1.03,2.47] | 0.038 | Validation | |
| pN classification | (Training Data): 0.65 | ||||
| ‘N1a’ vs ‘N0’ | 1.66 | [1.05,2.62] | 0.031 | ||
| ‘N1b’ vs ‘N0’ | 2.00 | [1.23,3.27] | 0.005 | ||
| ‘N2a’ vs ‘N0’ | 2.14 | [1.21,3.80] | 0.009 | ||
| ‘N2b’ vs ‘N0’ | 2.56 | [1.23,5.32] | 0.012 | ||
| CEA | |||||
| >5 vs < = 5 | 1.26 | [0.96,1.64] | 0.093 | ||
| LNR | |||||
| Continuous* | 1.11 | [1.02,1.20] | 0.013 | ||
| Overall Survival | |||||
| Gender | |||||
| Male vs Female | 1.37 | [1.06, 1.78] | 0.017 | ||
| Age (years) | |||||
| 50–69 vs < = 49 | 1.03 | [0.77,1.36] | 0.854 | ||
| > = 70 vs < = 49 | 1.42 | [1.00,2.02] | 0.049 | ||
| Tumor location | |||||
| Mid ([5 cm, 10 cm]) vs Low (<5cm) | 0.68 | [0.52, 0.90] | 0.007 | ||
| High (>10 cm) vs Low (<5 cm) | 0.61 | [0.37, 1.01] | 0.053 | ||
| Adjuvant chemotherapy | |||||
| Yes vs No | 0.56 | [0.42, 0.73] | <0.0001 | ||
| Adjuvant chemoradiotherapy | |||||
| Yes vs No | 0.75 | [0.57, 0.98] | 0.033 | Training data: 0.70 | [0.66, 0.73] |
| pT stage | Validation data: 0.76 | [0.67, 0.86] | |||
| ‘T3’ vs ‘T1–T2’ | 1.20 | [0.77, 1.87] | 0.414 | Ten-fold Cross | |
| ‘T4’ vs ‘T1–T2’ | 1.68 | [1.11,2.52] | 0.013 | Validation | |
| pN stage | (Training Data): 0.67 | ||||
| ‘N1a’ vs ‘N0’ | 1.67 | [1.08, 2.59] | 0.021 | ||
| ‘N1b’ vs ‘N0’ | 2.42 | [1.54, 3.79] | 0.00012 | ||
| ‘N2a’ vs ‘N0’ | 2.28 | [1.33, 3.91] | 0.0028 | ||
| ‘N2b’ vs ‘N0’ | 2.75 | [1.40, 5.44] | 0.0035 | ||
| CEA | |||||
| >5 vs < = 5 | 1.24 | [0.96, 1.59] | 0.097 | ||
| LNR | |||||
| Continuous* | 1.12 | [1.03, 1.20] | 0.0046 | ||
Note: The concordance index (c-index) for the training and external validation are given for the nomogram as a performance measure; Tumor location was determined the distance from anal verge by preoperative colonoscopy or digital examination.
*LNR was analyzed as a continuous variable.
Abbreviations: HR, hazard ratio; PH, proportional hazards; c-index, concordance index; CEA, carcinoembryonic antigen; LNR, metastatic lymph nodes ratio.
Predicted events within each AJCC stage classification
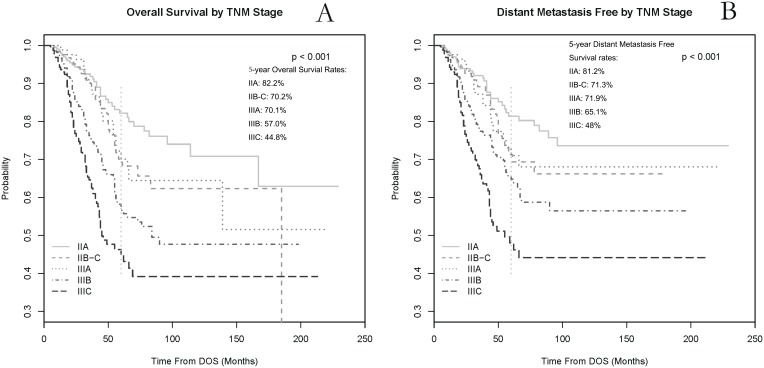
Within each AJCC stage (7th Edition), the 5-year OS rates were 82.2% (stage IIA), 70.2% (stage IIB–C), 70.1% (stage IIIA), 57.0% (stage IIIB) and 44.8% (stage IIIC); and the 5-year DM rates were 19.8% (stage IIA), 28.7% (stage IIB–C), 28.1% (stage IIIA), 34.9% (stage IIIB), and 52.0% (stage IIIC), respectively. The Kaplan-Meier survival probability curves by AJCC stage were plotted for OS and DM in Figure 2. The overall log-rank tests for testing whether the survival curves are the same among all AJCC stage groups are significant for both OS and DM (p<0.001).
