Abstract
Objective
To determine whether geographical elevation is inversely associated with diabetes, while adjusting for multiple risk factors.
Design and Methods
This is a cross-sectional analysis of publicly available online data from the Behavioral Risk Factor Surveillance System, 2009. Final dataset included 285,196 US adult subjects. Odds ratios were obtained from multilevel mixed-effects logistic regression analysis.
Results
Among US adults (≥20 years old), the odds ratio for diabetes were 1.00 between 0−499 m of altitude (reference), 0.95 (95% confidence interval, 0.90 to 1.01) between 500−1,499 m, and 0.88 (0.81 to 0.96) between 1,500−3,500 m, adjusting for age, sex, body mass index, ethnicity, self-reported fruit and vegetable consumption, self-reported physical activity, current smoking status, level of education, income, health status, employment status, and county-level information on migration rate, urbanization, and latitude. The inverse association between altitude and diabetes in the US was found among men [0.84 (0.76 to 0.94)], but not women [1.09 (0.97 to 1.22)].
Conclusions
Among US adults, living at high altitude (1,500−3,500 m) is associated with lower odds of having diabetes than living between 0−499 m, while adjusting for multiple risk factors. Our findings suggest that geographical elevation may be an important factor linked to diabetes.
Keywords: Altitude, diabetes, high altitude, obesity, odds, odds ratio
Introduction
Diabetes mellitus is the 7th leading cause of death in the United States (US) (1). The World Health Organization have estimated that ~346 million adult people worldwide have diabetes, of which 90-95% belong to the group of type 2 diabetes (2). The global prevalence of diabetes has been estimated at 6.4%, and it is projected to increase to 7.7% by 2030 (3).
Abnormal elevation of blood glucose levels is the hallmark of diabetes. Intriguingly, male residents at high altitude, compared with residents at sea level, have lower fasting glycemia (4-6). Similarly, lower fasting glycemia has been reported for pregnant (7-9) and non-pregnant women (9,10) residing at high altitude. Residents of high altitude also show a better glucose tolerance (11,12) compared with residents at sea level.
An inverse association between prevalence of diabetes mellitus and altitude has likewise been reported among hospital adult inpatients (13). Another study reported a lower prevalence of diabetes in a community located at high altitude (3,052 m) compared with those from other five communities located near sea level (14). In North America, the age-adjusted incidence of type 2 diabetes among Mexican-Americans living in San Antonio, Texas (198 m) was higher than that among Mexicans living in Mexico City (2,240 m), both in men and in women (15), suggesting that ethnicity may not explain the lower prevalence of diabetes at higher altitudes.
Although numerous reports suggest beneficial effects of living at high altitude on glucose homeostasis, no study has investigated the potential contribution of altitude to the odds of prevalent diabetes while adjusting for multiple risk factors and potential confounders. In the present study, we re-examined publicly available online data from a survey conducted in a nationally representative sample of the adult population from the US. The aim of this study was to determine whether geographical elevation is inversely associated with diabetes, while adjusting for age, sex, body mass index (BMI), ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, health status, employment status, and county-level information on migration rate, urbanization, and latitude. Our findings indicate that US adult individuals living at high altitude (1,500−3,500 m) had lower odds of having diabetes, while adjusting for multiple risk factors. The mechanism(s) underlying this interesting finding remains unknown.
Methods
In the present study, high altitude was defined as an elevation between 1,500 m and 3,500 m, according to the classification recommended by the International Society for Mountain Medicine (www.ismmed.org).
This study did not require approval or exemption from the Institutional Review Board at Cedars-Sinai Medical Center because it involved a cross-sectional analysis of publicly available, de-identified online data.
Data from the Centers of Disease Control and Prevention (CDC)
Database from the CDC (apps.nccd.cdc.gov/ddtstrs) was utilized to compare the age-adjusted self-reported prevalence of obesity and diabetes for 2009 in the US adult population (20 years or older) between low- and high-altitude counties. This database was also utilized to determine the prevalence trends of obesity and diabetes in low- and high-altitude counties from 2004 to 2009. Prevalence estimates reported by the CDC included all US contiguous states, Puerto Rico, and the District of Columbia. Since data for Alaska and Hawaii were not available, Puerto Rico data were also excluded for not being part of the contiguous US. Therefore, 3,109 counties were analyzed. CDC estimated the prevalence of obesity and diabetes by county using data from the Behavioral Risk Factor Surveillance System (BRFSS) and data from the United States Census Bureau's Population Estimates Program. Diabetes (type 1 and type 2 together) and obesity prevalences for 2009 were estimated using three years of data (2008, 2009, and 2010) to improve the precision of the estimates. Further details on the methodology are available online (apps.nccd.cdc.gov/ddtstrs).
Data from the BRFSS
Data from the BRFSS (www.cdc.gov/brfss), a telephone-based survey conducted in 432,607 subjects ≥18 years old, was utilized to estimate the odds ratios for overweight, obesity, and diabetes at different altitude bands. To be consistent with the analysis of the prevalence estimates of obesity and diabetes for 2009 (latest report) from the CDC, we chose the BRFSS database for 2009. Subjects from Alaska, Hawaii, Guam, Puerto Rico, and Virgin Islands were not included. Individuals aged 18−19 years and self-reported pregnant cases were also excluded. Thus, final dataset with complete information comprised 285,196 subjects for overweight and obesity analyses and 284,945 subjects for diabetes analyses. The odds ratios for overweight, obesity, and diabetes were adjusted for age, sex, ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, general health status, employment status, migration rate, urbanization, and latitude. Because obesity is a well established risk factor for type 2 diabetes (16), BMI was also included as a mediator to estimate the odds ratio for diabetes. Overweight was defined as a BMI (weight/height2) of 25−29.9. Obesity was defined as a BMI of 30 or higher. Obesity classes were divided as follow: Class I (BMI 30.0−34.9), class II (BMI 35−39.9), and class III (BMI 40 or higher) (17). Ethnicity included 8 categories: White, Black, Asian, Native Hawaiian or other Pacific Islander, American Indian or Alaskan Native, Other ethnicity, Hispanic, and Multiracial. Self-reported fruit and vegetable consumption represented the total number of servings of fruits and vegetables consumed per day, grouped in 4 categories, according to the frequency distribution of number of servings in the population studied: 0−1.99; 2−3.99; 4−4.99; and ≥5.00 servings. Moderate or vigorous physical activity beyond the recommended 150 min per week (18) has been related to more sustained weight loss (19). Therefore, we analyzed the self-reported total minutes per week of moderate and vigorous activity (at home or during recreation), grouped in 4 categories: 0−149 min, 150−299 min, 300−449 min, and ≥ 450 min. Current smoking status, level of education (if completed high school), income level (if income was less than 10,000 US dollars a year per household), general health status, and employment status (if currently employed or self-employed only), and health insurance status (if insured) were treated as dichotomous variables. Self-reported health status was grouped as poor or good. Data for all these variables were obtained from the BRFSS database. Although smoking cessation is also linked to weight gain (20), information on this variable was only available in ~15% of the population sample, therefore, it was not included in the analysis. Sample size for individuals living in counties with a mean altitude above 3,000 m was small to included them in a separate category, Therefore, altitude was grouped only in 3 categories: 0−499 m (~83% of the sample size), 500−1,499 m (~11%), and 1,500−3,500 m.
