Abstract
Background
Encapsulating peritoneal sclerosis (EPS) commonly presents after peritoneal dialysis has been stopped, either post-transplantation (PT-EPS) or after switching to hemodialysis (classical EPS, cEPS). The aim of the present study was to investigate whether PT-EPS and cEPS differ in morphology and clinical course.
Methods
In this European multicenter study we included fifty-six EPS patients, retrospectively paired-matched for peritoneal dialysis (PD) duration. Twenty-eight patients developed EPS after renal transplantation, whereas the other twenty-eight patients were classical EPS patients. Demographic data, PD details, and course of disease were documented. Peritoneal biopsies of all patients were investigated using histological criteria.
Results
Eighteen patients from the Netherlands and thirty-eight patients from Germany were included. Time on PD was 78(64–95) in the PT-EPS and 72(50–89) months in the cEPS group (p>0.05). There were no significant differences between the morphological findings of cEPS and PT-EPS. Podoplanin positive cells were a prominent feature in both groups, but with a similar distribution of the podoplanin patterns. Time between cessation of PD to the clinical diagnosis of EPS was significantly shorter in the PT-EPS group as compared to cEPS (4(2–9) months versus 23(7–24) months, p<0.001). Peritonitis rate was significantly higher in cEPS.
Conclusions
In peritoneal biopsies PT-EPS and cEPS are not distinguishable by histomorphology and immunohistochemistry, which argues against different entities. The critical phase for PT-EPS is during the first year after transplantation and therefore earlier after PD cessation then in cEPS.
Introduction
Prolonged time on peritoneal dialysis (PD) could be complicated by encapsulating peritoneal sclerosis (EPS), a rare but severe complication [1]–[6]. Nowadays, three diagnostic hallmarks are used, i.e. clinical symptoms, radiologic findings and macroscopical/histological criteria [7]–[9]. In 2000, the International Society for Peritoneal Dialysis (ISPD) defined EPS by clinical signs of abdominal pain, bowel obstruction or weight loss in late stages of the disease [10]. Vlijm et al. [11] and Tarzi et al. [12] published computed tomography (CT)-based scores to diagnose EPS by radiological findings. Several working groups studied histological findings in EPS. However, diagnostic criteria are not well defined [13]–[15]. Recently, we established a scoring system based on morphological and immunohistochemical features [8]. This study was performed to distinguish simple sclerosis from EPS, more than 20 histological findings were studied and described.
Several risk factors for development of EPS have been reported. The risk of EPS increases with longer time on PD. Additionally younger age, glucose load, peritonitis rate, and cessation of PD are factors illustrated in some studies [5], [16], [17]. EPS may occur when patients are on dialysis (classical EPS, cEPS) or after undergoing a kidney transplantation (post-transplantation EPS, PT-EPS). The prevalence of PT-EPS has been reported to be between 1 and 3%. This presentation of EPS seems to occur shortly after kidney transplantation in former PD patients [18]–[21].
The pathophysiology of EPS is still unknown. The widely discussed second-hit theory assumes that the peritoneal membrane is “preconditioned” by the prolonged use of dialysis fluids resulting in a repair process with inflammation and fibrosis, so called simple fibrosis [21]–[23]. When the second-hit occurs, for example an inflammatory stimulus like bacterial peritonitis, or discontinuation of PD, EPS can develop [24]. There are several hypotheses how transplantation might act as a “second-hit”. These include discontinuation of peritoneal lavage of proinflammatory factors, direct apposition of damaged peritoneal membrane or, after successful kidney transplantation, concomitant use of profibrotic calcineurin inhibitors (CNIs) [7], [20], [21], [25]. Previously, Khanna et al. showed that both, Ciclosporine and Tacrolimus can enhance TGB-ß expression and subsequent fibrosis [26].
From a clinical point of view, both cEPS and PT-EPS are similar with regard to clinical presentation and radiological findings. However, post-transplantation EPS seems to be associated with less systemic inflammation at time of presentation and a better outcome [19], [21]. The purpose of the current analysis was to determine whether the morphological features of patients presenting with PT-EPS are different to cEPS, thus suggesting a different clinical entity. The clinical course following cessation of PD is also compared. For this purpose we combined peritoneal biopsies from two countries of an European consortium [7].
Materials and Methods
Study population
In the present study, 56 peritoneal biopsies were studied. All biopsies (n = 9) of PT-EPS cases in the biobank of the Dutch EPS registry and Rotterdam PA database (Netherlands) were selected and for each PT-EPS biopsy, one biopsy of a cEPS case was selected resulting in a total number of 18 biopsies [3]. Likewise, a total number of 38 biopsies (including 19 PT-EPS biopsies and 19 cEPS biopsies) were selected from the biobank of the Robert Bosch Hospital in Stuttgart (Germany).
In total, 28 biopsies from patients with PT-EPS were included in the present study. PT-EPS was defined as EPS in former PD patients undergoing a kidney transplantation, after which they developed EPS while having a functioning renal allograft. All 28 biopsies of cEPS patients were paired-matched for PD duration. After cessation of PD, none of the patients performed peritoneal lavage. Classical EPS was defined as EPS in patients who had been or were treated with PD without undergoing prior kidney transplantation.
