Abstract
Background
Few population-based studies have described characteristics and management of patients with chronic hepatitis B (CHB) in the USA.
Methods
We retrospectively studied adults with CHB in the Northern California Kaiser Permanente Medical Care Program (KPNC) from July 2009 to December 2010 (n = 12,016). Laboratory tests, treatment patterns, and hepatocellular carcinoma (HCC) surveillance were ascertained during a “recent” 18-month study window (July 2009–December 2010), or as “ever” based on records dating to 1995.
Results
The mean age was 49 years; 51 % were men, 83 % Asian, and 87 % KPNC members >5 years. Overall, 51 % had ≥1 liver-related visit, 14 % with gastroenterology or infectious disease specialists, and 37 % with primary care providers (PCP) only. Less than 40 % of patients had both hepatitis B virus (HBV) DNA and ALT testing conducted recently, while 56 % of eligible patients had received HCC surveillance. Recent laboratory testing and HCC surveillance were more frequent in patients seen by a specialist versus PCP only (90 vs. 47 % and 92 vs. 73 %, respectively, p values <0.001). During the study period, 1,649 (14 %) received HBV treatment, while 5 % of untreated patients had evidence of treatment eligibility. Among 599 patients newly initiated on HBV therapy, 76 % had guideline-based indications for treatment.
Conclusions
Most patients initiated on HBV treatment met eligibility, and very few patients with evidence of needing treatment were left untreated. However, monitoring of ALT and HBV DNA levels, as well as HCC surveillance, were not frequent, underestimating the proportion of patients that warranted HBV therapy. Viral monitoring and cancer surveillance are therefore important targets for improving the scope of CHB care in the community setting.
Keywords: Community, Management, Provider, Treatment
Introduction
Worldwide, over 2 billion people have been acutely infected with the hepatitis B virus (HBV), and approximately 350 million have chronic infection [1, 2]. Globally, the Asian Pacific and sub-Saharan Africa carry the highest disease burden of chronic hepatitis B (CHB), with prevalence rates>8 % [2, 3]. In the USA, widespread HBV vaccination has reduced the incidence of acute HBV infection over the past two decades. However, the prevalence of CHB has not declined, due in part to continued migration of infected individuals from endemic countries [4].
A recent study incorporating estimates of CHB among foreign-born (FB) individuals in the USA reports CHB prevalence of 3.5 %, or 2.2 million people [5]. This reflects a higher CHB prevalence than the 0.4 % reported by the National Health and Nutrition Examination Surveys (NHANES), which under-represent several high-risk groups, including Asian Pacific Islanders (APIs). Complications of CHB represent an important area of health care disparities in the USA, as those at highest risk remain foreign-born individuals who comprise 40–70 % of the population living with CHB. Studies among APIs living in the San Francisco Bay Area have highlighted the disproportionately high risk of subsequent development of hepatocellular carcinoma (HCC) in this population [6].
The morbidity and mortality associated with CHB in the USA are marked, with up to 40 % of patients developing serious complications, including end-stage liver disease, liver cancer, and/or death [7]. Prior studies in a large integrated care population in Northern California (where the current study is based) found that 8 % of chronic liver disease-related deaths were attributed to CHB [8]. In 2010, an Institute of Medicine report called for dedicated interventions to improve the morbidity and mortality associated with viral hepatitis in the USA [9]. For hepatitis B, morbidity and mortality may be prevented by monitoring disease progression, surveillance for HCC, and initiation of HBV treatment in appropriate candidates. Several major societies, including The American Association of the Study of Liver Diseases (AASLD), have released guidelines on the management of CHB, which detail recommended measures for laboratory monitoring, HCC surveillance, and treatment initiation [7, 10–12].
Few population-based studies have characterized the demographic and clinical parameters of patients with chronic hepatitis B (CHB) in the USA, including adherence to guidelines for CHB management. In this study, we describe patient characteristics and management patterns in a large cohort of adult patients with CHB, followed in an integrative care system. Understanding the population at risk, as well as current practice patterns, is critical to help inform interventions to improve chronic HBV care.
Methods
Study Design and Source Population
We performed a retrospective study of adult CHB patients followed in the Northern California Kaiser Permanente Medical Care Program (KPNC). KPNC is a comprehensive, integrated health care delivery system that serves over 3.2 million members in the San Francisco and Sacramento Greater Metropolitan areas. The membership includes over 25 % of the area’s total insured population and is representative except for persons with extremes in income [13, 14]. During the study period, the age-adjusted prevalence of CHB among adult health plan members was 0.6 %.
