Abstract
Published data provide strong evidence that heparin treatment of proliferating vascular smooth muscle cells results in decreased signaling through the ERK pathway and decreases in cell proliferation. In addition, these changes have been shown to be mimicked by antibodies that block heparin binding to the cell surface. Here we provide evidence that the activity of protein kinase G is required for these heparin effects. Specifically, a chemical inhibitor of protein kinase G, Rp-8-pCPT-cGMS, eliminates heparin and anti-heparin receptor antibody effects on bromodeoxyuridine incorporation into growth factor stimulated cells. In addition, protein kinase G inhibitors decrease heparin effects on ERK activity, phosphorylation of the transcription factor ELK-1, and heparin induced MKP-1 synthesis. Although transient, the levels of cGMP increase in heparin treated cells. Finally, knock down of protein kinase G also significantly decreases heparin effects in growth factor activated vascular smooth muscle cells. Together, these data indicate that heparin effects on vascular smooth muscle cell proliferation depend, at least in part, on signaling through protein kinase G.
INTRODUCTION
Following injury, migration of vascular smooth muscle cells (VSMCs) from the tunica intima into the vessel lumen and subsequent hyperplasia, are key events in the development of atherosclerosis. Molecules that can decrease VSMC proliferation have been examined for possible treatments to slow disease advancement. The discovery that heparin suppresses VSMC growth was reported more than 30 years ago (Clowes and Karnovsky, 1977); yet the mechanism by which heparin treatment of VSMCs inhibits their proliferation remains unclear. Heparin blocks PKC-dependent c-fos induction and activation of ERK, a MAPK activated in response to numerous treatments of sub-cultured VSMCs (Castellot et al., 1989; Ottlinger et al., 1993). In addition, heparin treatment results in decreases in cyclin dependent kinase 2 activity by increasing levels of p27kip1 (Fasciano et al., 2005). However, sequestration of growth factors is not likely to explain all of the effects of heparin on VSMCs (Blaukovitch et al., 2010; Pukac et al., 1997; Reilly et al., 1989; Savage et al., 2001).
VSMCs specifically bind and endocytose heparin (Castellot et al., 1985). This specific binding activity, in combination with heparin’s effects on cell signaling pathways, supports a model whereby heparin binds to cell surface proteins and initiates its own signaling pathways. To identify putative heparin receptor proteins, Patton et al. (1995) produced monoclonal antibodies that specifically inhibit heparin binding to cells in vitro. These antibodies identified a single heparin-binding cell surface protein and act as agonists of heparin, mimicking both heparin’s effects on cell signaling and its anti-proliferative effects in cultured VSMCs (Blaukovitch et al., 2010; Savage et al., 2001).
MAPK activity is regulated by the reversible phosphorylation of specific tyrosine and threonine residues, and active ERK accumulation in the nucleus is critical in cell cycle progression through G1 (Brunet et al., 1999) where the sustained ERK activity results in ELK-1 phosphorylation (Shin et al., 2003). Dual-specificity protein phosphatases play important roles in ERK inactivation, and MAPK Phosphatase-1 (MKP-1) localizes to the nucleus (Rohan et al., 1993) where it is able to regulate ERK signaling in VSMCs. Loss of active ERK in the nucleus results in decreased Elk-1 activity (Shin et al., 2003). Our laboratory has demonstrated that heparin and the anti-heparin receptor antibodies increase MKP-1 levels in VSMCs, playing an important role in heparin-induced ERK activity decreases (Blaukovitch et al., 2010). However, the signaling intermediates between heparin’s interaction with its receptor and induction of MKP-1 expression remain unknown. One possibility is suggested by studies of insulin signaling (Begum et al., 1998; Jacob et al., 2002). These studies report the expression of MKP-1 in VSMCs in response to insulin and insulin-related growth factor (IGF) where insulin and IGF induce the expression of inducible NOS (iNOS), eventually increasing the levels of cGMP in response to the NO activation of soluble guanylyl cyclase. The increase in cGMP levels was shown to be sufficient to induce MKP-1 expression and attenuate ERK activity. Similarly, leptin treatment induces decreased VSMC proliferation, and this depends on iNOS induction (Rodríguez et al., 2010). As well as in heparin treated cells, p27kip is induced in response to cGMP rises and cGMP-dependent protein kinase (PKG) activity in VSMCs (Sato et al., 2000).
Another agent that elevates cGMP in VSMCs is atrial natriuretic factor or peptide (ANF or ANP). Upon ligand binding, the ANP receptor undergoes a conformational change that activates an intracellular guanylate cyclase domain thereby elevating levels of cGMP. Although better known for their role in vasorelaxation, both ANP and cGMP have been shown to decrease the proliferation of VSMCs (Baldini et al., 2002; Tantini et al., 2005). These effects on VSMCs imply that cGMP mediates a coordinated control of acute cellular function. Elevations in cGMP (by cGMP analogues, activators of guanylate cyclase, or ANP) induce MKP-1 expression in smooth muscle, mesangial and endothelial cells by way of PKG (Baldini et al., 2002; Furst et al., 2005; Jacob et al., 2002; Sugimoto et al., 1996). This increase in MKP-1 expression results in a decrease of ERK activation, and thus provides a mechanism for the anti-proliferative activity of ANP in VSMC and mesangial cells (Baldini et al., 2002; Sugimoto et al., 1996; Tantini et al., 2005). As with heparin, ANP treatment induces increases in p27kip1 levels (Tete et al., 2001).
Heparin and cGMP affect VSMCs similarly. First, both inhibit growth of VSMCs late in the G1 phase of the cell cycle. Second, the proximity of the endothelium to VSMCs in vivo provides a source for both endogenous heparin and cGMP-elevating agents such as NO. Endogenous heparin from endothelial cells could maintain quiescence in VSMCs (Castellot et al., 1981). Third, in reducing VSMC growth, both cGMP and heparin cause an inactivation of ERK due, at least in part, to the induction of MKP-1 (Baldini et al., 2002; Blaukovitch et al., 2010). Because of the similarities in the way that heparin, ANP, and NO-induced cGMP increases affect VSMCs, we hypothesize that heparin’s cellular effects are mediated through the second messenger cGMP target, PKG. Consistent with this idea is evidence that reductions in cGMP signaling occur with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis (Melichar et al., 2004). Also consistent with this hypothesis is the fact that expression of constitutively active PKG inhibits VSMC proliferation in response to high glucose (Wang and Li, 2009). In the present report, we present evidence that PKG activity is indeed required for heparin-induced decreases in VSMC ERK activity, Elk-1 phosphorylation, and VSMC proliferation.