Figure 2. The overall survival (A) and distant metastases free (B) Kaplan-Meier probability curves within each stage (AJCC 7th Edition) classification in locally advanced rectal cancer.
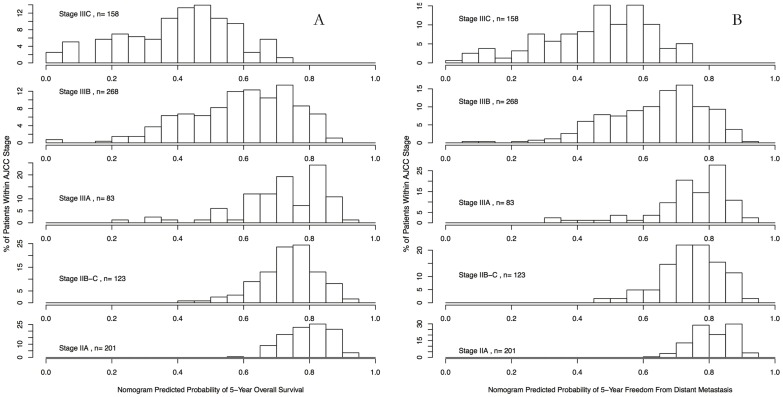
Based on our developed nomograms, the predicted probability of 5-year overall survival and distant control for each patient was computed, and the corresponding histograms were produced by AJCC stage classification from stage IIA to stage IIIC, respectively (Figure 3). The histograms showed that even within the same AJCC stage category, there are still a substantive amount of variability in terms of the predicted 5-year OS and DM-free probabilities, while in average the later stage patients have a smaller probabilities compared to earlier stage patients for both survival outcomes. Greater variations were observed for later stage patients (stage IIIB and IIIC) than earlier stage patients (stage IIA to IIIC) in terms of both 5-year OS and DM-free predicted probabilities.
Figure 3. Histogram of nomogram-predicted 5-year overall survival rate (A) and distant control rate (B) within each subgroup of the 7th edition of American Joint Committee on Cancer (AJCC) stage.
Discussion
In current study, for AJCC stage II–III (7th edition) rectal cancers without neoadjuvant treatment, we have developed prognostic monograms with independent validation samples for predicting OS and DM, based on demographic, clinicopathological and adjuvant treatment information. Our models were developed using a 20-year period institutional database; during that time, neoadjuvant RT or CRT was not well applied in China. Our predictive models are helpful to support decision-making in clinical practice and follow-up protocols, especially in patients with rectal cancer who are preoperatively under-staged and undergo surgical resection first.
The purpose of treatment in rectal cancer is to potentially improve symptoms through local control, increase chance of cure, or prolong survival. Although the German Rectal Cancer Study Group established the significant improvements in local control and toxicity for patients with locally advanced rectal cancer treated with preoperative CRT [4], long-term follow-up and other clinical trials didn’t show benefit in overall survival and distant control for patients undergoing preoperative CRT[18]–[21]. A variety of factors ultimately influence a patient’s decision to receive preoperative CRT, such as proximal tumor location, suboptimal preoperative staging methods, inaccessible facilities for optimal radiotherapy, patient preference, and/or financial considerations. The potential benefits of receiving preoperative CRT must be carefully evaluated with the potential risks. Currently, there is no nationwide or international report about the accurate proportion of preoperative CRT in locally advanced rectal cancer. The US National Cancer Database (NCDB) reported that in 2008, 41% of patients with stage I–II rectal cancer received proctocolectomy with chemotherapy or radiotherapy, in which 80% of chemotherapy, which is mainly accompanied by radiotherapy, was delivered preoperatively. However, the percentage of preoperative CRT in stage II–III rectal cancer was not reported [2]. In Canada, only an average of 45% of stage II–III rectal cancers treated in 2007–2008 were reported to undergo preoperative RT or CRT in a Canadian nationwide cancer performance report [1], [22]. In Asian countries, much lower percentage of stage II–III rectal cancers undergo preoperative RT or CRT, as most surgeons in Asia do not usually recommend preoperative CRT for clinical T2 or T3 rectal cancers [3]. The wide variation in indications and clinical applications of neoadjuvant RT or CRT reflect the complexity of the disease, which should alert international rectal cancer expert organizations as well as health-care administrators. Therefore, in current clinical circumstance, there are still a great number of patients with locally advanced rectal cancer receiving curative surgical treatment prior to RT or CRT. Our study will help rectal cancer patients and physicians to pursue more individualized postoperative treatment according to their risks of disease control and survival expectations.