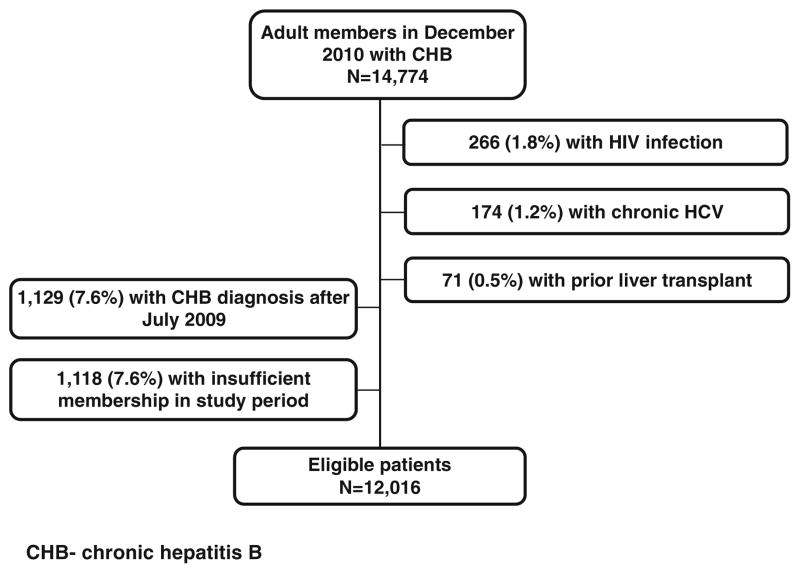
The study cohort was identified from a base population of 14,774 health plan members with known chronic hepatitis B (CHB) in December 2010, which reflected the most recent and complete cross-sectional data at the time of study. CHB was defined by laboratory confirmation (positive HBV surface antigen), history of treatment for chronic HBV, and/or a diagnosis code for CHB. Eligible adult patients had health plan membership for ≥15 months from July 2009 to December 2010. Patients with known human immunodeficiency virus (HIV), hepatitis C virus (HCV), or history of liver transplant were excluded. We excluded patients whose CHB confirmation was documented after the start of the study window in July 2009. In a subset analysis, we describe treatment patterns of the 599 patients who were newly initiated on oral HBV therapy during the study period 1/1/2009–12/31/2010. This electronic-records-based study protocol was approved by the Institutional Review Board of the Kaiser Foundation Research Institute in March 2011, with subsequent yearly re-approval. All data were extracted from the KPNC electronic records, which included administrative, clinical, and research databases. Complete information was available for years 1995–2010. All laboratory testing was conducted through The Permanente Medical Group Regional Laboratory.
Baseline Characteristics
Baseline patient characteristics included demographics, comorbid conditions, and liver-related characteristics. Race/ethnicity was defined primarily by self-report in the medical record. History of obesity [body mass index (BMI) >30) [15] and alcohol abuse was defined by the presence of corresponding diagnosis during the study period. Diabetes was defined as any prior diagnosis noted in the Kaiser Permanente Diabetes Registry [16]. Incident HCC diagnosis was obtained from the KPNC Cancer Registry. Cirrhosis was defined by a clinical diagnosis and/or pathologic confirmation.
General Care Measures
For patients with CHB, the AASLD recommends the following baseline evaluation: (1) tests to assess the state of liver disease including complete blood count, platelet count, hepatic panel, and INR, (2) tests to assess for viral replication, including HBeAg, anti-HBe, and HBV DNA level, and (3) tests to rule out viral coinfections, including anti-HCV, anti-HDV, anti-HAV, and anti-HIV [7]. We determined the proportion of patients with record of ever receiving such tests during KPNC membership from 1995 to 2010. Assessment of hepatitis A immune status was defined by the record of anti-HAV testing and/or vaccination.
Recent HBV-Related Monitoring
For patients with CHB, the AASLD recommends monitoring HBV disease activity with ALT and HBV DNA testing. At the time of the study, hepatocellular carcinoma surveillance by abdominal imaging and alpha fetaprotein (AFP) was recommended every 6–12 months in appropriate surveillance candidates [7, 17]. We assessed “recent” HCC surveillance and laboratory monitoring of chronic hepatitis B activity with outpatient ALT and HBV DNA levels during an 18-month study window (July 2009–December 2010). As most guideline-based testing is recommended at 6–12-month intervals, an 18-month window was chosen to allow for flexibility within a clinically reasonable period. HBV DNA qualitative levels were sensitive to 60 copies/ml, and quantitative levels were performed using COBAS AmpliPrep-TaqMan PCR (Roche Diagnostics). We report HCC surveillance using AFP alone, abdominal imaging alone, or both modalities among CHB patients eligible for surveillance in KPNC. Eligibility was based on AASLD guidelines and included patients (regardless of race) with cirrhosis, women >50 years old, men>40 years old, and patients with a clinical diagnosis of alcohol abuse [18, 19].