For the diagnosis of EPS we used clinical criteria stated by Nakamoto et al. [27], radiological criteria by Vlijm et al. [11] and histological criteria by Braun and Honda et al [13], [28].
Data collection included demographic data, PD details at start of dialysis. The study protocol was approved by the medical ethics committee of Erasmus Medical Center and by the local ethics committee in Germany (#322/2009BO1, Eberhard-Karls University Tuebingen, Germany). All patients gave written informed consent before participating in the study.
Peritoneal biopsies and analysis
Biopsies from the visceral peritoneum were formalin-fixed in 4% buffered formalin and paraffin-embedded following routine protocols [29]. All peritoneal biopsies were taken from patients at the time of catheter removal or during abdominal surgery (e.g. enterolysis, peritonectomy and enterolysis (PEEL)) following the protocol published by Williams et al. [30] in the time period from February 2002 to December 2012. Staining for podoplanin with the monoclonal antibody D2-40 has been used in several previous studies demonstrating the expression and pattern in EPS [8], [28], [31]. A monoclonal mouse antihuman podoplanin antibody (D2-40, DAKO, Baar, Switzerland) was used on all biopsies [8], [32]. A negative control specimen was created by omitting the primary antibody. Podoplanin was evaluated as either vascular or podoplanin avascular (0, 1, 2, 3). Furthermore, the histological description and pattern(s) of podoplanin-positive cells in peritoneal biopsies were investigated. The biopsies were separated into four groups (“low” podoplanin pattern, “organized” pattern, “diffuse” pattern and “mixed” pattern with features of both “organized” and “diffuse” patterns) [31].
From each slide hematoxylin and eosin staining was done for morphological analysis as previously described [8]: fibrosis: absent, 1–10%/low-power field (LPF), 11–50%/LPF, >51%/LPF (0, 1, 2, 3). Fibroblast-like cells (FLC): absent, 1/5 high-power fields (HPFs), 2–4/5 HPFs, >5/5 HPFs (0, 1, 2, 3); exudation: absent, 1 small area in 1 MPF, 1 area <50%/MPF, 1 area >50%/medium-power field (MPF) (0, 1, 2, 3); cellularity was evaluated as 0(1–2 nuclei/HPF), 1(3–5 nuclei/HPF) 2(6–20 nuclei/HPF) and 3(>20 nuclei/HPF); vessel density: absent, 1–5/HPF, 6–10/HPF, >10/HPF in the submesothelial cell layer (0, 1, 2, 3), acute inflammation (neutrophiles): absent, 1/HPF, 2–5/HPF, >5/HPF (0, 1, 2, 3); chronic inflammation (round cells): absent, 1–5/HPF, 6–20/HPF, >20/HPF (0, 1, 2, 3); hemorrhage: absent extravasal erythrocytes, 1 area <10%/5 LPF, 2+3 area/5 LPF or 1 area 11–30%/LPF, 4+5 area/5 LPF or 1 area >30%/LPF (0, 1, 2, 3); fibrin deposits: absent eosinophilic area, 1 area <5%/5 MPF, 1 area 6–20%/5 MPF, 1 area >20%/5 MPF (0, 1, 2, 3); presence of vasculopathy: thickening of vessel walls and/or inflammation of the vessel wall (0, 1); mesothelial denudation: no visible mesothelium (0, 1); presence of acellular areas (0, 1); presence of brown, probably iron deposits (0, 1); presence of blue, probably calcium deposits (0, 1), and osseous metaplasia (0, 1). FLC were defined as elongated cells, separated from vessel lumen with vesicular nucleus and one to three nucleoli. Acute inflammatory reaction was defined by the presence of neutrophilic granulocytes. Chronic inflammatory reaction was defined by the presence of round cells without taking into consideration further subclasses such as lymphocytes, plasma cells, monocytes and histiocytes. Furthermore thickness of the sub mesothelial cell (SMC) zone was measured as was descripted previously [30], [33], [34]. HPF = 0.26 mm2, MPF = 0.91 mm2, LPF = 3.2 mm2. Two experienced observer (one pathologist and one nephrologist) blinded to the specimen’s diagnosis evaluated each section.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD). Variables were classified as either binary (present or absent) or ordinal. The ordinal variables were discriminated as absent, low grade, moderate grade and high grade. We compared a four level classification system with a two level classification system. Each parameter was analyzed for its inter-observer variability. Comparisons between different disease groups were made using analysis of variances (ANOVA) and the Fisher-test. Statistical results with a p-value≤0.05 were considered as statistically significant.