Materials & Methods
Materials
Cell culture chemicals, DMEM and MEM, 2.5% trypsin/EDTA, gelatin, heparin, penicillin/streptomycin, phorbal myristic acid (PMA), PDGF and glutamate were obtained from Sigma Chemical Co. (St. Louis, MO). Pre-tested FBS was obtained from Invitrogen (Gaithersburg, MD), Atlanta Biologicals (Atlanta, GA) or Biowest (St. Louis MO). Anti-active ERK (rabbit, against phosphorylated ERK, but called active ERK in the text to distinguish it from the mouse antibody) and anti-phospho Elk-1 (pElk) antibodies were from Cell Signaling (Beverly, MA). Anti-MKP-1 (V-15), anti-phospho ERK (pERK, mouse, employed when both pERK and pElk were detected using double-immunofluorescence) and anti-PKG (a mixture of antibodies against PKG Iα and Iβ was used) were from Santa Cruz Biotechnology (La Jolla, CA). siRNA (species specific) for PKG was also from Santa Cruz. Anti-smooth muscle myosin, and Extra-avidin-alkaline phosphatase™ were obtained from Sigma. Biotin-labeled and fluorescent-tagged secondary antibodies (in donkey or bovine, with minimal cross-reactivity) were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). 8-Br-cAMP, 8-Br-cGMP, the PKG inhibitor KT5823, 8-pCPT-cAMS and Rp-8-pCPT-cGMS and Mowiol were from Calbiochem (EMD, San Diego, CA). cGMP ELISA kits were from R & D Systems, Inc. (Minneapolis, MN) or Cayman Chemical (Ann Arbor, MI).
Cell Culture
A7r5 rat smooth muscle cells were obtained from ATCC (Rockville, MD). Porcine aortic smooth muscle cells were obtained from Clonetics, a division of BioWhitaker (Walkersville, MD) or Cell Applications, Inc. (San Diego, CA). Commercially available VSMCs were grown as recommended by the supplier and porcine cells were exchanged into MEM media over time before experiments. For some experiments, VSMCs were isolated from porcine aortas, characterized as smooth muscle, and cultured as described previously (Blaukovitch et al., 2010; Savage et al., 2001).
BrdU Incorporation Assays
The effects of heparin and other reagents on cell growth were examined by monitoring BrdU incorporation as described previously (Savage et al., 2001). Cells were routinely activated with 1.5 μg/ml PDGF after 48 h culture in media without serum. Prior to activation, indicated cells were treated for 10 min with 200 μg/ml heparin, 100 nM ANP, 100 μM 8-Br-cAMP, 100 μM 3-isobutyl-1-methylxanthine (IBMX), or 100 μM 8-Br-cGMP. cAMP-dependent protein kinase (PKA) or PKG inhibitors, Rp-8-pCPT-cAMS and Rp-8-pCPT-cGMS, were added at a concentration of 2 μM for 10 min before the addition of heparin or analogous treatments. After 24 to 48 h, the cells were processed, and cell staining was visualized with the addition of 0.66 mg/ml 3,3′-diaminibenzidine (DAB) in TBS with 0.1% Tween-20 (TBST) with 0.8 μl/ml hydrogen peroxide (Savage et al., 2001). Data collection and statistical analysis of these experiments were also carried out as described (Savage et al., 2001). Briefly, treatments were run in duplicate or triplicate within an experiment and tested in at least three independent experiments. Data collection was carried out by individuals blind to experimental treatments. For BrdU incorporation in cells treated with siRNA for knock down of PKG, the cell staining was visualized by Alexa 488 labeled secondary antibodies and intensity analysis using Image J. as described for immunofluorescence below. The relative effects of each treatment from each independent experiment were analyzed by unpaired t-tests as appropriate.
Western Blotting
For immunoblotting, cells starved as above were incubated with 200 μg/ml heparin, 100 nM ANP, 100 μM 8-Br-cAMP, 100 μM 8-Br-cGMP or anti-heparin receptor antibodies (at 0.8 μg/ml) for 10–20 min as noted, and then activated with PMA (at 50 ng/ml), 10% serum, or 1.5 μg/ml PDGF as noted. The PKG inhibitor, Rp-8-pCPT-cGMS, was added at a concentration of 2 μM for 10 min before the addition of heparin or analogous treatments. Cells were harvested and ERK activity or MKP-1 levels were determined as described previously (Blaukovitch et al., 2010; Savage et al., 2001).
Measurement of Intracellular cGMP Levels by ELISA
Porcine VSMCs or A7r5s were treated with heparin (200 μg/ml), anti-receptor antibodies (0.8 μg/ml) or ANP (100 nM) for the indicated time periods. Samples were extracted with ice-cold ethanol, and concentrated to volumes required according to the kits. The concentration of GMP present in each sample was measured according to the protocol of the kit used.
Indirect immunofluorescence analysis of Elk phosphorylation and ERK phosphorylation
A7r5 or porcine VSMC were cultured on gelatin-coated glass cover slips individual wells of a 6 well plate and starved as above. Starved cells were activated with 1.5 μg/ml PDGF at 37°C for 10 or 20 min and cells were fixed with 4% paraformaldehyde for 15 min, washed 3 times in PBS and permeabilized with 0.3% Triton X100 in PBS for 5 min. Some cell samples were treated with 200 μg/ml heparin 10 min prior to PDGF activation. Rp-8-pCPT-cGMS (2 μM final) or KT5823 (0.2 μM final) was added 10 min prior to heparin in cell samples so labeled. Fixed and permeabilized cells were treated with primary antibodies against pElk and pERK (1:100 in 1%BSA/PBS) overnight at 4°C. In some cases, cells were treated with heparin as above without PDGF and stained for MKP-1. After three washes with PBS, Tetramethyl Rhodamine Isothiocyanate (TRITC) -labeled anti-rabbit and FITC or Alexa 488-labeled anti-mouse secondary antibodies (1:200) were added and incubated for 1 hr at room temperature in the dark. Cover slips were washed 3 times in PBS, mounted with mowoil or Vectashield (Vector Laboratories, Burlingame, CA), observed using a Nikon eclipse TE 2000-U fluorescence microscope (Nikon, Tokyo Japan), and micrographs were captured with a SPOT RT KE camera. Fluorescent intensity in cell nuclei was determined using Image J.