Provider Visits
We report the proportion of patients that attended at least one liver-related clinic visit within the study window. Liver-related visits were defined as any visit with at least one diagnosis that included the words “hepatitis,” “liver,” or any known complications of liver disease. This also captured visits for which CHB was included among secondary visit diagnoses. We compared the performance of HCC surveillance, as well as of ALT and HBV DNA testing, between patients with at least one liver-related visit with a specialist and those with a liver-related visit with a PCP only. Specialists were defined as infectious disease providers as well as gastroenterologists. We further described the characteristics of patients with specialist follow-up.
HBV Treatment Patterns
Patients ever treated for HBV were defined as those dispensed HBV antiviral medication covering at least 28 continuous days, dating from 1995 to 2010. This definition also applied to patients on recent antiviral therapy during the study window. Based on most recent HBeAg testing, as well as ALT and HBV DNA levels reported during the study window, we determined the proportion of untreated patients with guideline-based indications for the treatment (defined below).
We identified patients who were newly initiated on oral HBV therapy during the study period. They were dispensed oral HBV antiviral medication covering at least 28 continuous days between 1/1/2009 and 12/31/2010, and no HBV medication within 365 days prior to treatment initiation. For patients with more than one eligible treatment course, the first course in the study window was investigated. We evaluated on-treatment laboratory monitoring including ALT and HBV DNA testing at treatment months 3–6 (10–26 weeks) among patients treated for at least 6 months. HBeAg testing was determined at months 6–12 (8–57 weeks) among HBeAg-positive patients treated for at least 1 year.
Guideline-Based Treatment Criteria
Among patients not on recent treatment, we evaluated the proportion with guideline-based indications for therapy [7, 12]. This was defined as having ALT >1× ULN and HBV DNA>20,000 IU/ml, and/or having HCC or cirrhosis with detectable HBV DNA. ALT and HBV DNA levels were based on the highest reported values within the 18-month study period. For all analyses, ALT levels reflect standardized levels using the upper limit of normal (ULN) of 19 U/L for women and 30 U/L for men [20].
To evaluate eligibility of patients newly initiated on HBV treatment, we also identified the proportion of HBeAg-negative patients that met broader treatment criteria using an HBV DNA threshold of 2,000 rather than 20,000 IU/ml. Broader treatment criteria were assessed due to considerable variation in the published treatment guidelines on viral load thresholds for HBeAg-negative patients [7, 10–12]. For this analysis, laboratory thresholds for patients with HBeAg-positive disease were applied to patients without documented HBeAg status to ensure laboratory indications for treatment regardless of HBeAg status. Patients without laboratory indications for the treatment, but with stage 2–3 fibrosis or grade 2–4 necroinflammation on prior liver biopsy, were also considered treatment eligible (<1 % of patients evaluated), as were patients with HCC or cirrhosis with detectable HBV DNA. ALT and HBV DNA levels were based on the highest reported values within 1 year prior to treatment initiation, and HBeAg status was based on most recent available results within 2 years prior to treatment initiation.
Statistical Analyses/Laboratory Testing
Descriptive statistics included means, medians, and proportions. Bivariate analyses of dichotomous outcomes were performed using chi-square tests, employing exact methods as needed. Analyses were conducted with Stata software, version 12.0 (College Station, TX) or SAS, version 9.1.3.
Results
Baseline Characteristics
From the initial base cohort of 14,774 patients, 12,016 met final inclusion criteria (Fig. 1). Most (76 %) had strict laboratory evidence of chronicity (positive HBV DNA and/or HBsAg persisting for over 6 months), while the remainder had a positive HBsAg with no subsequent laboratory results inconsistent with HBV chronicity. The mean age was 49 years (±13.0), half of the patients were men (51 %), and most were Asian (83 %). Over 75 % were health plan members for at least 60 months (Table 1). Less than 9 % carried a recent diagnosis of CHB from less than 12 months prior to the start of the study period. Though alcohol abuse was infrequently diagnosed (<1 %), this is likely an underestimation, as standardized alcohol assessments were not conducted uniformly during the study period. Approximately 11 % of patients had diabetes, and 12 % were obese.
Fig. 1.
Selection of study cohort
Table 1.