With the wide utilization of neoadjuvant CRT in clinical practice and randomized clinical trials, several studies focused on the outcome prediction in patients with combined modality treatment. Recently, a prediction nomogram was developed to predict local recurrence, distant metastases, and survival for patients with locally advanced rectal cancer treated with long-course chemoradiotherapy (CRT) followed by surgery in five European phase III clinical trials [16]. Postoperative ypT stage and ypN stage were most relevant to overall survival. However, as downstaged by preoperative CRT, the two most important prognostic factors (ypT and ypN classfications) could not be well applied to patients treated with curative surgery prior to adjuvant treatment. Otherwise, the decision of neoadjuvant CRT mainly relies on preoperative staging of the primary tumor. The accuracy of T and N stage by preoperative MRI or endorectal ultrasound varies, especially in N stage. A number of patients with locally advanced rectal cancer will be under-staged preoperatively and undergo surgery first. The postoperative treatment and outcome prediction for this group of patients are currently lacking. Moreover, although perioperative CRT or CT has been proved to be effective in rectal cancer, in real clinical circumstance, there are still a part of patients with locally advanced rectal cancer undergoing surgery alone. According to a large-scale population-based study through the California Cancer Registry, there were still 33% and 18.6% of patients with stage II and stage III rectal cancer undergoing surgery alone from the year 1994 to 2008 [23]. Similarly, 57.4% and 13.0% of patients with stage II and stage III rectal cancer underwent surgery alone in our study. Currently, we are lacking of studies in defining characteristics of patients who have good outcomes without neoadjuvant therapy, particularly with surgery alone. Our nomogram provides a helpful tool for identifying patients with good outcomes if they were preoperatively under-staged and underwent surgery first. Meanwhile, as preoperative CRT contributed small improvements in overall survival and distant metastases, our study provided helpful tools and comparable dataset for predicting patients’ distant control and overall survival in locally advanced rectal cancer with multiple treatment modalities.
The goal of our study is to develop monograms to predict overall survival and distant metastases for patients without preoperative treatment. To our knowledge, using the 7th edition of AJCC staging system was the first predicting model for OS and distant control in rectal cancer (Figure 2), especially in Asian patients who were less represented in the AJCC stage system. Similar survival differences among different AJCC stage categories were observed in our patient cohort, as compared with Surveillance, Epidemiology, and End Results (SEER) population-based data [24]. Postoperative T stage and N stage were still most significant factors to predict OS and DM rates. However, from the predicted outcomes based on our nomograms, heterogeneities in the risk of death and distant metastases still largely existed within each sub-category stage from stage IIA to stage IIIC. Specifically, from the histograms in Figure 3, the variability of predicted OS and DM rates was observed greater in patients in stage IIIB and IIIC than patients in stage IIA–IIIA. This suggests that the prediction value of OS and DM may be better in patients with stage IIIB and IIIC rectal cancer when adding these demographic and clinicopathological variables which were not included in TNM staging system; while for patients with stage IIA to IIIA, molecular markers (eg. microsatellite instability, loss of heterozygosity, etc.), rather than adding more clinicopathological variables, may be benefit to further improve the accuracy of outcome prediction. By integrating important demographic and clinicopathological features, our nomogram helped further individualize the outcome prediction based on current TNM staging system. More personalized postoperative treatment may be utilized for preoperatively under-staged patients with rectal cancer in the same AJCC stage.