PKG Knockdown
A7r5 cells were transfected using 20 μg/ml of siRNA in the Bio-Rad Gene Pulser X-Cell System (Hercules, CA). Briefly, 100 mm confluent plates of cells were trypsinized, rinsed with PBS, suspended in HEPES-buffered saline, electroporated with the preset HeLa protocol (modified to 170 volts), seeded onto glass cover slips in 6-well plates as described above, and fresh media was added after approximately 24 h, and monitored for PKG knockdown after various times by immunofluorescent staining for PKG. To evaluate effects of knockdown on heparin induced signaling, cells exposed to siRNA for approximately 72 hours total exposure were employed. PKG knock-down cells were treated with heparin at 200 μg/ml heparin for 20 min, followed by PDGF for 10, 15, or 20 min. Controls were left untreated, or treated with PDGF alone. Identical cells were electroporated with control siRNA and additional control cells were not electroporated, but were treated identically. PDGF was not included in cells where MKP-1 synthesis was monitored. Each cell sample was monitored to measure PKG knockdown by staining for PKG, and cells were also stained for pERK, pElk, or MKP-1 as above to determine heparin effects. PKG knockdown was also carried out on cells that were then analyzed for BrdU incorporation as above. As in the pERK, pElk and MKP-1 experiments, cells were not starved before treatments to maximize PKG knockdown. To determine statistics with knockdown experiments, cells where PKG was knocked down were evaluated. Cells in knockdown samples were not counted if bright PKG staining remained in the treated samples.
Results
Heparin, ANP & 8-Br-cGMP all block PDGF-stimulated DNA Synthesis
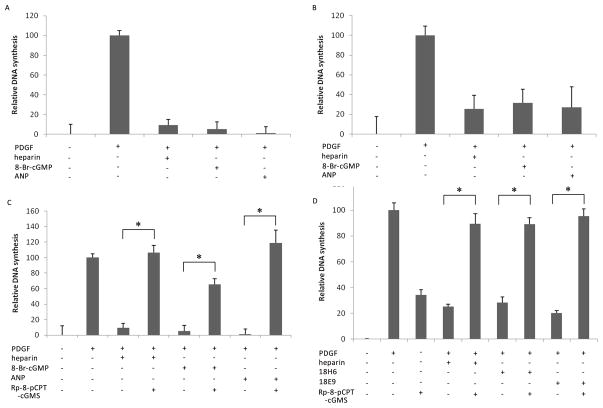
To test the hypothesis that heparin and ANP function similarly in VSMCs, the effects of heparin, ANP and 8-Br-cGMP on PDGF-stimulated BrdU incorporation were examined as a measure of DNA replication. Cells were serum-starved for 48 h and then grown in the presence of 20 μM BrdU for 4 h. Heparin, ANP, and 8-Br-cGMP were added for 10 min prior to stimulation of the cells with PDGF. Figure 1 (A&B) demonstrates that heparin, ANP, and 8-Br-cGMP added 10 min prior to PDGF all block the incorporation of BrdU during PDGF-stimulated DNA synthesis in both rat and porcine VSMCs. In rat A7r5 cells, all three treatments blocked 90% or more of the PDGF-stimulated BrdU incorporation (Figure 1A). In porcine VSMCs, these treatments blocked approximately 75% of the BrdU incorporation (Figure 1B).
Figure 1.
cGMP mimics heparin and PKG inhibition blocks heparin effects on VSMC DNA synthesis. A7r5 VSMC (A, C, D) and porcine VSMC (B) were grown to approximately 60% confluence and synchronized by starvation. Cells were incubated with 20 μM BrdU for at least four hours and inhibitors were added (200 μg/ml heparin [n = 5 in A, 6 in B], 100 μM 8-Br-cGMP [n = 4 in A, 3 in B], 100 nM ANP [n = 3 in A, 3 in B]. After 10 min, 1.5 μg/ml PDGF was added. After 48 h incubation, cells were fixed and the percentage of the total cells demonstrating BrdU incorporation was measured histochemically. The values reported reflect the average (± SE) relative BrdU incorporation from separate experiments compared to non-treated controls (0%) and PDGF treatments (100%). All three treatments in A and B blocked PDGF-stimulated DNA synthesis (p < 0.0001). For panel C, indicated samples were pretreated with 2 μM Rp-8-pCPT-cGMS for 20 min prior to the PDGF addition (n=3). Inhibition of PKG with Rp-8-pCPT-cGMS prevented the effects of anti-proliferative agents (heparin, 8-Br-cGMP & ANP) on PDGF-stimulated DNA synthesis (p < 0.0001, 0.0002, and 0.0028, respectively). For panel D, anti-heparin receptor antibodies (18H6 or 18E9, 0.8 μg/ml) were added to some cells, as noted. Rp-8-pCPT-cGMS was added, as described for panel C. The inhibitor blocked the anti-heparin receptor antibody effects on BrdU incorporation (p < 0.0001). Comparisons with significant differences are noted by *.
To further examine anti-proliferative effects in this pathway, the ability of a PKG inhibitor to attenuate heparin’s effects was tested. Cells were treated as above, but the indicated cells were treated with the PKG inhibitor, Rp-8-pCPT-cGMS, for 20 min prior to treatment with PDGF. Addition of Rp-8-pCPT-cGMS almost completely reversed the effects of heparin and ANP (Figure 1C). The inhibitor also attenuated the ability of 8-Br-cGMP to block PDGF-stimulated BrdU incorporation, but BrdU incorporation remained lower than in the other inhibitor treatments. This may be the result of the direct competition for PKG between the PKG inhibitor and 8-Br-cGMP.