Demographics of CHB study cohort (n = 12,016)
| n | % | |
|---|---|---|
| Agea | ||
| 18–35 | 1,556 | 12.9 |
| 35–49 | 4,723 | 39.3 |
| 50–65 | 4,490 | 37.4 |
| >65 | 1,247 | 10.4 |
| Men | 6,070 | 50.5 |
| Race/ethnicity | ||
| Asianb | 9,945 | 82.8 |
| Chinese | 4,935 | 41.1 |
| Vietnamese | 1,570 | 13.1 |
| Filipino | 1,626 | 13.5 |
| Korean | 190 | 1.6 |
| Hmong | 170 | 1.4 |
| Japanese | 63 | 0.5 |
| Other/Unknown | 1,391 | 11.6 |
| Black | 607 | 5.1 |
| White | 716 | 6.0 |
| Hispanic | 221 | 1.8 |
| Native American | 16 | 0.1 |
| Unknown | 511 | 4.3 |
| Primary language English | 8,894 | 74.0 |
| Health plan membership (months) | ||
| 12–59 | 1,606 | 13.4 |
| 60–119 | 3,389 | 28.2 |
| 120+ | 7,021 | 58.4 |
CHB denotes chronic hepatitis B
Age as of December 31, 2010
Includes Pacific Islanders
Most (70 %) patients were HBeAg negative, and less than 2 % of patients had a diagnosis of cirrhosis (Table 2). Among patients with ALT testing (77 %), the most recent level was within normal range among 58 % using standardized ALT (sALT), compared with 84 % using laboratory-based ALT thresholds (not shown). The most recent sALT level was within normal range in 58 % of patients not on the treatment, and similarly, 57 % of those on the treatment. As shown in Table 2, 61 % of patients had no recent HBV DNA testing, which reflects 69 % of patients not on the treatment, and only 9 % of patients on HBV therapy during the study window.
Table 2.
Clinical characteristics of cohort (n = 12,016)
| n | % | |
|---|---|---|
| General characteristics | ||
| Body mass index (BMI) | ||
| <25 | 5,723 | 47.6 |
| ≥25 to <30 | 3,804 | 31.7 |
| ≥30 | 1,468 | 12.2 |
| Missing | 1,021 | 8.5 |
| Alcohol abuse diagnosed | 76 | 0.6 |
| Diabetes | 1,327 | 11.0 |
| Liver-specific characteristics | ||
| Months since first CHB diagnosisa | ||
| <12 | 1,040 | 8.6 |
| 12–59 | 3,686 | 30.7 |
| 60–119 | 4,287 | 35.7 |
| 120+ | 3,003 | 25.0 |
| Most recent ALT levelsb, c | ||
| Normal | 5,337 | 44.4 |
| >1–2× ULN | 3,154 | 26.3 |
| >2× ULN | 758 | 6.3 |
| Not tested | 2,767 | 23.0 |
| Most recent viral load (IU/ml)b | ||
| <2 k | 3,316 | 27.6 |
| 2–20 k | 711 | 5.9 |
| >20 k | 706 | 5.9 |
| Not tested | 7,283 | 60.6 |
| Most recent HBeAg statusc | ||
| Positive | 1,157 | 9.6 |
| Negative | 8,437 | 70.2 |
| Not tested | 2,422 | 20.2 |
| Cirrhosisb | 195 | 1.6 |
| Incident HCCb | 28 | 0.2 |
CHB denotes chronic hepatitis B. Data reflect 10,367 individuals on treatment and 1,649 not on treatment during the 18-month study window
As of July 1, 2009
Documented during 18-month study window
Standardized upper limit of normal (ULN) ALT of 19 for women and 30 for men
Most recent since 1995
Care Measures
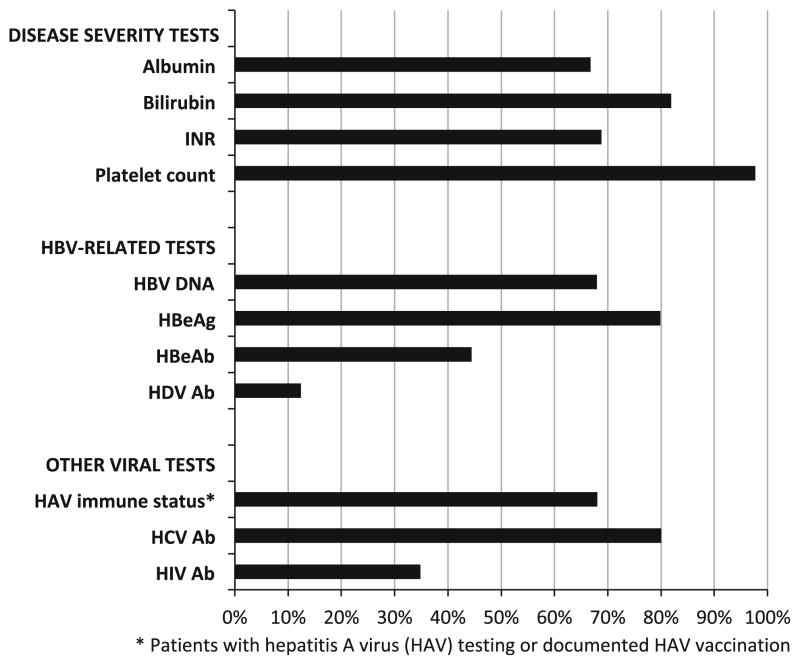
In this cohort, the majority had ever been tested for albumin, bilirubin, and INR, and almost all had ever received a platelet count (Fig. 2). Approximately 80 % had at least one assessment of HBeAg status, as well as HCV antibody testing. Hepatitis A immune status was documented in 68 %, while testing for HIV (35 %) and hepatitis D (12 %) was less frequent. Not surprisingly, 98 % of patients had ever received ALT testing. Just 8 % of patients had ever received a liver biopsy.