Data from the United States Census Bureau
Since data about migration and urbanization were not available from the BRFSS, instead county-level information of these variables were obtained from the United States Census Bureau (factfinder2.census.gov) and used for multivariate analysis. Out-migration rate data represented flow counts, that is movers moving from one county to other different destination counties during less than one year before the time they were surveyed (in a 5-year period estimate from 2005−2009). Information about urbanization (we used the percentage of rural population) was estimated from the most current data available for 2000. Out-migration rate and urbanization were treated as continuous variables.
Additional data sources
Altitude of every US county (mean altitude of city-level elevations of a given county) was obtained from the database Zip-codes.com (Datasheer, L.L.C., Hopewell Junction, NY), and missing data were completed utilizing the National Elevation Dataset from the US Geological Survey (ned.usgs.gov). Because ambient temperature is mainly determined by altitude and latitude (21), to avoid collinearity, latitude was included as a confounder instead of ambient temperature. County-level latitude data were also obtained from Zip-codes.com, and grouped in 3 categories: 20−29.99° N; 30−39.99° N; and 40−49.99° N. County-level air pollution data of fine particulate matter (PM 2.5, ≤2.5 Rm diameter) and ozone (O3) levels for 2009 were obtained from the United States Environmental Protection Agency (www.epa.gov). PM 2.5 values represented the highest weighted annual mean concentration. O3 represented the highest fourth daily maximum 8-hour concentration. Air pollution data represented the quality of the air in the vicinity of the monitoring sites within a county.
Statistical analysis
Prevalence data were expressed as medians and interquartile ranges. Bivariate associations were determined using Spearman rank order correlation. Multilevel mixed-effects logistic regression was used to determine the odds ratios for overweight, obesity, overweight and obesity together, obesity classes, and diabetes, while adjusting for multiple risk factors and potential confounders. This approach accounted for nested data (state, county, and individual level) and the random effects between subjects. Health insurance status and air pollution were included as covariates in subset analyses as data availability were restricted to fewer counties (~50% only). All calculations were performed using Statistica 7.0 (StatSoft Inc., Tulsa, OK) and Stata/SE 13.0 for Windows (StataCorp LP, College Station, TX).
Results
Adult prevalence of obesity and diabetes in low' and high'altitude US counties
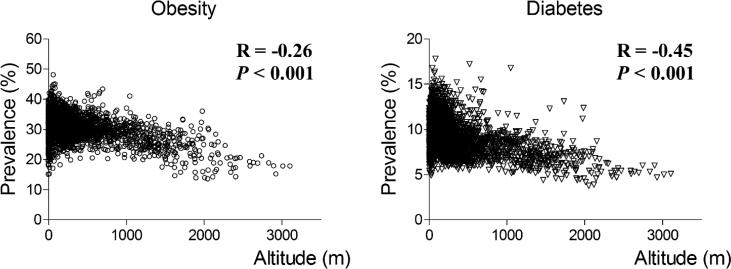
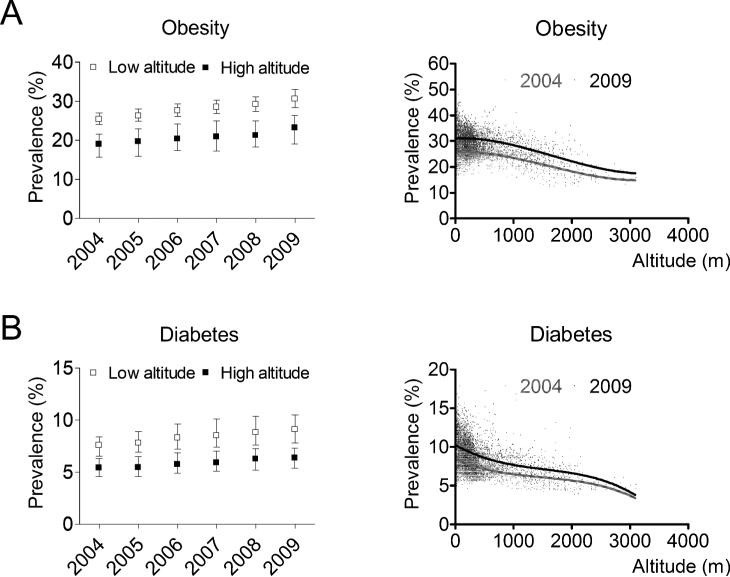
One hundred and thirty-two high-altitude counties compared with 2,977 low-altitude counties had considerably lower age-adjusted median prevalences of obesity [23.3% (interquartile range, 19.1 to 26.4) and 30.6% (28.4 to 33.0)]. Similarly, diabetes was substantially less prevalent in high-altitude counties [6.4% (5.4 to 7.3) and 9.1% (7.8 to 10.5)]. Altitude and prevalence of obesity (Figure 1A) were negatively correlated (R=−0.26; P<0.001). Likewise, altitude and prevalence of diabetes (Figure 1B) were negatively correlated (R=−0.45; P<0.001). Figure 2 shows the trends of obesity (Figure 2A) and diabetes (Figure 2B) prevalences at low- and high-altitude US counties during the period 2004−2009.
Figure 1.
Correlation between altitude and county-level prevalence of self-reported obesity and diabetes among US adults. Data of obesity prevalence (A) and diabetes prevalence (B) where age-adjusted and corresponded to estimates from the Centers for Disease Control and Prevention for 2009. Associations were estimated using Spearman rank order correlation.