Results
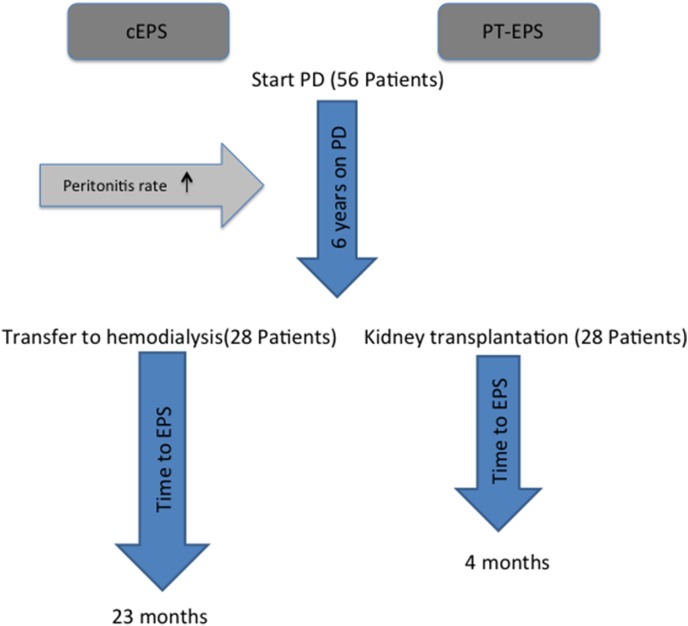
The baseline clinical characteristics of the study population are shown in table 1. A total of 56 EPS patients were included (28 PT-EPS and 28 cEPS patients, Figure 1). Eighteen patients from the Netherlands and thirty-eight patients from Germany were included. Time on PD was 78(64–95) months in the PT-EPS and 72(50–89) months in the cEPS group without a significant difference between the groups, indicating successful matching. In both groups, there were more female than male (p>0.05). Patients with cEPS demonstrated a significant higher rate of peritonitis episodes (most common organisms were Staphylococcus aureus followed by coagulase negative Staphylococci in both groups) and a more frequent use of Icodextrin (table 1).
Table 1. Clinical data of PT-EPS and cEPS patients; PD, peritoneal dialysis; EPS, encapsulating peritoneal sclerosis; PET, peritoneal equilibrium test, PDF, peritoneal dialysis fluid, *p<0.05, **p<0.001, #median and interquartile range.
| Variable | Post-transplantation EPS | Classical EPS |
| N | 28 | 28 |
| Age (years)# | 52 (46–58) | 55 (52–63) |
| Female/Male | 17/11 | 21/7 |
| CT diagnostic | 28 | 28 |
| Peritoneal thickening | 13 | 12 |
| Bowel dilatation | 15 | 16 |
| Calcification | 7 | 9 |
| Ascites | 19 | 14 |
| Clinical features | ||
| Bowel obstruction | ||
| Nausea and vomiting | 23 | 22 |
| Loss of appetite | 18 | 15 |
| Abdominal pain | 28 | 26 |
| Diarrhea | 9 | 10 |
| Inflammation | ||
| Fever | 10 | 7 |
| PD details | ||
| PD-duration at time of EPS diagnosis in months# | 78 (64–95) | 72 (50–89) |
| PET (switch to HD/NTx) | 21 | 22 |
| Low/low average | 7 | 5 |
| High average/high | 14 | 17 |
| Composition of PDF | ||
| Neutral pH | 6 | 10 |
| Acidic pH | 11 | 11 |
| Both | 8 | 3 |
| N.D. | 3 | 4 |
| Icodextrin* | 13/24 | 22/25 |
| Peritonitis* | 45 in 1990 months 1:44.2 | 103 in 1913 months 1:18.6 |
| No peritonitis episodes | 8/28 | 3/28 |
| 1–4 peritonitis episodes | 19/28 | 17/28 |
| >4 peritonitis episodes | 1/28 | 8/28 |
| Reason for cessation PD | ||
| Peritonitis | 10 | |
| Ultrafiltration failure | 13 | |
| Technical failure | 5 | |
| Age at time of NTx# | 39 (32–47) | |
| Transfer to HD or NTx to diagnosis EPS (months)**# | 4 (2–9) | 23 (7–24) |
| Treatment after transplantation | ||
| Tacrolimus | 13 | |
| Ciclosporin | 9 | |
| Both | 6 | |
| Follow-up | ||
| Follow-up time (months)# | 29 (22–74) months | 31 (20–63) months |
| Alive/Dead | 19/9 | 14/14 |
Figure 1. Schematic course of the studied patients.
Fifty-six patients started PD. After a mean of approximately six years, twenty-eight patients were transferred to HD, whereas the other twenty-eight patients received a functioning renal allograft. Peritonitis rate was higher and the use of Icodextrin more common in the cEPS compared to the PT-EPS group. Time between transfer to hemodialysis and development of EPS was significantly longer, compared to time between transplantation and development of EPS (23(7–24) months vs. 4(2–9) months, p<0.001; cEPS classical EPS, PT-EPS post-transplantation EPS).
The time between cessation of PD and diagnosis of EPS was significantly longer in cEPS compared to time after transplantation in PT-EPS group (23(7–24) months vs. 4(2–9) months, p<0.001) (table 1 and figure 1). Time from onset of symptoms associated with EPS to requirement of surgery was 8(5–11) months in the PT-EPS and 5(4–8) months in the cEPS group (p = 0.7). Other parameters including outcome were not significantly different between the groups. All patients in the PT-EPS group were treated with CNIs as part of the transplant immunosuppressive protocol. Six out of twenty-eight patients in the PT-EPS group were exposed to both, Ciclosporin and Tacrolimus.