Fig. 2.
Percent of cohort ever receiving recommended tests (n = 12,016)
Recent Monitoring of HBV Disease Activity
As shown in Table 3, less than 14 % of patients had a liver-related visit with a specialist; 37 % had such visit with a PCP alone, and almost half had no recent liver-related visit. Patients seen for specialist follow-up in the study period were similar demographically to the remaining cohort, but more likely to have ever received HBV treatment (55 vs. 11 %, p <0.001), and more likely to have a diagnosis of cirrhosis (7 vs. 0.7 %, p <0.001).
Table 3.
Recommended care during 18-month period by provider type (n = 12,016)
| Overall | Patients with liver-related visit (51 %)
|
Patients without liver-related visit | ||||||
|---|---|---|---|---|---|---|---|---|
| Specialist | PCP only | |||||||
|
|
|
|
|
|||||
| (n = 12,016) | (n = 1,676) | (n = 4,494) | (n = 5,846) | |||||
|
|
|
|
|
|||||
| n | % | n | % | n | % | n | % | |
| HBV disease monitoring | ||||||||
| ALT only | 4,595 | 38.2 | 158 | 9.4 | 2,090 | 46.5 | 2,347 | 40.1 |
| HBV DNA only | 79 | 0.7 | 8 | 0.5 | 47 | 1.0 | 24 | 0.4 |
| Both ALT and HBV DNA | 4,654 | 38.7 | 1,501 | 89.6 | 2,093 | 46.6 | 1,060 | 18.1 |
| (n = 7,256)
|
(n = 1,098)
|
(n = 2,934)
|
(n = 3,224)
|
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| HCC surveillance | ||||||||
| Imaging and AFP | 3,537 | 48.8 | 953 | 86.8 | 1,901 | 64.8 | 683 | 21.2 |
| Imaging alone | 541 | 7.5 | 57 | 5.2 | 240 | 8.2 | 244 | 7.6 |
| AFP alone | 686 | 9.5 | 58 | 5.3 | 383 | 13.1 | 245 | 7.6 |
| None | 2,492 | 34.3 | 30 | 2.7 | 410 | 14.0 | 2,052 | 63.7 |
Among surveillance candidates defined by AASLD-based criteria
Overall, ALT and HBV DNA levels were not frequently performed (<40 %). Among the 51 % of patients with a recent liver-related visit, testing for both was more common if seen by a specialist compared to PCP alone (90 vs. 47 %, p <0.001). Few patients (1 %) had received a recent liver biopsy.
Among HCC surveillance candidates (n = 7,256), 56 % had received abdominal imaging within 18 months; this was more frequent in those seen for liver disease by specialists compared to PCPs alone (92 vs. 73 %, p <0.001). Almost half (49 %) had received dual screening modalities (AFP testing and imaging). AFP testing alone was performed in 686 (10 %) of HCC surveillance candidates. Patients seen by PCPs were less likely to receive both modalities than those seen by specialists and more likely to receive AFP alone (65 vs. 87 % and 13 vs. 5 %, respectively, p values <0.001).
HBV Treatment
Overall, 14 % of the cohort had been recently treated (during the study window), and 17 % had ever received HBV therapy. Among all patients without recent treatment, 5 % had evidence of guideline-based treatment indications. However, just 30 % (n = 3,161) of untreated patients had recent testing of both HBV DNA and ALT levels to assess for treatment eligibility, and 17 % of those patients had indications for the treatment.
To describe current treatment patterns, we identified the 599 patients that were newly started on oral HBV medications during the study window. Characteristics of the new treatment initiators were similar to those of the overall study cohort, although a somewhat larger proportion was male (61 %). The majority (85 %) of patients were being treated by specialists (78 % gastroenterologists and 7 % infectious disease physicians). Most (71 %) met stringent treatment criteria, and 76 % met broader criteria (HBV DNA threshold of 2,000 IU for HBeAg-negative patients). Fulfillment of broader criteria was more common in patients treated by specialists than PCPs (85 vs. 40 %, p <0.001). Entecavir (71 %) was the most commonly prescribed medication followed by tenofovir (15 %), lamivudine (9 %), and adefovir (4 %). Of note, entecavir was on the health plan formulary for HBV treatment several years prior to the study period, with tenofovir added in early 2009. Both GI/ID specialists and PCPs most commonly prescribed entecavir (75 and 49 % of patients, respectively). However, the second most commonly prescribed medication among PCPs was lamivudine (37 %) versus tenofovir among GI/ID specialists (17 %).