Figure 2.
Trends of age-adjusted prevalences of obesity (A) and diabetes (B) among US adults aged 20 years or older from low- and high-altitude counties. Plots and bars represent median and interquartile range, respectively. In the insets showing the prevalence of obesity and diabetes as a function of altitude, lines represent the non-linear (third-order polynomial) fit of the plots.
Odds ratios for overweight, obesity, and diabetes at high altitude
In the simplest multilevel model, including altitude as the only independent variable, the odds ratios for diabetes among US adult subjects living at different elevations were as follows: 1.00 between 0−499 m (reference); 0.99 (95% confidence interval 0.92 to 1.05) between 500−1,499 m; and 0.82 (0.73 to 0.91) between 1,500−3,500 m. When BMI was also included, the odds ratios for diabetes were 1.00 between 0−499 m (reference), 1.00 (0.94 to 1.06) between 500−1,499 m, and 0.87 (0.78 to 0.96) between 1,500−3,500 m.
Prevalence of risk factors included in the full models for overweight, obesity, and diabetes, by altitude bands, are shown in Table 1. Odds ratios for overweight, obesity, and diabetes, adjusted for multiple risk factors and potential confounders, are shown in Table 2. This study found no significant association between overweight and altitude. Using the category 0−499 m as reference (odds ratio = 1.00), the odds ratio for obesity and diabetes, respectively, were 0.96 (95% confidence interval 0.92 to 1.01) and 0.95 (0.90 to 1.01) between 500−1,499 m, and 0.77 (0.70 to 0.83) and 0.88 (0.81 to 0.96) between 1,500−3,500 m. Intriguingly, the lower odds of having diabetes in individuals living between 1,500−3,500 m, compared with those living between 0−499 m, was not mediated by BMI alone.
Table 1.
Prevalence of risk factors* for overweight, obesity, and diabetes in US adults aged 20 years or older, by altitude bands.
| Overweight | Obesity | Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–499 m | 500–1,499 m | 1,500–3,500 | 0–499 m | 500–1,499 m | 1,500–3,500 | 0–499 m | 500–1,499 m | 1,500–3,500 | |
| Variable | |||||||||
| Health status | |||||||||
| Poor | 16.6 | 16.5 | 14.2 | 28.0 | 26.6 | 25.0 | 46.4 | 45.4 | 43.1 |
| Good | 83.4 | 83.5 | 85.8 | 72.0 | 73.4 | 75.0 | 53.6 | 54.7 | 56.9 |
| Current smoker | |||||||||
| No | 84.2 | 85.6 | 85.8 | 85.1 | 86.2 | 87.0 | 85.8 | 86.9 | 87.3 |
| Yes | 15.8 | 14.4 | 14.2 | 14.9 | 13.8 | 13.0 | 14.2 | 13.1 | 12.7 |
| Level of education | |||||||||
| Yes high school | 8.6 | 8.1 | 6.1 | 11.3 | 10.2 | 8.9 | 16.3 | 14.8 | 11.4 |
| No high school | 91.4 | 91.9 | 93.9 | 88.7 | 89.8 | 91.1 | 83.7 | 85.2 | 88.6 |
| Currently employed | |||||||||
| No | 48.5 | 48.7 | 44.4 | 50.3 | 48.5 | 45.8 | 71.6 | 70.7 | 67.6 |
| Yes | 51.5 | 51.3 | 55.6 | 49.7 | 51.5 | 54.2 | 28.4 | 29.3 | 32.4 |
| Low Income | |||||||||
| No | 95.7 | 96.3 | 97.0 | 93.3 | 94.4 | 94.8 | 90.5 | 91.8 | 92.9 |
| Yes | 4.3 | 3.7 | 3.0 | 6.7 | 5.6 | 5.2 | 9.5 | 8.2 | 7.1 |
| Sex | |||||||||
| Male | 47.3 | 49.4 | 52.1 | 39.2 | 42.5 | 42.0 | 40.8 | 42.4 | 43.1 |
| Female | 52.7 | 50.6 | 47.9 | 60.8 | 57.5 | 58.0 | 59.2 | 57.6 | 56.9 |
| Ethnicity | |||||||||
| Multiracial** | 1.3 | 1.3 | 1.1 | 1.6 | 1.3 | 1.6 | 1.6 | 1.4 | 1.3 |
| White only** | 81.9 | 85.6 | 79.7 | 76.0 | 83.2 | 73.3 | 74.1 | 80.1 | 67.5 |
| Black only** | 9.1 | 0.9 | 1.2 | 14.8 | 1.3 | 1.7 | 15.9 | 1.6 | 2.1 |
| Asian only** | 1.1 | 0.6 | 0.6 | 0.4 | 0.2 | 0.2 | 0.9 | 0.6 | 0.6 |
| Hawaiian or other Pacific Islander only** | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 |
| American Indian or Alaskan Native only** | 0.8 | 1.4 | 3.6 | 1.1 | 2.2 | 6.5 | 1.3 | 2.9 | 6.7 |
| Other only** | 0.5 | 0.5 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.8 | 0.7 |
| Hispanic | 5.2 | 9.5 | 13.2 | 5.6 | 11.1 | 16.0 | 5.4 | 12.4 | 21.0 |
| Fruit/Vegetable | |||||||||
| 0-1.99 Servings/day | 17.3 | 17.6 | 17.1 | 20.9 | 20.4 | 21.3 | 19.0 | 18.9 | 20.3 |
| 2-3.99 Servings/day | 43.2 | 42.8 | 42.7 | 43.3 | 44.0 | 42.5 | 42.5 | 42.2 | 40.5 |
| 4-4.99 Servings/day | 16.2 | 15.7 | 15.5 | 14.8 | 14.4 | 13.6 | 15.4 | 14.8 | 13.8 |
| 5+ Servings/day | 23.3 | 23.9 | 24.7 | 21.0 | 21.3 | 22.6 | 23.0 | 24.0 | 25.4 |
| Latitude | |||||||||
| 20–29.99° N | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 30–39.99° N | 52.4 | 38.5 | 65.9 | 55.3 | 40.2 | 65.3 | 57.6 | 43.5 | 68.2 |
| 40–49.99° N | 47.6 | 61.5 | 34.1 | 44.7 | 59.8 | 34.7 | 42.4 | 56.5 | 31.8 |
| Physical activity | |||||||||
| 0–149 min/week | 39.7 | 35.9 | 32.6 | 20.9 | 20.4 | 21.3 | 58.5 | 54.6 | 49.8 |
| 150–299 min/week | 23.7 | 23.9 | 25.3 | 43.3 | 44.0 | 42.5 | 18.6 | 19.9 | 21.4 |
| 300–449 min/week | 14.2 | 14.8 | 16.2 | 14.8 | 14.4 | 13.6 | 9.5 | 9.9 | 11.8 |
| ≥ 450 min/week | 22.4 | 25.4 | 25.9 | 21.0 | 21.3 | 22.6 | 13.4 | 15.7 | 17.1 |
Prevalences are percentages.