A detailed evaluation of the biopsies from 28 PT-EPS patients and of 28 patients with cEPS was performed (Table 2, Figure 2). Two observer blinded to the diagnosis studied the slides. Examples of the 17 parameters were illustrated in figure 2. As previously described the majority of EPS biopsies demonstrated severe fibrosis, accumulation of fibroblast like cells, mesothelial denudation, fibrin deposits and chronic inflammation (Table 2). The degree of fibrosis was measured and additionally analyzed semiquantitatively. Measurement of fibrosis revealed a fibrosis zone of 1369 µm [IQR 946–2551] in cEPS and 1690 µm [IQR 1356–2598] in PT-EPS (p = 0.17). Furthermore, podoplanin positive cells and podoplanin positive lymphatic vessels were prominent features (Figure 2).
Table 2. Histological findings in patients with post-transplantation EPS and classical EPS; Fibrosis (0, 1 vs. 2, 3), Fibroblast-like-cells (FLC) (0, 1 vs. 2, 3), Exudation (0, 1 vs. 2, 3), Mesothelial denudation (0 vs. 1), Acellular areas (0 vs. 1), Cellularity (0, 1 vs. 2, 3), Vessel density (0, 1 vs. 2, 3), Acute inflammation (0, 1 vs. 2, 3), Chronic inflammation (0, 1 vs. 2, 3), Vasculopathy (0 vs. 1), Hemorrhage (0, 1 vs. 2, 3), Fibrin deposits (0, 1 vs. 2, 3), Calcification (0 vs. 1), Iron deposits (0 vs. 1), Ossification (0 vs. 1), Podoplanin vascular (0, 1 vs. 2, 3), Podoplanin avscular (0, 1 vs. 2, 3).
| Variable | PT-EPS (n = 28) | cEPS (n = 28) | p |
| Fibrosis | 1/27 | 2/26 | 1 |
| FLC | 14/14 | 10/18 | 0.4 |
| Exudation | 12/16 | 14/14 | 0.8 |
| Mesothelial denudation | 0/28 | 0/28 | 1 |
| Acellular areas | 16/12 | 21/7 | 0.3 |
| Cellularity | 12/16 | 8/20 | 0.4 |
| Vessel density | 9/19 | 5/23 | 0.4 |
| Acute inflammation | 24/4 | 23/5 | 0.3 |
| Chronic inflammation | 16/12 | 13/15 | 1 |
| Vasculopathy | 5/23 | 6/22 | 1 |
| Hemorrhage | 17/11 | 15/13 | 0.8 |
| Fibrin deposits | 11/17 | 10/18 | 1 |
| Calcification | 25/3 | 26/2 | 1 |
| Iron deposits | 11/17 | 10/18 | 1 |
| Ossification | 0/0 | 0/0 | 1 |
| Podoplanin vascular | 16/12 | 17/11 | 1 |
| Podoplanin avascular | 11/17 | 10/18 | 1 |
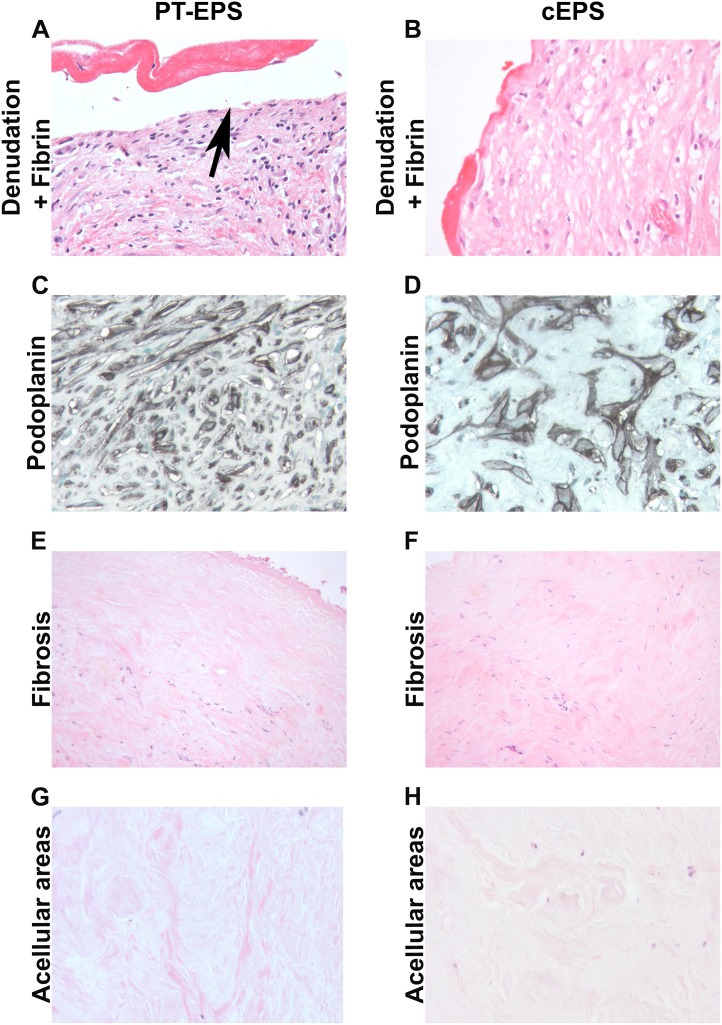
Figure 2. Morphologogical evaluation of peritoneal biopsies in PT-EPS and cEPS.