Among the 68 HBeAg-positive patients with at least 1 year of treatment, 44 % received follow-up HBeAg testing. Among patients treated for at least 6 months (n = 355), HBV DNA testing was more common in those treated by specialists compared to PCPs alone (70 vs. 48 %, p = 0.003). ALT testing did not differ by provider type (79 vs. 80 %, p = 0.9).
Discussion
In this large US-based integrated care cohort, we describe patient characteristics and management of chronic hepatitis B (CHB). Among patients with available laboratory data, adherence to guideline-based treatment recommendations was common. However, ALT and HBV DNA testing in these chronically infected patients was not frequently performed. Just over half of HCC surveillance candidates received appropriate liver imaging. Laboratory monitoring as well as HCC surveillance was more commonly performed among patients with a liver-related visit with a specialist, as compared to a liver-related visit with a primary care provider only.
In non-cirrhotic patients, guidelines recommend HBV treatment based on both ALT and HBV DNA levels [7, 10]. Accordingly, elevated HBV DNA in the absence of elevated ALT may prompt further evaluation by liver biopsy, but not necessarily treatment initiation. In the current study, we found that most CHB patients received ALT measurement, but were less likely to have both ALT and HBV DNA tested within a recent 18-month period. This raises the question whether a cost-effective approach for monitoring disease activity could be based on reflexive HBV DNA testing prompted by an elevated ALT result. This is supported by our findings in which <5 % of untreated patients with dual testing had elevated HBV DNA with an otherwise normal ALT (not shown).
Most patients that were newly initiated on oral HBV medication met guideline-based treatment criteria. Among untreated patients with available laboratory testing, we found few patients with clear indications for therapy (5 %). These findings contrast with the recent publication by Zhang et al. [21] in which antiviral therapy was initiated in only 50 % of treatment eligible Asian Americans followed in a community setting. These differences may reflect HBV treatment patterns outside of an integrative care setting. However, given that just 30 % of untreated patients in the current study had received both ALT and HBV DNA testing, we have likely underestimated the true proportion of untreated patients that warranted hepatitis B treatment by current published guidelines. Perhaps closer to 15 % of untreated cohort members may have been eligible for the treatment. While possible that patients had ALT and/or HBV DNA testing outside of the Kaiser Permanente system, this is unlikely a large factor in this cohort of mostly long-standing health plan members. The implications of the study findings include potential delay in timely treatment of CHB, which has been shown to decrease the risk of progressive fibrosis and the development of hepatocellular carcinoma [22, 23].
Current AASLD guidelines recommend abdominal imaging every 6 months, without requiring AFP, in those patients at highest risk for developing HCC [24]. At the time of the current study, the recommendations included HCC surveillance by abdominal imaging every 6–12 months with concurrent AFP [17]. Imaging for HCC occurred within a recent 18-month period in only 56 % of patients eligible for surveillance within KPNC. Most people that had imaging also received AFP testing. AFP alone is not advised [7], nor FDA approved, for HCC surveillance. However, we found that isolated AFP was performed in 10 % of patients that warranted surveillance and was most common among patients seen by PCPs alone. Although AFPL3 and DCP are FDA-approved for HCC surveillance, these serum tests are currently not widely used in clinical practice and rarely measured in our study population.
HCC surveillance and laboratory testing were more frequent in patients with recent liver-related visit(s). Among those with at least one liver-related visit, both HCC surveillance and laboratory testing were more common in patients seen by a specialist compared to PCP alone. Though some patients may have been newly diagnosed with CHB (requiring time to gather data on treatment eligibility or specialist referral), most patients (91 %) carried a diagnosis of CHB for more than a year. Furthermore, over 60 % of the cohort had record of CHB for at least 5 years prior to this study, allowing for adequate follow-up time to present for recommended testing and referral. In this integrated care setting, we did not assess the type of provider ordering tests, as we were interested in contrasting the care of patients with and without current specialty care for CHB. Interestingly, a recent publication on community-based CHB management among primarily Asian Americans noted similar trends among ordering providers, with ALT and HBV DNA levels more likely to have been ordered by specialists as compared to PCPs [25]. Treatment patterns were also markedly different by provider type, with a high rate of lamivudine use among patients seen by PCPs alone. Given that lamivudine is not standard of care for CHB management, it would be interesting to further investigate these prescription patterns by surveying providers to determine whether lamivudine use stems from presumed virologic control and/or provider-based knowledge barriers regarding optimal CHB treatment.
A recent AASLD report describing barriers to hepatitis management cites inadequate numbers of providers trained to treat infected patients, as well as low referral rates for chronic HBV management [26]. Although CHB management entails several straightforward laboratory and imaging tests, a recent survey of primary care providers caring for a predominately Asian American population found that only 43 % were familiar with guidelines on the management of HBV [27]. These findings highlight the role for targeted education among PCPs managing chronic HBV. Early identification of patients that warrant specialist referral may also help to improve current gaps in care.