In addition to the TN stage, metastatic lymph nodes ratio (LNR) was reported to be a reliable prognostic factor both in colon and rectal cancer[25]–[28]. However, utilization LNR in clinical practice is relatively difficult, as optimal cut-off of the continuous LNR value has not been established. We also found LNR was one of most important prognostic factors for predicting DM and OS, in addition to patients' N stage. LNR was treated as a continuous variable in our predicting nomograms, which contributed to improve the performance of our model in predicting patients’ survival outcomes. Data from the five European trials found small but statistical significant improvement in distant control for patients with neoadjuvant CRT [16]. A recent meta-analysis of 21 randomized controlled trials from 1975 to 2011 concluded that adjuvant 5-Fu-based chemotherapy was beneficial for rectal cancer patients in improving overall survival and disease-free survival [29]. However, the benefit of adjuvant chemotherapy after combined treatment of rectal cancer is still not well defined in single randomized trials [4], [19], [21]. In our patient cohort, we only found improvements in local control in patients with any adjuvant treatment, compared with no adjuvant treatment. Further clinical trials are needed to explore the effect of adjuvant chemotherapy (single agent or combination) in improving distant control and overall survival.
Currently, there are emerged debates about adding adjuvant radiotherapy to node positive patients who receive surgical treatment first because of under-staged disease by preoperative imaging. Although randomized clinical trials proved the improvement of local control in node positive rectal cancer [30], [31], the risks of treatment toxicities and decremented quality of life limited its clinical use [32], [33]. A predicted nomogram for local recurrence including demographic and clincopathological variables may help physicians to choose patients who may benefit more from adjuvant radiotherapy. In our study, improved local control was observed in patients with any adjuvant treatment in univariate analysis, and the most optimal local control were observed in patients with adjuvant chemoradiotherapy followed by chemotherapy (Table 2). Unfortunately, our study was not able to develop a reliable nomogram for predicting local recurrence. Treatment variations in adjuvant setting, heterogeneous data, lacking of statistical power, less events in the validation group may be attributed to this. Further studies are needed to develop a reliable predictive model for local recurrence in preoperatively under-staged patients.
As a retrospective study, there are other limitations: detailed regimens of adjuvant chemotherapy could not be clearly provided for each patient; techniques of radiotherapy are changing over the 20 years; detailed information of recurrence may be unclear for part of patients, as well as loss of follow-up problems. However, our study still provides a valuable tool to help clinicians manage under-staged patients with rectal cancer who undergo surgery first. Further study is needed to provide optimal postoperative treatment for these patients.
Conclusions
The prognostic nomograms integrated demographic and clinicopathological factors to account for tumor and patient heterogeneity, and thereby provided a more individualized outcome prognostication than that by the AJCC staging system alone. Our individualized prediction nomograms could help physicians counsel and advise patients about their personalized treatment strategies and follow-up protocols, especially in patients with preoperatively under-staged rectal cancer.
Funding Statement
This work was supported by the grants from Shanghai Municipal Commission of Health and Family Planning Program KJ201204. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rahal R, Forte T, Lockwood G, Klein-Geltink J, Bryant H (2012) Recently published indicators allow for comparison of radiation treatment rates relative to evidence-based guidelines for rectal cancer. Current oncology 19: 175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Database NC American College of Surgeons Commission on Cancer, 2008 Data Submission.
- 3. Hyodo I, Suzuki H, Takahashi K, Saito Y, Tanaka S, et al. (2010) Present status and perspectives of colorectal cancer in Asia: Colorectal Cancer Working Group report in 30th Asia-Pacific Cancer Conference. Japanese journal of clinical oncology 40 Suppl 1 i38–43. [DOI] [PubMed] [Google Scholar]
- 4. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 5. Lai LL, Fuller CD, Kachnic LA, Thomas CR Jr (2006) Can pelvic radiotherapy be omitted in select patients with rectal cancer? Seminars in oncology 33: S70–74. [DOI] [PubMed] [Google Scholar]
- 6. Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, et al. (2005) Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23: 6199–6206. [DOI] [PubMed] [Google Scholar]
- 7. Tepper JE, O’Connell M, Niedzwiecki D, Hollis DR, Benson AB 3rd, et al. (2002) Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control–final report of intergroup 0114. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 20: 1744–1750. [DOI] [PubMed] [Google Scholar]
- 8.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, et al. (2010) AJCC Cancer Staging Manual (7th Edition). New York: Springer.
- 9.Green FL, Page DL, Fleming ID, Fritz A, Balch CM, et al. (2002) AJCC Cancer Staging Manual (6th edition). Chicago, IL, American Joint Committee on Cancer.