Peritoneal biopsies were either stained with PAS (A, B, E–H) or by immunohistochemistry with a monoclonal antibody against podoplanin (D2-40, C, D, original magnifications X 400 in A–D, G, H, X200 in E, F). The morphological evaluation demonstrated similar degrees of denodation and fibrin deposition (A, B), podoplanin positive cells (C, D), fibrosis (E, F) and acellular ares (G, H). (left column post transplant EPS; PT-EPS, right column classical EPS, cEPS).
Based on the morphological evaluation the biopsies with PT-EPS could not be separated from cEPS (Figure 2). There was no significant difference in any of the scored parameters (Table 2). The prominent staining for podoplanin in EPS biopsies was seen to be identical in both country sources, reproducing previous findings. The scores for podoplanin were not significantly different. The peritoneal biopsies were separated into four groups: “low” podoplanin pattern, “organized” podoplanin pattern, “diffuse” pattern and “mixed” pattern with features of both “organized” and “diffuse” patterns). Using this detailed analysis, no differences could be detected between the PT-EPS and cEPS group (all p>0.05) (table 3). Importantly, the percentages of the various patterns were similar as previously described [31].
Table 3. Podoplanin patterns [31] in post-transplantation and classical EPS patients; Organized pattern (0 vs. 1), Diffuse pattern (0 vs. 1), Low pattern (0 vs. 1), Mixed pattern (0 vs. 1).
| Variable | Post-transplantation EPS (n = 28) | Classical EPS (n = 28) | p |
| Organized pattern | 10 | 8 | 0.8 |
| Diffuse pattern | 7 | 6 | 1 |
| Low pattern | 1 | 5 | 0.2 |
| Mixed pattern | 4 | 4 | 1 |
To avoid a systematic bias between the peritoneal biopsies from different sources (i.e. the Netherlands and Germany), the results were compared between the biopsies from different countries. There was no statistically significant difference between the peritoneal biopsies from the German patient cohort compared to the patients from the Netherlands (table 4).
Table 4. Histological findings in patients with EPS (post-transplantation EPS and classical EPS) in the study population of the Netherlands and Germany; Fibrosis (0, 1 vs. 2, 3), Fibroblast-like-cells (FLC) (0, 1 vs. 2, 3), Exudation (0, 1 vs. 2, 3), Mesothelial denudation (0 vs. 1), Acellular areas (0 vs. 1), Cellularity (0, 1 vs. 2, 3), Vessel density (0, 1 vs. 2, 3), Acute inflammation (0, 1 vs. 2, 3), Chronic inflammation (0, 1 vs. 2, 3), Vasculopathy (0 vs. 1), Hemorrhage (0, 1 vs. 2, 3), Fibrin deposits (0, 1 vs. 2, 3), Calcification (0 vs. 1), Iron deposits (0 vs. 1), Ossification (0 vs. 1), Podoplanin vascular (0, 1 vs. 2, 3), Podoplanin avscular (0, 1 vs. 2, 3).
| Variable | Netherlands (n = 18) | Germany (n = 38) | p |
| Fibrosis | 2/16 | 1/37 | 0.2 |
| FLC | 10/8 | 14/24 | 0.3 |
| Exudation | 8/10 | 18/20 | 1 |
| Mesothelial denudation | 0/18 | 0/38 | 0.3 |
| Acellular areas | 13/5 | 24/14 | 0.6 |
| Cellularity | 6/12 | 14/24 | 1 |
| Vessel density | 3/15 | 11/38 | 0.5 |
| Acute inflammation | 14/4 | 33/5 | 0.4 |
| Chronic inflammation | 11/7 | 18/20 | 0.4 |
| Vasculopathy | 5/13 | 6/32 | 0.3 |
| Hemorrhage | 12/6 | 20/18 | 0.8 |
| Fibrin deposits | 9/9 | 12/26 | 0.2 |
| Calcification | 18/0 | 33/5 | 0.2 |
| Iron deposits | 4/14 | 17/21 | 0.1 |
| Ossification | 0/0 | 0/0 | 1 |
| Podoplanin vascular | 13/5 | 20/18 | 0.2 |
| Podoplanin avascular | 9/9 | 12/26 | 0.2 |
Discussion
Previous studies suggest, that the clinical course of cEPS differs from patients who develop EPS after transplantation. Therefore, the goal of this study was to compare the morphology of a high number of peritoneal biopsies from patients with PT-EPS and cEPS to detect possible histological differences between the two groups. We matched the groups according to “time on PD”, the most relevant risk factor for the development of EPS. A separation of the two groups on morphological grounds would provide evidence that these reflect two different pathological entities. Inflammation, angiogenesis and fibrosis are the main features of EPS, resulting in exudations of fibrin and chronic inflammation of the peritoneal membrane [35]. This leads to adhesions, development of a fibrous cocoon covering the intestines and results in symptoms of bowel obstruction [10], [36]. The biopsies included in our study demonstrated the typical (but non-specific) morphological features described for EPS. We could not confirm the hypothesis that PT-EPS and cEPS are two different entities, as the histological evaluation could not separate biopsies from the groups. Although, we applied all ever published histological features in EPS, we found no significant differences between the two groups [8]. Immunohistochemstry with podoplanin, including an extensive pattern analysis revealed no difference between the groups. Recently, Kinashi et al. showed that peritoneal tissue from patients with ultrafiltration failure (UFF) contained more lymphatic vessels than tissue from patients without UFF [37]. Podoplanin was found to be a good marker for lymphatic endothelial cells, but is expressed by peritoneal mesothelial and fibroblast-like-cells (FLC) too [8], [38]. In our patient cohort, approximately fifty-percent of the patients in both groups showed a strong expression of podoplanin, but no difference in the expression pattern. Hence, podoplanin seems to play an role in the pathogenesis of EPS, but does not differentiate between cEPS and PT-EPS.