Categorical variables included in the multilevel mixed-effects logistic regression.
Non-Hispanic.
Table 2.
Odds ratios for overweight, obesity, and diabetes in US adults aged 20 years or older.
| All* | Women** | Men** | |
|---|---|---|---|
| Overweight | |||
| N | 285,196 | 285,196 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 1.01 (0.98 to 1.04) | 1.02 (0.97 to 1.08) | 1.00 (0.96 to 1.04) |
| 1,500–3,500 m | 0.98 (0.94 to 1.02) | 0.89 (0.847 to 0.95) | 1.04 (0.98 to 1.10) |
| Obesity | |||
| n | 285,196 | 285,109 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.96 (0.92 to 1.01) | 0.97 (0.92 to 1.02) | 0.98 (0.92 to 1.04) |
| 1,500–3,500 m | 0.77 (0.70 to 0.83) | 1.05 (0.98 to 1.13) | 0.75 (0.68 to 0.82) |
| Overweight or obesity | |||
| n | 285,196 | 285,109 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.97 (0.93 to 1.02) | 1.01 (0.96 to 1.07) | 0.96 (0.91 to 1.02) |
| 1,500–3,500 m | 0.78 (0.72 to 0.84) | 0.98 (0.92 to 1.05) | 0.79 (0.72 to 0.86) |
| Obesity class I | |||
| n | 285,109 | 285,109 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.98 (0.94 to 1.03) | 0.94 (0.89 to 1.00) | 1.01 (0.96 to 1.07) |
| 1,500–3,500 m | 0.82 (0.76 to 0.88) | 1.07 (0.98 to 1.16) | 0.79 (0.72 to 0.86) |
| Obesity class II | |||
| n | 285,109 | 285,109 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.95 (0.89 to 1.02) | 1.08 (0.97 to 1.19) | 0.91 (0.83 to 1.00) |
| 1,500–3,500 m | 0.79 (0.71 to 0.88) | 1.06 (0.92 to 1.21) | 0.77 (0.67 to 0.88) |
| Obesity class III | |||
| n | 285,109 | 285,109 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.94 (0.86 to 1.03) | 1.04 (0.91 to 1.19) | 0.91 (0.80 to 1.04) |
| 1,500–3,500 m | 0.79 (0.68 to 0.91) | 1.06 (0.87 to 1.30) | 0.75 (0.62 to 0.92) |
| Diabetes | |||
| n | 284,945 | 284,945 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.95 (0.90 to 1.01) | 1.04 (0.96 to 1.13) | 0.93 (0.87 to 1.00) |
| 1,500–3,500 m | 0.88 (0.81 to 0.96) | 1.09 (0.97 to 1.22) | 0.84 (0.76 to 0.94) |
95% Confidence intervals are shown in parentheses.
Odds ratios were adjusted for age, sex, ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, out-migration rate, health status, employment status, urbanization, and latitude, including body mass index to estimate the odds ratios for diabetes.
Odds ratios were adjusted for age, ethnicity, fruit and vegetable consumption, physical activity, current smoking, level of education, income, out-migration rate, health status, employment status, urbanization, and latitude, including body mass index to estimate the odds ratios for diabetes.
Odds ratios for other covariates included in the full models are shown in Table 3. When logistic regression was performed only in subjects with a BMI between 18.5 and 24.99 (n=93,032), while adjusting for other risk factors, the odds ratio for diabetes between 1,500−3,500 m was 0.85 (95% confidence interval 0.72 to 1.00), relative to 0−499 m, suggesting a trend for lower odds of having diabetes in lean individuals living at high altitude compared with lean individuals living below 500 m. To explain the association between diabetes and altitude independent of BMI, we tested the possibility of an interaction among current smoking status, BMI, and altitude. There were no interaction between these covariates, nor did these interactions lower the association between altitude and diabetes [0.67 (95% CI 0.50 to 0.90)], suggesting that smoking status and BMI do not moderate the relationship between diabetes and altitude.
Table 3.
Odds ratios for covariates included in the full multilevel models for overweight, obesity, and diabetes in US adults aged 20 years or older (all women and men).