The effects of anti-heparin receptor antibodies on VSMCs are PKG-dependent
Heparin as well as anti-receptor antibodies, 18H6 & 18E9, blocked PDGF-stimulated BrdU incorporation. Inhibition of PKG with Rp-8-pCPT-cGMS reversed the anti-proliferative effects of heparin and the anti-receptor antibodies (Figure 1D). Therefore, as in studies of the effects of these anti-heparin receptor antibodies on cell proliferation and MAPK activity (Blaukovitch et al., 2010; Savage et al., 2001), the sensitivity of these anti-heparin receptor antibody effects to the PKG blocker matches the sensitivity of heparin effects. Interestingly, Rp-8-pCPT-cGMS also increased the level of BrdU incorporation in cells not treated with PDGF (Figure 1D). The reason for this remains unclear.
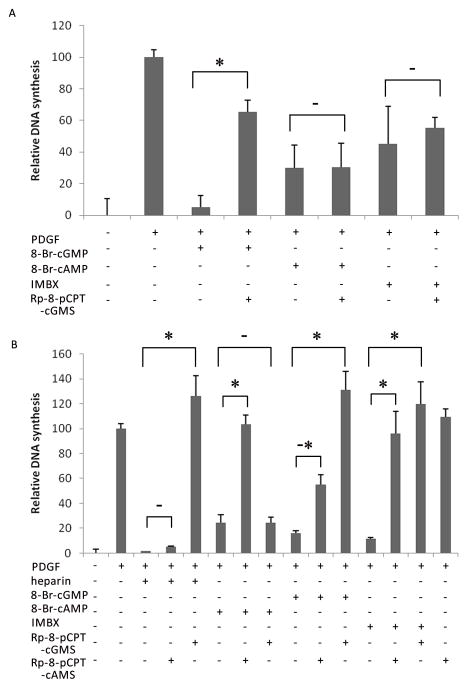
PKA is not involved in heparin effects
To rule out the possibility that cAMP cross-talk was responsible for the anti-proliferative effects of heparin, the ability of the PKG inhibitor to block cAMP-dependent inhibition of BrdU incorporation was tested. Cells were treated as in Figure 1; 10 min prior to PDGF stimulation, cells were treated with 8-Br-cGMP, 8-Br-cAMP, or IBMX. IBMX is a non-specific phosphodiesterase inhibitor, and treatment with this agent should result in elevations of cAMP and cGMP simultaneously. Indicated cells were treated with the PKG inhibitor, Rp-8-pCPT-cGMS for 20 min prior to treatment with PDGF. Each treatment (8-Br-cGMP, 8-Br-cAMP, and IBMX) significantly reduced PDGF-stimulated BrdU incorporation in the A7r5 cells (Figure 2A). 8-Br-cGMP inhibited almost 95% of the BrdU incorporated into cells treated with PDGF alone. 8-Br-cAMP and IBMX inhibited PDGF-stimulated BrdU incorporation by 70% and 55% respectively. When the PKG inhibitor was added to each of these treatments, Rp-8-pCPT-cGMS only reversed the inhibition of BrdU incorporation by 8-Br-cGMP. Both 8-Br-cAMP and IBMX still inhibited PDGF-stimulated BrdU incorporation in the presence of the PKG inhibitor in the A7r5 cells. These results demonstrate that there are also PKG-independent signaling pathways capable of blocking PDGF stimulation of VSMCs. To further support the PKG-dependence of heparin’s effects on PDGF-induced BrdU incorporation into DNA, we tested the ability of a PKA inhibitor to block heparin’s effects in porcine VSMCs (Figure 2B). Heparin’s inhibitory effects were only blocked by Rp-8-pCPT-cGMS, but not by Rp-8-pCPT-cAMS. Interestingly, the effects of IBMX were almost totally decreased by the inhibitors of both PKG and PKA. In each case, the effect of the 8-Br-form of the cyclic nucleotide was only completely blocked by the Rp-8-form of that nucleotide and only the PKG inhibitor blocked the heparin effect. These results strongly support the role of PKG, not PKA, in mediating the anti-proliferative effects of both cGMP and heparin in VSMCs.
Figure 2.
PKG, and not PKA, mediates heparin inhibition of DNA synthesis. A7r5 (A, n = 3) and porcine (B, n = 3) VSMC were treated as in Figure 1. In these assays, some cells were treated with 100 μM 8-Br-cAMP, 100 μM IBMX, and/or Rp-8-pCPT-cAMS at 2 μM. Heparin, 8-Br-cGMP, 8-Br-cAMP and IBMX blocked PDGF-stimulated BrdU incorporation (p < 0.0001). Inhibition of PKA with Rp-8-pCPT-cAMS blocked the effects of 8-Br-cAMP (p < 0.0001). However, only Rp-8-pCPT-cGMS completely blocked the effects of heparin and 8-Br-cGMP (p < 0.0001). Interestingly, Rp-8-pCPT-cAMS did have a partial effect on 8-Br-cGMP in the porcine cells (-*). Both inhibitors decreased the effects of IBMX (p < 0.0001).
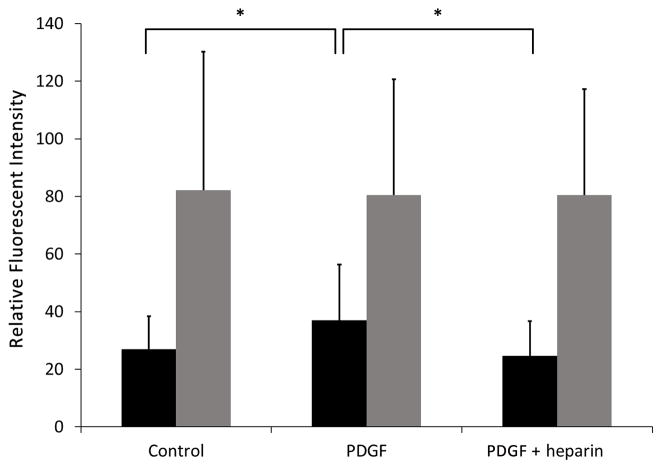
Decreasing PKG levels results in decreased heparin effects on BrdU incorporation
To further confirm the results obtained using kinase inhibitors, A7r5 cells were treated with siRNA for PKG. BrdU incorporation was monitored by immunofluorescence detection of BrdU to allow simultaneous detection of PKG levels. Consistent knock down of PKG required maintaining cells in standard culture medium. Therefore, cells were not starved for these studies. Under these conditions, BrdU incorporation was observed in control and PDGF treated cells that had been subjected to knock down of PKG with siRNA or treated with control siRNA. In control siRNA treated cells, PDGF significantly increased BrdU incorporation. It was interesting that cells treated with siRNA for PKG exhibited a much higher than control level of BrdU incorporation, and PDGF induced limited additional incorporation (see Figure 3). This result is consistent with the data using inhibitor alone in Figure 1D that resulted in BrdU incorporation without PDGF. In cells treated with control siRNA, heparin was able to significantly decrease BrdU incorporation. However, heparin had little effect on BrdU incorporation if the cells had reduced PKG levels after treatment with PKG siRNA (Figure 3).