- 10. Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, et al. (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. International journal of radiation oncology, biology, physics 72: 99–107. [DOI] [PubMed] [Google Scholar]
- 11. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, et al. (2008) Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 113: 57–64. [DOI] [PubMed] [Google Scholar]
- 12.Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, et al. (2013) Outcome Prediction in Primary Resected Retroperitoneal Soft Tissue Sarcoma: Histology-Specific Overall Survival and Disease-Free Survival Nomograms Built on Major Sarcoma Center Data Sets. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [DOI] [PubMed]
- 13. Meretoja TJ, Strien L, Heikkila PS, Leidenius MH (2012) A simple nomogram to evaluate the risk of nonsentinel node metastases in breast cancer patients with minimal sentinel node involvement. Annals of surgical oncology 19: 567–576. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Li J, Xia Y, Gong R, Wang K, et al. (2013) Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 15. Giordano A, Egleston BL, Hajage D, Bland J, Hortobagyi GN, et al. (2013) Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 19: 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, et al. (2011) Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29: 3163–3172. [DOI] [PubMed] [Google Scholar]
- 17. Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 18. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. (2007) Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370: 2020–2029. [DOI] [PubMed] [Google Scholar]
- 19. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. The New England journal of medicine 355: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 20. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, et al. (2009) Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 373: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, et al. (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30: 1926–1933. [DOI] [PubMed] [Google Scholar]
- 22.Canadian Partnership Against Cancer (2011) The 2011 Cancer System Performance Report. Toronto, ON: CPAC.
- 23. Cho MM, Morgan JW, Knutsen R, Oda K, Shavlik D, et al. (2013) Outcomes of Multimodality Therapies for Patients With Stage II or III Rectal Cancer in California, 1994–2009. Diseases of the colon and rectum 56: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 24. Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A (2010) Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferri M, Lorenzon L, Onelli MR, La Torre M, Mercantini P, et al. (2013) Lymph node ratio is a stronger prognotic factor than microsatellite instability in colorectal cancer patients: Results from a 7 years follow-up study. International journal of surgery. [DOI] [PubMed]
- 26. Lu YJ, Lin PC, Lin CC, Wang HS, Yang SH, et al. (2013) The Impact of the Lymph Node Ratio is Greater than Traditional Lymph Node Status in Stage III Colorectal Cancer Patients. World journal of surgery 37: 1927–1933. [DOI] [PubMed] [Google Scholar]
- 27. Greenberg R, Itah R, Ghinea R, Sacham-Shmueli E, Inbar R, et al. (2011) Metastatic lymph node ratio (LNR) as a prognostic variable in colorectal cancer patients undergoing laparoscopic resection. Techniques in coloproctology 15: 273–279. [DOI] [PubMed] [Google Scholar]
- 28. Peng J, Xu Y, Guan Z, Zhu J, Wang M, et al. (2008) Prognostic significance of the metastatic lymph node ratio in node-positive rectal cancer. Annals of surgical oncology 15: 3118–3123. [DOI] [PubMed] [Google Scholar]
- 29. Petersen SH, Harling H, Kirkeby LT, Wille-Jorgensen P, Mocellin S (2012) Postoperative adjuvant chemotherapy in rectal cancer operated for cure. The Cochrane database of systematic reviews 3: CD004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tveit KM, Guldvog I, Hagen S, Trondsen E, Harbitz T, et al. (1997) Randomized controlled trial of postoperative radiotherapy and short-term time-scheduled 5-fluorouracil against surgery alone in the treatment of Dukes B and C rectal cancer. Norwegian Adjuvant Rectal Cancer Project Group. The British journal of surgery 84: 1130–1135. [PubMed] [Google Scholar]
- 31. Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, et al. (1988) Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. Journal of the National Cancer Institute 80: 21–29. [DOI] [PubMed] [Google Scholar]
- 32. Kollmorgen CF, Meagher AP, Wolff BG, Pemberton JH, Martenson JA, et al. (1994) The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Annals of surgery 220: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundby L, Krogh K, Jensen VJ, Gandrup P, Qvist N, et al. (2005) Long-term anorectal dysfunction after postoperative radiotherapy for rectal cancer. Diseases of the colon and rectum 48: 1343–1349; discussion 1349–1352; author reply 1352. [DOI] [PubMed]