| Overweight | Obesity | Diabetes | |
|---|---|---|---|
| Variable | |||
| BMI | -- | -- | 1.11 (1.11 to 1.11) |
| Urbanization | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| Migration rate | 0.92 (0.75 to 1.12) | 0.96 (0.71 to 1.29) | 0.83 (0.59 to 1.17) |
| Health status | |||
| Poor | 1.00 | 1.00 | 1.00 |
| Good | 1.34 (1.31 to 1.37) | 0.52 (0.50 to 0.53) | 0.36 (0.35 to 0.37) |
| Age | 1.01 (1.01 to 1.01) | 0.99 (0.99 to 0.99) | 1.04 (1.04 to 1.04) |
| Current smoker | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 0.96 (0.94 to 0.98) | 0.64 (0.63 to 0.66) | 1.01 (0.97 to 1.05) |
| Level of education | |||
| Yes high school | 1.00 | 1.00 | 1.00 |
| No high school | 0.99 (0.96 to 1.02) | 0.94 (0.91 to 0.97) | 0.98 (0.94 to 1.02) |
| Currently employed | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.10 (1.08 to 1.12) | 1.06 (1.04 to 1.08) | 0.76 (0.74 to 0.79) |
| Low Income | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 0.91 (0.88 to 0.95) | 1.10 (1.06 to 1.15) | 1.18 (1.12 to 1.24) |
| Sex | |||
| Male | 1.00 | 1.00 | 1.00 |
| Female | 0.56 (0.56 to 0.57) | 0.92 (0.90 to 0.93) | 0.71 (0.69 to 0.73) |
| Ethnicity | |||
| Multiracial* | 1.00 | 1.00 | 1.00 |
| White only* | 1.02 (0.95 to 1.09) | 0.78 (0.73 to 0.84) | 0.79 (0.71 to 0.87) |
| Black only* | 1.05 (0.97 to 1.13) | 1.44 (1.34 to 1.56) | 1.33 (1.20 to 1.48) |
| Asian only* | 0.79 (0.71 to 0.87) | 0.20 (0.17 to 0.23) | 1.62 (1.38 to 1.91) |
| Hawaiian or other Pacific Islander only* | 1.17 (0.94 to 1.47) | 0.84 (0.66 to 1.07) | 1.34 (0.93 to 1.93) |
| American Indian or Alaskan Native only* | 1.04 (0.94 to 1.15) | 1.19 (1.07 to 1.31) | 1.46 (1.27 to 1.68) |
| Other only* | 0.94 (0.82 to 1.08) | 0.76 (0.66 to 0.88) | 1.07 (0.88 to 1.31) |
| Hispanic | 1.26 (1.17 to 1.36) | 0.89 (0.82 to 0.96) | 1.28 (1.14 to 1.43) |
| Fruit/Vegetable intake | |||
| 0-1.99 Servings/day | 1.00 | 1.00 | 1.00 |
| 2-3.99 Servings/day | 1.04 (1.02 to 1.06) | 0.93 (0.91 to 0.95) | 1.04 (1.01 to 1.08) |
| 4-4.99 Servings/day | 1.00 (0.98 to 1.03) | 0.83 (0.81 to 0.86) | 1.03 (0.98 to 1.07) |
| 5+ Servings/day | 0.97 (0.94 to 0.99) | 0.80 (0.78 to 0.82) | 1.12 (1.07 to 1.16) |
| Altitude | |||
| 0–499 m | 1.00 | 1.00 | 1.00 |
| 500–1,499 m | 1.01 (0.98 to 1.04) | 0.96 (0.92 to 1.01) | 0.95 (0.90 to 1.01) |
| 1,500–3500 m | 0.98 (0.94 to 1.02) | 0.77 (0.70 to 0.83) | 0.88 (0.81 to 0.96) |
| Latitude | |||
| 20–29.99° N | 1.00 | 1.00 | 1.00 |
| 30–39.99° N | 0.98 (0.93 to 1.03) | 1.02 (0.94 to 1.11) | 1.08 (0.98 to 1.18) |
| 40–49.99° N | 1.00 (0.95 to 1.06) | 1.01 (0.92 to 1.11) | 1.02 (0.92 to 1.13) |
| Physical activity | |||
| 0–149 min/week | 1.00 | 1.00 | 1.00 |
| 150–299 min/week | 1.13 (1.11 to 1.16) | 0.70 (0.69 to 0.72) | 0.92 (0.89 to 0.95) |
| 300–449 min/week | 1.12 (1.10 to 1.15) | 0.59 (0.58 to 0.61) | 0.82 (0.79 to 0.86) |
| ≥ 450 min/week | 1.09 (1.06 to 1.11) | 0.53 (0.52 to 0.54) | 0.74 (0.71 to 0.77) |
95% Confidence intervals are shown in parentheses.
Non-Hispanic.
The odds of being obese between 1,500−3,500 m was lower among men only. Likewise, the odds of being obese at high altitude was lower among men, but not women, in all obesity classes (Table 2). Of note, the odds of having diabetes between 1,500−3,500 m was also lower among men only [Men: 0.84 (95% confidence interval 0.76 to 0.94); women: 1.09 (0.97 to 1.22)]. Likewise, analysis by age groups showed an inverse association between altitude and diabetes among men, but not women (Table 4). It should be noted that while adjusting for multiple covariates, including altitude, women had lower odds of being obese (OR=0.92, 95% CI=0.90 to 0.93) or having diabetes (OR=0.71, 95% CI=0.69 to 0.73) compared with men (Table 3).
Table 4.
Odds ratios for obesity by age category among US adults.
| All* | Women** | Men** | |
|---|---|---|---|
| 20–39 years old | |||
| n | 51,714 | 51,714 | |
| 0–499 m | 1.00 | 1.00 | 1.00 |
| 500–1,499 m | 1.04 (0.94 to 1.14) | 0.95 (0.83 to 1.07) | 1.07 (0.95 to 1.21) |
| 1,500–3,500 m | 0.81 (0.70 to 0.94) | 1.12 (0.94 to 1.34) | 0.76 (0.64 to 0.90) |
| 40–59 years old | |||
| n | 119,105 | 119,105 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.97 (0.91 to 1.04) | 1.00 (0.92 to 1.08) | 0.97 (0.89 to 1.06) |
| 1,500–3,500 m | 0.74 (0.67 to 0.83) | 1.01 (0.91 to 1.13) | 0.74 (0.65 to 0.84) |
| 60–79 years old | |||
| n | 95,346 | 95,346 | |
| 0–499 m | 1.00 | ||
| 500–1,499 m | 0.91 (0.85 to 0.97) | 0.93 (0.84 to 1.02) | 0.95 (0.87 to 1.04) |
| 1,500–3,500 m | 0.73 (0.66 to 0.82) | 1.05 (0.92 to 1.20) | 0.71 (0.63 to 0.81) |
95% Confidence intervals are shown in parentheses.
Odds ratios were adjusted for sex, ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, out-migration rate, health status, employment status, urbanization, and latitude.
Odds ratios were adjusted for ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, out-migration rate, health status, employment status, urbanization, and latitude.
Analysis of subjects belonging to age category ≥ 80 years old did not yield outputs because of missing data.
We found no lower odds of having diabetes at high altitude, relative to 0−499 m, among men younger than 40 years of age or ≥80 years old. Moreover, among men and women together, the strongest inverse association between altitude and diabetes was found in the age category 40−59 years old (Table 5).
Table 5.