Figure 3.
Knockdown of PKG decreases the ability of heparin to block DNA synthesis. A7r5 cells were treated with siRNA designed to knock down PKG or non-specific siRNA as described in Methods. After 48 hr, BrdU was added to all cells. Cells were left untreated or treated with PDGF in the presence or absence of 200 μg/ml heparin. Cells were stained with antibodies against PKG (not shown) or antibodies against BrdU followed by appropriate secondary antibodies. Fluorescent intensity as a percentage of the highest intensity sample from the experiment was calculated for each cell. Data from control siRNA-treated cells (black bars) are shown from one experiment run in duplicate (more than 100 cells/treatment). For control siRNA-treated cells (black bars), PDGF treated cells had significantly increased fluorescent intensity compared to controls (p<0.05,*), and these cells also treated with heparin had significantly decreased fluorescent intensity compared to cells treated with PDGF alone (p<0.05,*). Data from two experiments (more than 150 cells/treatment) are shown for PKG siRNA treated cells (grey bars). There were no significant differences in fluorescent intensity between treatments for knockdown cells.
Deactivation of ERK by heparin is PKG-dependent
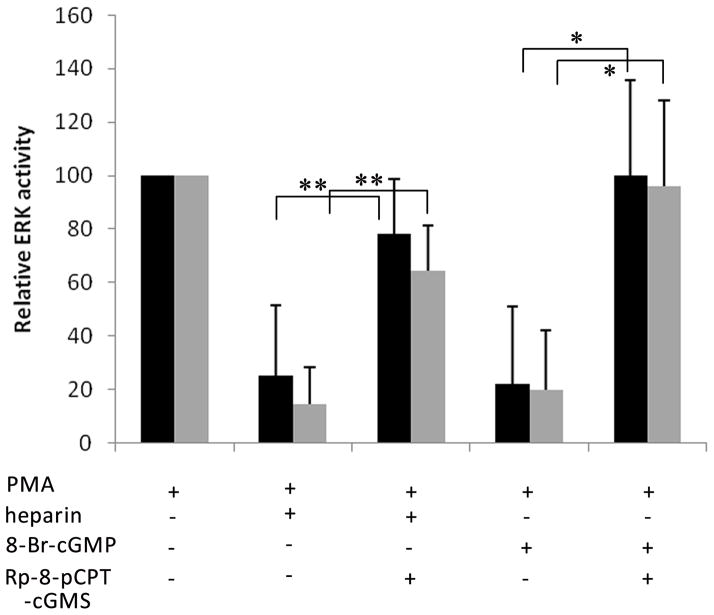
Treatment of VSMCs with heparin leads to an accelerated deactivation of PMA-stimulated ERK activation (Ottlinger et al., 1993; Pukac et al., 1997; Savage et al., 2001) which is dependent upon heparin induction of MKP-1 expression that in turn deactivates ERK (Blaukovitch et al., 2010). To establish the role of PKG in deactivating ERK, porcine VSMCs and A7r5 cells were treated with the PKG inhibitor Rp-8-pCPT-cGMS for 10 min before the addition of heparin or 8-Br-cGMP. Again, heparin and 8-Br-cGMP decreased PMA-stimulated ERK2 activation (Figure 4). Inhibition of PKG with Rp-8-pCPT-cGMS partly blocked the effects of 8-Br-cGMP and heparin on ERK phosphorylation. Similarly, PKG inhibition caused serum activated A7r5 cells to exhibit little heparin-induced change in ERK phosphorylation (data not shown). Variability in PMA induced activation between experiments, relatively small heparin-induced decreases, and complexity of the serum system led us to develop assays to monitor heparin signaling under conditions where we could examine responses in individual cells.
Figure 4.
Inhibiting PKG negates heparin effects on ERK activity. A7r5 (A, black) and porcine VSMC (A, grey) were synchronized by starvation and stimulated for 30 min with 20 μM PMA. Indicated cells were pretreated for 10 min (A) with heparin at 200 μg/ml or 100 μM 8-Br-cGMP. Indicated cells were incubated with 2 μM Rp-8-pCPT-cGMS for ten min prior to heparin addition. Treated cells were analyzed by Western Blotting for active ERK as described in Methods. Each mean (± SE) represents the activation of ERK2 (n=3) compared to untreated controls (0% activation) and stimulated controls (100% activation). Heparin and 8-Br-cGMP both decreased PMA stimulated ERK activity (p<0.005). The effects of the PKG inhibitor in PMA-activated cells reached significance for the 8-Br-cGMP treated cells (p<0.01). Rp-8-pCPT-cGMS effects on heparin treated cells (**) in these three repeats were not quite at the significance level (p<0.058).