Several well-established CHB cohorts have described CHB disease progression and HBV-related outcomes. The HBV REVEAL study, a cohort study based in Taiwan dating to 1991, includes 4155 HBsAg-seropositive patients. Since its inception, the study has contributed extensive findings on the natural history of CHB in Asians [3, 23]. In the USA, the Chronic Hepatitis Cohort Study (CHeCS) includes several sites across the USA, with 2,202 CHB patients covered by a variety of health plans. CHeCS has provided important estimates of the burden of CHB and its related mortality [28]. Unlike CHeCS, the current study cohort is primarily Asian American, and one quarter of patients’ primary language was not English. Our findings from a Northern California population contribute to existing cohort data by reflecting practice patterns among foreign-born patients and their families, and may be helpful in addressing health care disparities, as foreign-born individuals remain at disproportionately high risk for complications related to CHB [5, 6, 29–31]. Additionally, our findings provide prevalence estimates for comorbid conditions that may influence liver disease progression such as obesity and diabetes in this primarily Asian study population. Our results also suggest that more uniform assessments of alcohol use may be due in this population, given the very low number with documented alcohol abuse.
A recent study among Asian Americans in Los Angeles County identified lack of insurance as the most common reason that patients newly diagnosed with CHB did not return for follow-up care [32]. Results of the current study may not generalize completely to uninsured patients or to those with other insurance plans. However, the uniformity of health plan coverage in our study cohort ensures that findings about health care delivery are not attributable to barriers in access to care. Previous data suggest that the performance of HCC surveillance is higher among providers that care for a large population of Asian Americans [33]. The observed need for improved HCC surveillance in the current, predominately Asian American cohort, likely reflects a greater need for improved CHB care in the community, which may extend more broadly to patients with chronic infection.
This study is inherently limited by a retrospective design, though it represents the largest investigation patterns of CHB management in a US community setting to date. Laboratory studies such as ALT, INR, CBC, and albumin levels, as well as abdominal imaging, may have been performed for indications other than hepatitis B. Nonetheless, these findings reflect “real-world” clinical practice patterns. An additional strength is the integrated care setting, with electronic medical records that can be accessed by all healthcare providers, with comprehensive documentation of clinic visits, pharmacy records, and radiologic and laboratory data. It is also likely that increased uptake of guideline-based management may develop over time, and the cross-sectional analysis of “recent” adherence to guidelines was not designed to capture such trends. Nonetheless, these results provide important insight into practice patterns in a large community cohort of CHB patients.
To improve care for Northern California Kaiser Permanente patients with chronic hepatitis B infection, a dedicated HBV Liver Care Program was instituted in May 2012. Informed by findings from the cohort described here, and on patient profiles maintained by the Viral Hepatitis Registry, the automated system of recall assures that appropriate individuals are contacted every 6 months for laboratory monitoring of disease activity and for HCC surveillance. The program includes specially trained nurse practitioners and medical assistants who order tests, report results to patients, and institute and monitor HBV treatment when indicated. Directed centrally by an expert hepatologist (JR), and managed by a regional Quality and Operations Support team, this system provides standardized, specialty level care efficiently to all patients with chronic hepatitis B. Future studies will investigate the impact of the program on quality of care measures, and on rates of cirrhosis and HCC in this population.
In summary, we describe patient characteristics, laboratory assessments, treatment practice, and HCC surveillance patterns in a large US-based CHB cohort. Adherence to guideline-based treatment patterns was common, although a large proportion of patients lacked recommended laboratory testing to assess for treatment eligibility. While the indicated HCC surveillance occurred in about half of patients, both HCC surveillance and laboratory monitoring of HBV disease activity were more common among patients seen by a specialist. These data reveal important practice patterns in the community management of CHB and suggest that viral monitoring and cancer surveillance are important targets for improving the scope of CHB care.
Acknowledgments
This research was funded by Gilead Sciences, Inc. [awarded to MM]. Additional support was received through the Sylvia Allison Kaplan Research Award [MS].
Abbreviations
- CHB
Chronic hepatitis B
- HBV
Hepatitis B virus
- AASLD
The American Association for the Study of Liver Diseases
- GI
Gastroenterologist
- PCP
Primary care provider
- ID
Infectious disease
- HBeAg
Hepatitis B e antigen
- anti-HBe
Hepatitis e antibody
- HCC
Hepatocellular carcinoma
- HIV
Human immunodeficiency virus
- HCV
Hepatitis C virus
- sALT
Standardized ALT
- ULN
Upper limit of normal
- HBsAg
Hepatitis B surface antigen
Footnotes
Conflict of interest Funding by Gilead Sciences, Inc. was awarded to Michele Manos. Norah Terrault has received grant support from Gilead Sciences, Inc., and served as a consultant for Bristol-Myers Squibb and Gilead Sciences, Inc.