Odds ratios for diabetes by age category among US adults.
| All* | Women** | Men** | |
|---|---|---|---|
| 20–39 years old | |||
| n | 51,696 | 51,696 | |
| 0–499 m | 1.00 | 1.00 | 1.00 |
| 500–1,499 m | 1.09 (0.90 to 1.31) | 1.28 (0.89 to 1.84) | 0.93 (0.68 to 1.26) |
| 1,500–3,500 m | 0.80 (0.61 to 1.06) | 0.96 (0.58 to 1.59) | 0.82 (0.55 to 1.23) |
| 40–59 years old | |||
| n | 119,043 | 119,043 | |
| 0–499 m | 1.00 | 1.00 | 1.00 |
| 500–1,499 m | 0.93 (0.85 to 1.02) | 1.16 (1.00 to 1.34) | 0.86 (0.77 to 0.97) |
| 1,500–3,500 m | 0.85 (0.74 to 0.96) | 1.09 (0.90 to 1.32) | 0.81 (0.69 to 0.96) |
| 60–79 years old | |||
| n | 95,288 | 95,288 | |
| 0–499 m | 1.00 | 1.00 | 1.00 |
| 500–1,499 m | 0.93 (0.87 to 1.00) | 0.99 (0.88 to 1.11) | 0.93 (0.85 to 1.03) |
| 1,500–3,500 m | 0.87 (0.78 to 0.98) | 1.08 (0.92 to 1.27) | 0.84 (0.73 to 0.97) |
| ≥80 years old | |||
| n | 19,004 | 19,004 | |
| 0–499 m | 1.00 | 1.00 | 1.00 |
| 500–1,499 m | 1.03 (0.89 to 1.18) | 0.85 (0.66 to 1.09) | 1.13 (0.92 to 1.38) |
| 1,500–3,500 m | 0.87 (0.70 to 1.09) | 1.27 (0.85 to 1.90) | 0.76 (0.54 to 1.05) |
95% Confidence intervals are shown in parentheses.
Odds ratios were adjusted for sex, body mass index, ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, out-migration rate, health status, employment status, urbanization, and latitude.
Odds ratios were adjusted for body mass index, ethnicity, fruit and vegetable consumption, physical activity, current smoking status, level of education, income, out-migration rate, health status, employment status, urbanization, and latitude.
After further adjustment for health insurance status and air pollution, including PM 2.5 and O3 levels together, the odds ratio for diabetes between 1,500−3,500 m remained similar [0.81 (0.69 to 0.95); n=101,246]. Because of the reduced number of observations having information on health insurance status and air pollution, analysis did not yield estimates of the odds ratios for obesity.
Discussion
Analysis of available database from a nationally representative sample from the United States revealed that adult individuals living at high altitude (1,500−3,500 m) had 12% lower odds of having diabetes than those living between 0−499 m, while adjusting for multiple risk factors, including age, sex, body mass index, ethnicity, and lifestyle-related factors. Likewise, US individuals living at high altitude had ~25% lower odds of being obese than those living between 0−499 m. Interestingly, the odds of being obese and having diabetes were lower among men only, although women had lower odds of being obese and having diabetes, as compared with men, regardless of altitude. The reasons for these differences remain unclear, and deserve further investigation. Our findings of an inverse association between altitude and diabetes in the US among men, but not women, demonstrate that this association interacts with gender.
We also found that the CDC modeled adult prevalence of obesity and diabetes in high-altitude US counties was ~25% and ~30% lower, respectively, compared with low-altitude counties. Our data confirm lower prevalences of obesity (22,23) and diabetes (14) at higher altitudes reported in smaller studies using the same diagnostic criteria. Interestingly, despite the lower US adult prevalence of obesity and diabetes at higher altitudes, both prevalences at high altitude appear to increase during the period 2004−2009 (Figure 2).
Our findings of an inverse association between altitude and obesity are consistent with those from a recent study that used data from the 2011 BRFSS (24). Authors reported odds ratios for obesity of 0.81 between 1.50−1.99 km, 0.37 between 2.50−2.99 km, and 0.22 between 3.00−3.49 km (24). Our findings are also consistent with those from a small study conducted in a non-representative sample of adult Tibetans, showing lower BMI at higher altitudes, while adjusting for several risk factors (22).
More importantly, the present study shows that living at high altitude is associated with lower odds of having diabetes, independent of obesity and other known risk factors, including physical activity. No previous study has explored the risk of diabetes in populations living at different altitudes, while adjusting for these risk factors. Of course, the interpretation of these findings has to be considered in the context of the limitations of our study. Our data originate from a cross-sectional study; thus, we cannot prove that the inverse association between altitude and obesity or between altitude and diabetes reflects causality. Certainly, it is possible that some residual confounding could influence our results. For example, despite our adjustment for physical activity, this information was self-reported. Information on total daily calorie intake was not available. Data for self-reported fruit and vegetable consumption were used instead. Although it might not be a surrogate of total calorie intake, fruit and vegetable consumption has been shown to be inversely associated with the incidence of diabetes (25).
One should consider whether reverse causality could explain our findings. Individuals with obesity or diabetes could migrate to lower altitudes. However, although altitude might represent a hostile environment for obese subjects, it seems unlikely that individuals with obesity class I, for example, selectively would tend to move to lower altitudes for medical reasons. Despite adjusting our findings for migration, information regarding residence period at high- or low-altitude was not available at the individual level. Interestingly, after further adjustment for health insurance status, the inverse association of altitude with diabetes was not affected, suggesting that access to health care may not explain the lower odds of having diabetes at high altitude.
Our findings originate from self-reported information collected by the BRFSS. The BRFSS slightly underestimates US obesity prevalences compared with the National Health and Nutrition Examination Survey, which does use standardized physical examination. However, diabetes prevalence estimates have been shown to be similar between surveys (26). Another limitation in our study is a potential bias due to lack of cell phone coverage. In addition, our multilevel analysis did not include final weight (frequency weighting) to account for the BRFSS sampling strategy. However, using binary logistic regression with clustered (at state level) robust standard errors, we found no substantial differences in the odds ratios for obesity and diabetes between weighted and unweighted data. Between 1,500−3,500 m, the odds ratio for obesity were 0.76 (95% confidence interval 0.68 to 0.86) and 0.73 (0.65 to 0.82) and for diabetes 0.84 (0.77 to 0.92) and 0.83 (0.76 to 0.91), weighted and unweighted data, respectively. Finally, BRFSS information on diabetes diagnosis does not discriminate the type of diabetes. Obesity is linked to type 2 diabetes, but not type 1 diabetes (16). However, it is unlikely that the lower odds of diabetes at higher altitude was explained by type 1 diabetes, since type 1 diabetes accounts for only 5-10% of the total cases of diabetes (16).