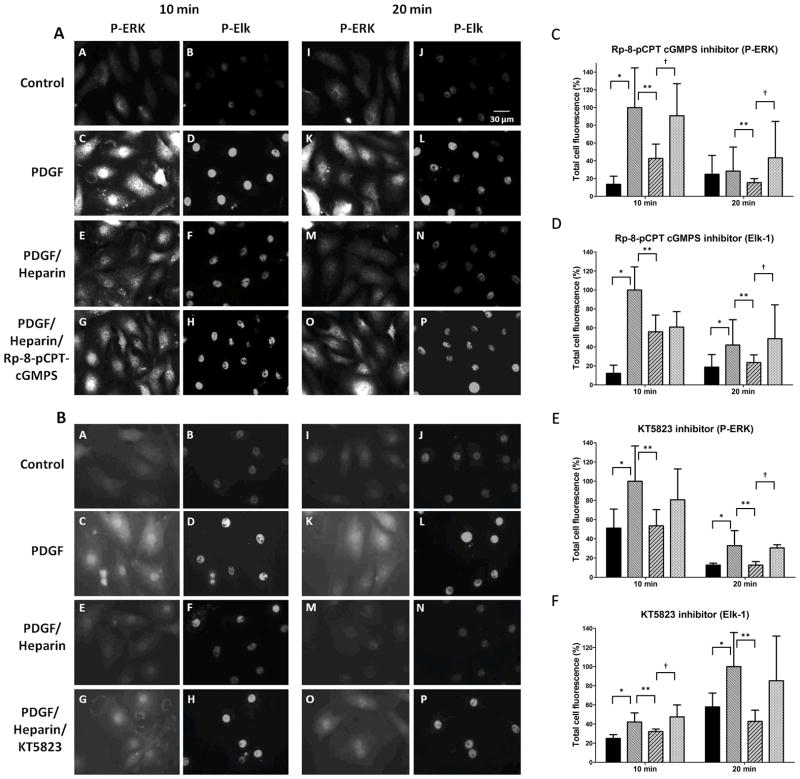
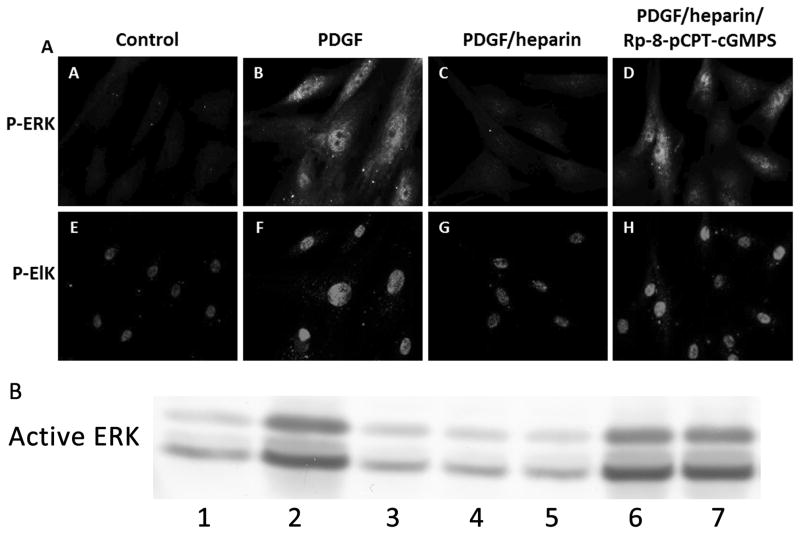
To further examine heparin effects on ERK activity, A7r5 VSMC were cultured on cover slips to facilitate examination signaling events in individual cells. Because heparin effects on ERK activity depend at least partly on nuclear dephosphorylation (Blaukovitch et al., 2010), we examined a nuclear transcription factor target of ERK in VSMC, Elk-1 (Shin et al., 2003; Yordy and Muise-Helmericks, 2000) for heparin sensitivity. The ability of heparin to cause decreased ERK activity and Elk phosphorylation was observed by immunofluorescence (Figure 5, e.g. compare images D with F and L with N in Panel A), where we found consistent heparin-induced decreases in ERK and Elk-1 PDGF-induced phosphorylation. Note that all heparin induced decreases in Elk-1 were significant in panels C through F. We then employed this fluorescence assay for active ERK and Elk-1 phosphorylation to examine the requirement of PKG activity in the heparin responses (Figure 5). PDGF treatment of starved A7r5 VSMCs induced significant increases in staining with antibodies to pERK at 10 and 20 min (e.g. compare Panel A pictures A to C and I to K). At both times (10 and 20 min PDGF treatment) heparin pre-treatment of cells decreased ERK activation and Elk phosphorylation. In the presence of Rp-8-pCPT-cGMPS (Figure 5, panel A) or KT5823, a second inhibitor of PKG, (Figure 5, panel B), heparin treatment had little effect on PDGF induced ERK activation and Elk-1 phosphorylation. Figure 5, panels C, D, E, and F show analysis of cells from three separate experiments (at least 50 cells/experiment) for each condition in panels A and B. In each condition, at least one of the time points indicated significant differences between the heparin treated cells with inhibitor and those without. The remaining time points in each condition were consistent, but did not reach significance. To confirm that PKG inhibition decreased active ERK and Elk-1 phosphorylation in primary VSMC, similar experiments were carried out with porcine VSMC (Figure 6, panels A and B). In these cells, PKG inhibitors Rp-8-pCPT-cGMPS and KT5823 (not shown) also decreased heparin effects on ERK activation and Elk-1 phosphorylation with the result that phosphorylation was similar to that in cells not treated with heparin.
Figure 5.
PKG inhibitors interfere with heparin effects on ERK activation and Elk phosphorylation. A7r5 cells were cultured on cover slips as described in Methods, starved and treated with PDGF in the presence or absence of 200 μg/ml heparin. Cells were fixed, permeabilized, stained with primary antibodies against pERK (mouse) and pElk (rabbit) followed by FITC-labeled anti-mouse secondary antibodies and TRITC-labeled anti-rabbit secondary antibodies, and visualized as described in Methods. Some cells were treated with Rp-8-cCPT-cGMPS (panel A) or KT5823 (panel B) 10 min prior to heparin treatment. Pictures are representative of at least 3 experiments. Cells were analyzed using image J to determine intensity of pERK and pElk staining. Three experiments with 50 to 80 cells per experiment in each treatment were analyzed. For statistical analysis black bars represent untreated cells, medium intensity bars represent PDGF activated cells, diagonal striped bars represent heparin treated cells stimulated with PDGF, and the lightest bars represent cells treated with inhibitor prior to heparin and PDGF treatments. In all cases except for pERK staining at 20 min in panel C PDGF stimulation resulted in significant (p<0.05) increases in staining (*). Heparin-induced decreases in staining were significant (p<0.05) in all cases (**). Panel C data indicate pERK staining with Rp-8-cCPT-cGMPS which significantly reversed heparin effects (p<0.05) at both 10 and 20 min (†). Panel D data indicate pElk staining with Rp-8-cCPT-cGMPS which significantly reversed heparin effects (p<0.05) at 20 min (†). Panel E data indicate pERK staining with KT5823 which significantly reversed heparin effects (p<0.05) at 20 min (†). Panel F data indicate pElk staining with KT5823 which significantly reversed heparin effects (p<0.05) at 10 min (†).