The incidence of EPS increases with time on PD (other factors like peritonitis rate, male gender, younger age, smoking and glucose exposure are under debate) [5], [16], [32], [39]–[44]. The group of patients with cEPS demonstrated a higher-rate of peritonitis, and the use of icodextrin was more common, whereas the time to diagnosis after cessation of PD was longer in the cEPS group. The shorter time to clinically symptomatic EPS in patients after kidney transplantation has previously been described [21], [45]. Interestingly, there were no differences regarding morphological findings, and particularly no differences in the severity of fibrosis, using both, semi-quantitative and quantitative analysis. This could argue that factors in the PT-EPS group resulted in a faster progression of the disease. It is likely that the time to the clinical manifestation is based on the ratio of pro fibrotic factors (e.g. time on PD, surgery, peritonitis, calcineurin-inhibitors) and factors which might inhibit the disease process (e.g. steroids, rinsing the abdominal cavity). The combination of pro-fibrotic factors after transplantation might result in a faster disease process, although the major players in the pathogenesis have not been defined [21].
It has been recently shown in a rat model of peritoneal exposure to dialysis fluid that additional administration of Ciclosporin leads to EPS like abnormalities [46]. The calcineurin-inhibitors (Ciclopsorin and Tacrolimus) can lead to enhanced expression of transforming growth factor-β (TGF-β), demonstrated in a preconditioned peritoneal membrane, which already demonstrated up-regulation of TGF-β. This results in increasing fibrosis and neoangiogenesis of the peritoneal membrane [21], [26], [47]. Due to the lack of biopsies at the time of transfer to either dialysis or transplantation we cannot prove the differences in progression. It is less likely that PT-EPS patients would have had more severe membrane injury at the time of modality transfer compared to the cEPS group who likely had complications leading to technique failure as evidenced by their higher rate of peritonitis episodes and greater need of Icodextrin, suggesting either UFF or less residual renal function. This raises an important question: could the morphological evaluation at the time of transfer to a different form of renal replacement therapy add value to an overall risk assessment (including time on treatment, peritonitis history and ultrafiltration failure) in predicting the development of EPS? This requires a high number of patients with biopsies at transfer when removing the PD catheter, and we will try to answer this question within the European patient cohort in the future.
An alternative explanation for the differences between the time to manifestation would be that patients after transplantation might be seen more often in the transplant clinics and/or symptoms might rather be ignored in patients on hemodialysis, as had been reported by patients [48]. In 9 interviews of patients with EPS the patients described a loss of trust in the doctors as symptoms while being on dialysis were not taken seriously [48].
If patients after transplantation would be detected earlier in the diseases course due to more frequent doctor visits, it would be expected that the histological finding would be less extensive in patients with PT-EPS. Particularly, the fibrosis scores should be less severe, but no differences could be detected and time from onset of symptoms associated with EPS to requirement of surgery was not different between both groups. Therefore this argument is less convincing than a faster disease process.
The limitations of the study design of course leaves unanswered questions and room for future studies. The available data from these two referral centers provided in the registers were limited. We could not provide more sophisticated information about membrane function (e.g. osmotic conductance or glucose exposure during PD) [49]. We were unable to fully comply with suggested standards for reporting clinical features of EPS as the data was collected prospectively, before the standards were suggested [50]. Survival was not significantly different between the groups, even so there was a trend towards a better outcome in the PT-EPS group (p = 0.3). This does not contradict previous reports, which demonstrated a better outcome of PT-EPS, as the study was not powered to find such a difference [19], [45]. The younger age of PT-EPS patients and the better overall condition of patients with a functioning kidney allograft would be likely explanations for a better outcome [19].
In conclusion, this analysis did not support the hypothesis that EPS following transplantation is a different clinico-pathological entity, despite differences in the time taken between stopping PD and diagnosis and possible differences in known risk factors such membrane failure.
Acknowledgments
The study was supported by Baxter.