Biological mechanisms that might explain the inverse association between altitude and obesity are speculative but deserve some comment. There is strong evidence for increased basal metabolic rate at high altitude (27,28). However, this has been demonstrated only under short-term exposure conditions. High-altitude residents do not seem to have increased basal metabolic rate compared with subjects who lived at low altitude (29). As aforementioned, there is an inverse relationship between elevation and ambient temperature (21). Thus, it is reasonable to speculate that highlanders has increased cold-induced thermogenesis. There is also strong evidence of decreased appetite at high altitude (30), although these data originate from short-term exposure. It is known that leptin favors energy expenditure and reduces appetite (31). However, fasting leptin is decreased in individuals residing at high altitude (10,32,33), probably indicating an adaptive mechanism to high altitude, or related to lower body adiposity at high altitude, as reported in Peruvian Amerindians (10). Therefore, leptin does not seem to support the idea that high-altitude residents have increased cold-induced thermogenesis. Since unintentional physical activity (e.g. daily walking) is an important component of the total energy expenditure (34), an alternative explanation is that individuals living at high altitude have increased energy expenditure due to non-intentional physical activity as they have to challenge the topographical terrain of highlands under hypoxic conditions.
It can be argued that given that obesity is a strong risk factor for type 2 diabetes (16), our findings of a lower prevalence of diabetes at high altitude would be accounted by the lower prevalence of obesity at high altitude. However, it is very interesting that lower prevalence of self-reported diabetes at high altitude was not mediated by obesity alone. We also found a trend for lower odds of having diabetes among lean subjects living at high altitude compared with lean subjects living below 500 m. These results suggest that the inverse association between diabetes and altitude does not appear to be explained by obesity. However, we cannot suggest that this association is independent of body fat. Possible explanations for the lower odds of prevalent diabetes at high altitude are the reported lower fasting glycemia (4,6,10) and the better glucose tolerance (11,12) in individuals living at high altitude compared to lowlanders. This is also supported by the evidence of increased glucose uptake in rodent and human skeletal muscle incubated under anoxic and hypoxic conditions (35-37). In addition, numerous studies have shown increased content of the glucose transporter GLUT4 in the plasma membrane of skeletal muscle cells incubated under anoxia conditions (35,38), and in skeletal muscle cells from rodents exposed to prolonged hypoxia (9% O2) (39). Together, the latter findings could explain the better glucose tolerance at high altitude, which may have an impact in the lower prevalence of diabetes at high altitude. Certainly, further studies are required to determine whether the highlander has better β-cell function or increased insulin sensitivity as compared with the lowlander. Of note, we did not find lower odds of being obese or having diabetes between 500−1,499 m compared with 0−499 m. This is consistent with the more significant adaptive changes occurring above 1,500 m (40).
In conclusion, among the US population, living between 1,500−3,500 m is associated with lower odds of being obese and having diabetes, than living below 500 m, while adjusting for multiple risk factors and potential confounders. The inverse association between altitude and obesity and between altitude and diabetes in the US was found among men, but not women. Our results suggest that geographical elevation may be an important factor not only linked to obesity but also diabetes.
What is already known about this subject?
Numerous studies have reported lower prevalence of diabetes at higher altitudes. Whether this is mediated by the relationship between obesity and altitude, related to confounders, or due to non-random migration is unknown.
What does this study add?
Among adults of the United States, living at high altitude (1,500–3,500 m elevation) is associated with lower odds of having diabetes than living between 0–499 m, while adjusting for multiple risk factors and potential confounders.
The inverse association between altitude and diabetes in the US is found among men, but not women.
Acknowledgements
We thank Dr. Deborah J. Clegg for critical review of this paper and Dr. Robert A. Farley for facilitating us the access to the resources from the Center for High-Performance Computing and Communications (HPCC, USC).
This study was supported by the National Institutes of Health, the Alexander von Humboldt Foundation, Bonn-Bad Godesberg, Germany, and the UCLA Clinical and Translational Science Institute.
OOW, OAC, and RNB conceived and designed the study. OOW, CG, and DS collected and assembled the data. OOW, OAC, CG, RE, DS, and RNB interpreted the data. OOW and DS performed the statistical analysis. OOW and RNB drafted the manuscript. OOW searched the literature and generated the figures. All authors have read and approved the submitted manuscript. OOW had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest
References
- 1.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. Natl Vital Stat Rep 2010. 58:1–135. [PubMed] [Google Scholar]
- 2.WHO [December 7, 2011];Media Center: Diabetes. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/index.html. 2011.