Figure 6.
Inhibitors of PKG interfere with heparin responses in primary VSMC. (Panel A) Porcine VSMC were treated identically to A7r5 cells with PDGF stimulation for 10 min. Heparin and inhibitor treatments and staining were identical to that in Figure 5. (Panel B) Porcine VSMC were treated identically and active ERK analyzed by western blotting. Lanes 1 – untreated control, 2 – PDGF 10 min, 3 – heparin only, 4 – heparin and PDGF, 5 – heparin and Rp8-cCPT-cGMPS, 6 – heparin, Rp8-cCPT-cGMPS and PDGF, 7 – Rp8-cCPT-cGMPS and PDGF.
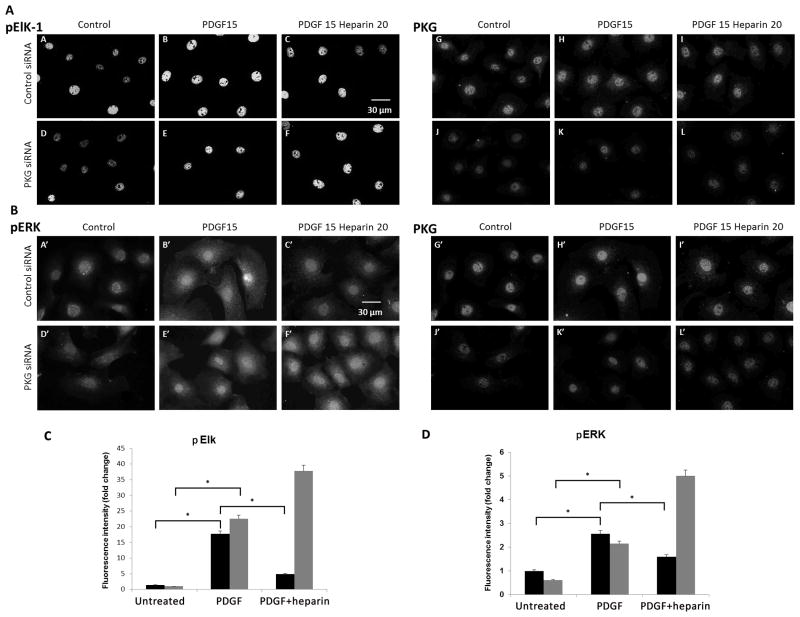
Because there is a short time frame required to add the inhibitors, heparin, and during which PDGF activation can be observed in ERK and Elk assays, we confirmed the importance of PKG activity in heparin effects on ERK phosphorylation in cells where PKG levels were significantly decreased through the use of siRNA. To ensure that siRNA was effectively getting into the cells, FITC-labeled control siRNA was used in one experiment. Essentially all cells took up the labeled control siRNA (data not shown). PKG knockdown efficiency varied between experiments from occasionally greater than 90% knockdown to about 50% decrease in staining in A7r5 cells treated with siRNA compared to a scrambled control as described in Methods. Cells allowed to recover in starvation media typically exhibited insignificant knockdown of PKG. Therefore, cells were treated with control siRNA or PKG siRNA and were cultured in regular growth media for 48 or 72 h and then treated with heparin for 20 min followed by PDGF for 15 min. Controls with PDGF or no treatment were compared. The presence of PKG, pERK and pElk-1 was determined by immunofluorescence staining (Figure 7A and 7B). Despite incomplete knockdown, it is clear that heparin induced decreases in pERK and pElk-1 staining were very limited in knockdown cells compared to cells with control siRNA. Western Blots confirm that heparin treatment does not have much effect on ERK activation in cells where PKG levels have been significantly decreased (data not shown). The maximum differences between untreated, PDGF-treated, and heparin/PDGF-treated cells are smaller because there is no starvation to decrease the basal ERK activity. These results are similar to those with BrdU incorporation in that whole cells treated with PKG siRNA retain more active ERK than cells treated with control siRNA. Repeats from immunofluorescence stained knockdown experiments were analyzed for nuclear staining and relative intensity comparisons are illustrated in (Figure 7C and D). Phosphorylation of ERK and Elk was significantly increased by PDGF in both cell types, but heparin significantly decreased nuclear ERK and Elk phosphorylation only in cells treated with control siRNA.
Figure 7.
Knockdown of PKG decreases heparin effects on ERK activity and pElk. A7r5 cells were electroporated with siRNA designed to knock down PKG in rat cells or scrambled RNA, and the cells were allowed to proliferate in growth media for 72 h with feeding at 24 h. At 72 h, cells were untreated, treated with PDGF for 15 min or heparin for 20 min before PDGF was added for 15 min. Panel A illustrates the pElk levels (pictures A–F). Staining for PKG is illustrated in the same cells in pictures G–L. Scrambled siRNA (A,B,C,G,H,I) as compared to PKG siRNA (D,E,F,J,K,L) is shown for cells not stimulated (A,D,G,J), PDGF treated cells (B,E,H,K) and heparin plus PDGF (C,F,I,L). Panel B illustrates an experiment where pERK was monitored (A′–F′) PKG staining for these cell samples is shown in pictures G′–L′. The treatment pattern is identical to that for panel A. These experiments are representative of two similar experiments each. Panel C provides quantitative analysis of Elk activation in PKG and scrambled siRNA. More than 100 cells from two separate experiments were analyzed and fluorescent intensity of pElk staining in the nucleus was determined. In both control (black bars) and PKG (grey) siRNA, pElk staining was significantly increased (p < 0.05,*). Heparin significantly (p<0.05,*) decreased pElk staining in control (black), but actually further increased pElk in PKG siRNA-treated cells. Panel D illustrates ERK activation as described for Elk in panel C. Nuclear pERK was determined for more than 100 cells from two experiments. As with Elk phosphorylation, PDGF increased ERK phosphorylation significantly (p<0.05,*) for both control siRNA (black bars) and PKG siRNA (grey bars) treated cells. In addition, heparin decreased PDGF-induced activation of ERK significantly (p<0.05,*) in control siRNA-treated cells, but not in PKG siRNA-treated cells.