European EPS study group: Andreas Vychytil, Division of Nephrology and Dialysis, Department of Medicine III, Medical University of Vienna, Vienna, Austria; Eric Goffin, Université catholique de Louvain, Brussels, Belgium; Guido Garosi, Azienda Ospedaliera Universitaria Senese, Siena, Italy; Rafael Selgas, Department of Nephrology, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain; Alferso C. Abrahams University Medical Center Utrecht,Utrecht, Netherlands; Bengt Lindholm, Divisions of Renal Medicine and Baxter Novum, Department of Clinical Science, Intervention and Technology, Karolinska University Hospital K56, Karolinska Institutet, 14186, Stockholm, Sweden; Paul Brenchley, Manchester Institute of Nephrology and Transplantation, Central Manchester University Hospital NHS Trust, Oxford Road, Manchester M13 9WL, UK; Angela Summers, Manchester Royal Infirmary, Manchester, UK.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
These authors have no support or funding to report.
References
- 1. Maruyama Y, Nakayama M (2008) Encapsulating peritoneal sclerosis in Japan. Perit Dial Int 28 Suppl 3S201–204. [PubMed] [Google Scholar]
- 2. Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D, et al. (2013) Encapsulating peritoneal sclerosis: a rare, serious but potentially curable complication of peritoneal dialysis-experience of a referral centre in Germany. Nephrol Dial Transplant 28: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 3. Korte MR, Boeschoten EW, Betjes MG (2009) The Dutch EPS Registry: increasing the knowledge of encapsulating peritoneal sclerosis. Neth J Med 67: 359–362. [PubMed] [Google Scholar]
- 4. Habib SM, Betjes MG, Fieren MW, Boeschoten EW, Abrahams AC, et al. (2011) Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med 69: 500–507. [PubMed] [Google Scholar]
- 5. Brown MC, Simpson K, Kerssens JJ, Mactier RA (2009) Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 4: 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sampimon DE, Korte MR, Barreto DL, Vlijm A, de Waart R, et al. (2010) Early diagnostic markers for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 30: 163–169. [DOI] [PubMed] [Google Scholar]
- 7. Summers AM, Abrahams AC, Alscher MD, Betjes M, Boeschoten EW, et al. (2011) A collaborative approach to understanding EPS: the European perspective. Biomarkers of EPS: can we go “back to the future”? Perit Dial Int 31: 245–248. [DOI] [PubMed] [Google Scholar]
- 8. Braun N, Fritz P, Ulmer C, Latus J, Kimmel M, et al. (2012) Histological criteria for encapsulating peritoneal sclerosis - a standardized approach. PLoS One 7: e48647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D, et al. (2013) Phenotypes of encapsulating peritoneal sclerosis–macroscopic appearance, histologic findings, and outcome. Perit Dial Int 33: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG (2000) Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 20 Suppl 4S43–55. [PubMed] [Google Scholar]
- 11. Vlijm A, Stoker J, Bipat S, Spijkerboer AM, Phoa SS, et al. (2009) Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 29: 517–522. [PubMed] [Google Scholar]
- 12. Tarzi RM, Lim A, Moser S, Ahmad S, George A, et al. (2008) Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol 3: 1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Honda K, Nitta K, Horita S, Tsukada M, Itabashi M, et al. (2003) Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 19: 169–175. [PubMed] [Google Scholar]
- 14. Garosi G, Di Paolo N, Sacchi G, Gaggiotti E (2005) Sclerosing peritonitis: a nosological entity. Perit Dial Int 25 Suppl 3S110–112. [PubMed] [Google Scholar]
- 15. Sherif AM, Yoshida H, Maruyama Y, Yamamoto H, Yokoyama K, et al. (2008) Comparison between the pathology of encapsulating sclerosis and simple sclerosis of the peritoneal membrane in chronic peritoneal dialysis. Ther Apher Dial 12: 33–41. [DOI] [PubMed] [Google Scholar]
- 16. Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, et al. (2010) Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int 77: 904–912. [DOI] [PubMed] [Google Scholar]
- 17. Oules R, Challah S, Brunner FP (1988) Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol Dial Transplant 3: 66–69. [PubMed] [Google Scholar]
- 18. Fontana I, Bertocchi M, Santori G, Ferrari G, Barabani C, et al. (2012) Encapsulating peritoneal sclerosis after kidney transplantation: a single-center experience from 1982 to 2010. Transplant Proc 44: 1918–1921. [DOI] [PubMed] [Google Scholar]
- 19. Habib SM, Korte MR, Betjes MG (2013) Lower mortality and inflammation from post-transplantation encapsulating peritoneal sclerosis compared to the classical form. Am J Nephrol 37: 223–230. [DOI] [PubMed] [Google Scholar]
- 20. Fieren MW, Betjes MG, Korte MR, Boer WH (2007) Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int 27: 619–624. [PubMed] [Google Scholar]
- 21. Korte MR, Habib SM, Lingsma H, Weimar W, Betjes MG (2011) Posttransplantation encapsulating peritoneal sclerosis contributes significantly to mortality after kidney transplantation. Am J Transplant 11: 599–605. [DOI] [PubMed] [Google Scholar]
- 22. Saito A (2005) Peritoneal dialysis in Japan: the issue of encapsulating peritoneal sclerosis and future challenges. Perit Dial Int 25 Suppl 4S77–82. [PubMed] [Google Scholar]