Contributor Information
Monika Sarkar, Email: monika.sarkar@ucsf.edu, Viral Hepatitis Registry, Kaiser Permanente Division of Research, Oakland, CA, USA. Division of Gastroenterology and Hepatology, Department of Medicine, University of California, San Francisco, 513 Parnassus Avenue, Room S-357, San Francisco, CA 94143-0358, USA.
Valentina A. Shvachko, Viral Hepatitis Registry, Kaiser Permanente Division of Research, Oakland, CA, USA
Joanna B. Ready, Viral Hepatitis Registry, Kaiser Permanente Division of Research, Oakland, CA, USA
Mary Pat Pauly, Viral Hepatitis Registry, Kaiser Permanente Division of Research, Oakland, CA, USA.
Norah A. Terrault, Division of Gastroenterology and Hepatology, Department of Medicine, University of California, San Francisco, 513 Parnassus Avenue, Room S-357, San Francisco, CA 94143-0358, USA
Marion G. Peters, Division of Gastroenterology and Hepatology, Department of Medicine, University of California, San Francisco, 513 Parnassus Avenue, Room S-357, San Francisco, CA 94143-0358, USA
M. Michele Manos, Viral Hepatitis Registry, Kaiser Permanente Division of Research, Oakland, CA, USA.
References
- 1.Gish RG, Gadano AC. Chronic hepatitis B: current epidemiology in the Americas and implications for management. J Viral Hepat. 2006;13:787–798. doi: 10.1111/j.1365-2893.2006.00787.x. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 3.Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: what exactly has REVEAL revealed? Liver Int. 2012;32:1333–1341. doi: 10.1111/j.1478-3231.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49:S28–S34. doi: 10.1002/hep.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–433. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 6.Chang ET, Keegan TH, Gomez SL, et al. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109:2100–2108. doi: 10.1002/cncr.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 8.Manos MM, Leyden WA, Murphy RC, et al. Limitations of conventionally derived chronic liver disease mortality rates: results of a comprehensive assessment. Hepatology. 2008;47:1150–1157. doi: 10.1002/hep.22181. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 10.European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: statistics from the 2007 California Health Interview Survey. Kaiser Permanente Division of Research; 2012. [Google Scholar]
- 15.Global Database on Body Mass Index. Vol. 2013. World Health Organization; http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. [Google Scholar]
- 16.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2013;36:574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruix JSM. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911–915. doi: 10.1016/j.gcb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Manos MM, Zhao WK, Shvachko VA, Quesenberry CP. Correlates of severe liver disease outcomes among chronic hepatitis B patients: a 9-year longitudinal study in a managed care setting. Hepatology. 2010;52:410A–411A. [Google Scholar]
- 20.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Ristau JT, Trinh HN, et al. Undertreatment of Asian chronic hepatitis B patients on the basis of standard guidelines: a community-based study. Dig Dis Sci. 2012;57:1373–1383. doi: 10.1007/s10620-012-2137-0. [DOI] [PubMed] [Google Scholar]
- 22.Sung JJ, Tsoi KK, Wong VW, et al. Meta-analysis: treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628–638. doi: 10.1111/j.1440-1746.2011.06695.x. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku KC, Li J, Ha NB, et al. Chronic hepatitis B management based on standard guidelines in community primary care and specialty clinics. Dig Dis Sci. 2013;58:3626–3633. doi: 10.1007/s10620-013-2889-1. [DOI] [PubMed] [Google Scholar]
- 26.Ward JW, Lok AS, Thomas DL, et al. Report on a single-topic conference on “Chronic viral hepatitis–strategies to improve effectiveness of screening and treatment”. Hepatology. 2012;55:307–315. doi: 10.1002/hep.24797. [DOI] [PubMed] [Google Scholar]
- 27.Burman BE, Mukhtar NA, Toy BC, et al. Hepatitis B management in vulnerable populations: gaps in disease monitoring and opportunities for improved care. Dig Dis Sci. 2014;59:46–56. doi: 10.1007/s10620-013-2870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56:40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 29.Cohen C, Caballero J, Martin M, et al. Eradication of hepatitis B: a nationwide community coalition approach to improving vaccination, screening, and linkage to care. J Community Health. 2013;38:799–804. doi: 10.1007/s10900-013-9699-4. [DOI] [PubMed] [Google Scholar]
- 30.Tong MJ, Pan CQ, Hann HW, et al. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. 2011;56:3143–3162. doi: 10.1007/s10620-011-1841-5. [DOI] [PubMed] [Google Scholar]
- 31.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 32.Xu JJ, Tien C, Chang M, et al. Demographic and serological characteristics of Asian Americans with hepatitis B infection diagnosed at community screenings. J Viral Hepat. 2013;20:575–581. doi: 10.1111/jvh.12073. [DOI] [PubMed] [Google Scholar]
- 33.Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56:1516–1523. doi: 10.1007/s10620-010-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]