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010. 87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Castillo O, Woolcott OO, Gonzales E, Tello V, Tello L, Villarreal C, et al. Residents at high altitude show a lower glucose profile than sea-level residents throughout 12-hour blood continuous monitoring. High Alt Med Biol. 2007;8:307–311. doi: 10.1089/ham.2007.8407. [DOI] [PubMed] [Google Scholar]
- 5.Garmendia F, Torres J, Tamayo R, Urdanivia E. Aportes al conocimiento de la glicemia de altura [Contributions to the knowledge of glycemia at high altitude]. Arch Inst Biol Andina. 1972;5:51–56. [PubMed] [Google Scholar]
- 6.Picon-Reategui E. Effect of chronic hypoxia on the action of insulin in carbohydrate metabolism. J Appl Physiol. 1966;21:1177–1180. doi: 10.1152/jappl.1966.21.4.1177. [DOI] [PubMed] [Google Scholar]
- 7.Calderon R, Llerena L, Munive L, Kruger F. Intravenous glucose tolerance test in pregnancy in women living in chronic hypoxia. Diabetes. 1966;15:130–132. doi: 10.2337/diab.15.2.130. [DOI] [PubMed] [Google Scholar]
- 8.Zamudio S, Torricos T, Fik E, Oyala M, Echalar L, Pullockaran J, et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 2010;5:e8551. doi: 10.1371/journal.pone.0008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krampl E, Kametas NA, Cacho Zegarra AM, Roden M, Nicolaides KH. Maternal plasma glucose at high altitude. Br J Obstet Gynaecol. 2001;108:254–257. doi: 10.1111/j.1471-0528.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindgarde F, Ercilla MB, Correa LR, Ahren B. Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt. Med. Biol. 2004;5:27–31. doi: 10.1089/152702904322963663. [DOI] [PubMed] [Google Scholar]
- 11.Picon-Reategui E. Intravenous glucose tolerance test at sea level and at high altitudes. J Clin Endocrinol Metab. 1963;23:1256–1261. doi: 10.1210/jcem-23-12-1256. [DOI] [PubMed] [Google Scholar]
- 12.Calderon R, Llerena A. Carbohydrate metabolism in people living in chronic hypoxia. Diabetes. 1965;14:100–105. doi: 10.2337/diab.14.2.100. [DOI] [PubMed] [Google Scholar]
- 13.Solís J, Guerra-García R. Prevalencia de diabetes mellitus en hospitalizados de las grandes alturas [Prevalence of diabetes mellitus in hospitalized patients from highlands]. Arch Inst Biol Andina. 1979;9:21–30. [Google Scholar]
- 14.Seclén S, Leey J, Villena A, Herrera B, Menacho J, Carrasco A, et al. Prevalencia de obesidad, diabetes mellitus, hipertensión arterial e hipercolesterolemia como factores de riesgo coronario y cerebro vascular en población adulta de la costa, sierra y selva del Perú [Prevalence of obesity, diabetes mellitus, arterial hypertension, and hypercholesterolemia as risk factors for coronary and cerebrovascular diseases in adult populations from the Coast, Mountain and the Forest in Peru]. Acta Médica Peruana. 1999;17:8–12. [Google Scholar]
- 15.Burke JP, Williams K, Haffner SM, Villalpando CG, Stern MP. Elevated incidence of type 2 diabetes in San Antonio, Texas, compared with that of Mexico City, Mexico. Diabetes Care. 2001;24:1573–1578. doi: 10.2337/diacare.24.9.1573. [DOI] [PubMed] [Google Scholar]
- 16.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 18.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005;99:1205–1213. doi: 10.1152/japplphysiol.00193.2005. [DOI] [PubMed] [Google Scholar]
- 19.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 20.Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8:e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery K. Variation in temperature with altitude and latitude. Journal of Geography. 2006;105:133–135. [Google Scholar]
- 22.Sherpa LY, Deji Stigum H, Chongsuvivatwong V, Thelle DS, Bjertness E. Obesity in Tibetans aged 30-70 living at different altitudes under the north and south faces of Mt. Everest. Int J Environ Res Public Health. 2010;7:1670–1680. doi: 10.3390/ijerph7041670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pajuelo-Ramírez J, Sánchez-Abanto J, Arbañil-Huamán H. Las enfermedades crónicas no transmisibles en el Perú y su relación con la altitud [Non transmissible chronic diseases in Peru and their relationship with altitude]. Revista de la Sociedad Peruana de Medicina Interna. 2010;23:45–52. [Google Scholar]
- 24.Voss JD, Masuoka P, Webber BJ, Scher AI, Atkinson RL. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int J Obes (Lond) 2013;37:1407–1412. doi: 10.1038/ijo.2013.5. [DOI] [PubMed] [Google Scholar]
- 25.Cooper AJ, Forouhi NG, Ye Z, Buijsse B, Arriola L, Balkau B, et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012;66:1082–1092. doi: 10.1038/ejcn.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Balluz LS, Ford ES, Okoro CA, Zhao G, Pierannunzi C. A comparison of prevalence estimates for selected health indicators and chronic diseases or conditions from the Behavioral Risk Factor Surveillance System, the National Health Interview Survey, and the National Health and Nutrition Examination Survey, 2007-2008. Prev Med. 2012;54:381–387. doi: 10.1016/j.ypmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Stock MJ, Norgan NG, Ferro-Luzzi A, Evans E. Effect of altitude on dietary- induced thermogenesis at rest and during light exercise in man. J Appl Physiol. 1978;45:345–349. doi: 10.1152/jappl.1978.45.3.345. [DOI] [PubMed] [Google Scholar]
- 28.Hannon JP, Sudman DM. Basal metabolic and cardiovascular function of women during altitude acclimatization. J Appl Physiol. 1973;34:471–477. doi: 10.1152/jappl.1973.34.4.471. [DOI] [PubMed] [Google Scholar]
- 29.Kashiwazaki H, Dejima Y, Orias-Rivera J, Coward WA. Energy expenditure determined by the doubly labeled water method in Bolivian Aymara living in a high altitude agropastoral community. Am J Clin Nutr. 1995;62:901–910. doi: 10.1093/ajcn/62.5.901. [DOI] [PubMed] [Google Scholar]
- 30.Palmer BF, Clegg DJ. Ascent to altitude as a weight loss method: The good and bad of hypoxia inducible factor activation. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolcott OO, Castillo OA, Torres J, Damas L, Florentini E. Serum leptin levels in dwellers from high altitude lands. High Alt Med Biol. 2002;3:245–246. doi: 10.1089/15270290260131975. [DOI] [PubMed] [Google Scholar]
- 33.Santos JL, Perez-Bravo F, Albala C, Calvillan M, Carrasco E. Plasma leptin and insulin levels in Aymara natives from Chile. Ann Hum Biol. 2000;27:271–279. doi: 10.1080/030144600282163. [DOI] [PubMed] [Google Scholar]
- 34.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 35.Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70:1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- 36.Lecoultre V, Peterson CM, Covington JD, Ebenezer PJ, Frost EA, Schwarz JM, et al. Ten nights of moderate hypoxia improves insulin sensitivity in obese humans. Diabetes Care. 2013;36:e197–198. doi: 10.2337/dc13-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azevedo JL, Jr., Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44:695–698. doi: 10.2337/diab.44.6.695. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds TH, IV., Brozinick JT, Jr., Rogers MA, Cushman SW. Effects of exercise training on glucose transport and cell surface GLUT-4 in isolated rat epitrochlearis muscle. Am J Physiol. 1997;272:E320–325. doi: 10.1152/ajpendo.1997.272.2.E320. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Warshaw JB, Haddad GG. Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am J Physiol. 1997;273:R1734–1741. doi: 10.1152/ajpregu.1997.273.5.R1734. [DOI] [PubMed] [Google Scholar]
- 40.Weil JV, Zwillich CW. Assessment of ventilatory response to hypoxia: methods and interpretation. Chest. 1976;70:124–128. [PubMed] [Google Scholar]