- 23. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, et al. (2004) Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 44: 729–737. [PubMed] [Google Scholar]
- 24. Honda K, Oda H (2005) Pathology of encapsulating peritoneal sclerosis. Perit Dial Int 25 Suppl 4S19–29. [PubMed] [Google Scholar]
- 25. Korte MR, Yo M, Betjes MG, Fieren MW, van Saase JC, et al. (2007) Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol Dial Transplant 22: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 26. Khanna A, Plummer M, Bromberek C, Bresnahan B, Hariharan S (2002) Expression of TGF-beta and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int 62: 2257–2263. [DOI] [PubMed] [Google Scholar]
- 27. Nakamoto H (2005) Encapsulating peritoneal sclerosis–a clinician's approach to diagnosis and medical treatment. Perit Dial Int 25 Suppl 4S30–38. [PubMed] [Google Scholar]
- 28. Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, et al. (2011) Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 26: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 29. Braun N, Reimold F, Biegger D, Fritz P, Kimmel M, et al. (2009) Fibrogenic growth factors in encapsulating peritoneal sclerosis. Nephron Clin Pract 113: c88–95. [DOI] [PubMed] [Google Scholar]
- 30. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, et al. (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479. [DOI] [PubMed] [Google Scholar]
- 31. Braun N, Alscher MD, Fritz P, Latus J, Edenhofer I, et al. (2012) The spectrum of podoplanin expression in encapsulating peritoneal sclerosis. PLoS One 7: e53382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, et al.. (2010) Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. [DOI] [PubMed]
- 33. Shimaoka T, Hamada C, Kaneko K, Io H, Sekiguchi Y, et al. (2010) Quantitative evaluation and assessment of peritoneal morphologic changes in peritoneal dialysis patients. Nephrol Dial Transplant 25: 3379–3385. [DOI] [PubMed] [Google Scholar]
- 34. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, et al. (2008) Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 3: 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bozkurt D, Sipahi S, Cetin P, Hur E, Ozdemir O, et al. (2009) Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit Dial Int 29 Suppl 2S206–210. [PubMed] [Google Scholar]
- 36. Alscher DM, Braun N, Biegger D, Fritz P (2007) Peritoneal mast cells in peritoneal dialysis patients, particularly in encapsulating peritoneal sclerosis patients. Am J Kidney Dis 49: 452–461. [DOI] [PubMed] [Google Scholar]
- 37. Kinashi H, Ito Y, Mizuno M, Suzuki Y, Terabayashi T, et al. (2013) TGF-beta1 promotes lymphangiogenesis during peritoneal fibrosis. J Am Soc Nephrol 24: 1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalof AN, Cooper K (2009) D2–40 immunohistochemistry–so far! Adv Anat Pathol. 16: 62–64. [DOI] [PubMed] [Google Scholar]
- 39. Guest S (2009) Hypothesis: gender and encapsulating peritoneal sclerosis. Perit Dial Int 29: 489–491. [PubMed] [Google Scholar]
- 40. Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW, et al. (2011) Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 31: 269–278. [DOI] [PubMed] [Google Scholar]
- 41. Martikainen TA, Teppo AM, Gronhagen-Riska C, Ekstrand AV (2005) Glucose-free dialysis solutions: inductors of inflammation or preservers of peritoneal membrane? Perit Dial Int 25: 453–460. [PubMed] [Google Scholar]
- 42. Posthuma N, Verbrugh HA, Donker AJ, van Dorp W, Dekker HA, et al. (2000) Peritoneal kinetics and mesothelial markers in CCPD using icodextrin for daytime dwell for two years. Perit Dial Int 20: 174–180. [PubMed] [Google Scholar]
- 43. Brown EA, Van Biesen W, Finkelstein FO, Hurst H, Johnson DW, et al. (2009) Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 29: 595–600. [PubMed] [Google Scholar]
- 44. Kawanishi H, Moriishi M (2005) Epidemiology of encapsulating peritoneal sclerosis in Japan. Perit Dial Int 25 Suppl 4S14–18. [PubMed] [Google Scholar]
- 45. Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, et al. (2009) The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 24: 3209–3215. [DOI] [PubMed] [Google Scholar]
- 46. van Westrhenen R, Aten J, Hajji N, de Boer OJ, Kunne C, et al. (2007) Cyclosporin A induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif 25: 466–472. [DOI] [PubMed] [Google Scholar]
- 47. Margetts PJ, Bonniaud P, Liu L, Hoff CM, Holmes CJ, et al. (2005) Transient overexpression of TGF-{beta}1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol 16: 425–436. [DOI] [PubMed] [Google Scholar]
- 48.Hurst H, Summers AM, Beaver K, Caress AL (2014) Living with Encapsulating Peritoneal Sclerosis (Eps): The Patient's Perspective. Perit Dial Int. [DOI] [PMC free article] [PubMed]
- 49. Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ (2010) The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int 78: 611–618. [DOI] [PubMed] [Google Scholar]
- 50. Lambie M, Braun N, Davies SJ (2013) Towards standardized reporting in studies of encapsulating peritoneal sclerosis. Perit Dial Int 33: 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.