Heparin-stimulated increases in MKP-1 expression are PKG-dependent
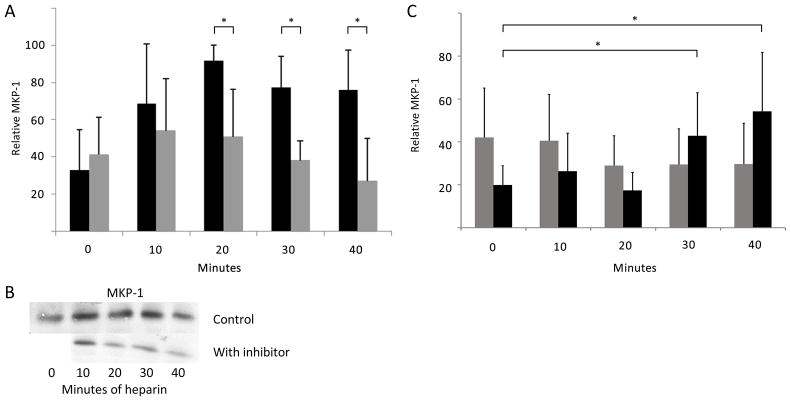
Because heparin-induced expression of MKP-1 in VSMCs was found to be important for heparin-induced decreases in ERK activity (Blaukovitch et al., 2010) and treatments that increase cGMP levels result in MKP-1 increases (Furst et al., 2005), the role of cGMP/PKG in the process of heparin induced MKP-1 expression was evaluated. Figure 8A demonstrates that the induction of MKP-1 expression by heparin was dependent on PKG; serum-starved rat A7r5 cells were treated with heparin with or without the PKG inhibitor Rp-8-pCPT-cGMS (added 10 min before the heparin). Inhibiting PKG significantly decreases the heparin-induced MKP-1 expression. This result was confirmed using PKG siRNA induced decreases in PKG levels. To maximize PKG decreases, cells were not starved after knockdown, and heparin-induced MKP-1 expression was compared between cells where PKG was knocked down and control cells where non-specific siRNA was employed (Figure 8C).
Figure 8.
Heparin-induced increases in MKP-1 require PKG activity. A. A7r5 cells were synchronized and left untreated or treated with 200 μg/ml heparin (dark bars). Identically treated cells were pre-treated with 2 μM Rp-8-pCPT-cGMS for 20 min prior to heparin (light bars) and cells were analyzed as described in Methods (n=7). MKP-1 expression was significantly different (*) between heparin and inhibited cells at 20, 30, and 40 min (p<0.05 in each case). B. One Western Blot experiment included in the data is illustrated. The blot was cut and aligned to show the same heparin points without inhibitor (above) and with inhibitor (below). No inhibitor without heparin was included in this blot. C. A7r5 cells were treated with siRNA to knock down PKG and treated with heparin as in A (light bars). For comparison, identical cells were treated with scrambled siRNA and then with heparin (dark bars). Cells were stained for PKG and MKP-1. Cells were analyzed using image J to determine intensity of MKP-1 staining. In knock down samples, cells were not analyzed if they stained strongly for PKG. Two experiments with more than 100 cells each were analyzed. In scrambled siRNA treated cells there were significant (*) increases in MKP-1 expression at 30 and 40 min (p<0.005). There were no increases in cells where PKG siRNA was used.
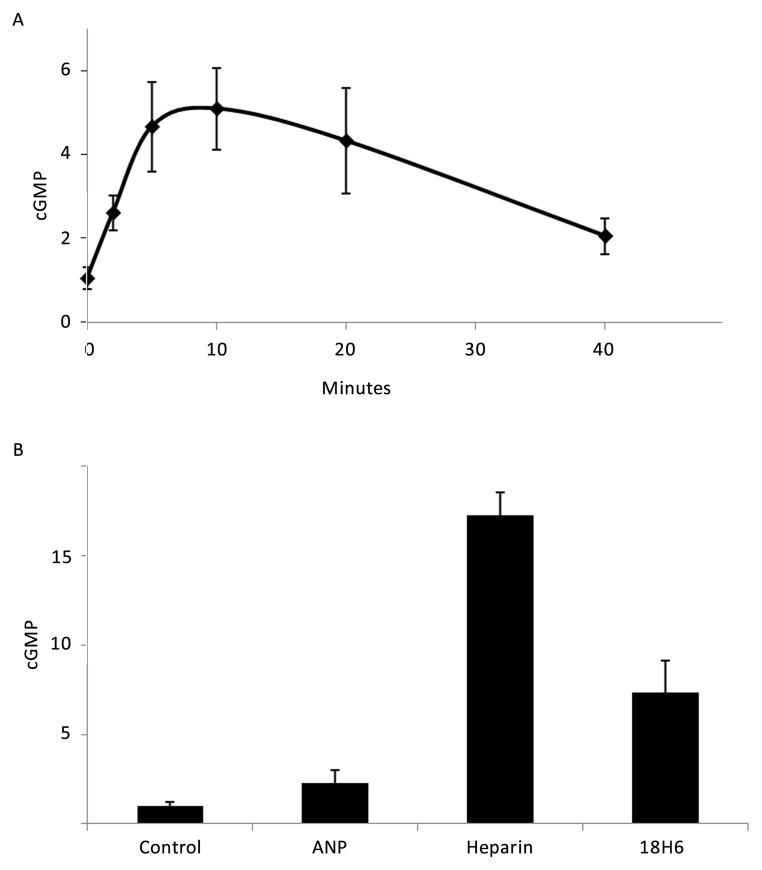
Heparin increases cGMP concentration in VSMCs
The ability of heparin to increase cGMP levels was tested in VSMCs using cGMP ELISA assays. In order to obtain measurable levels of cGMP, it was necessary to use 150 mm culture dishes of cells for each data point. Figure 9A illustrates the time course of heparin-stimulated cGMP increases in starved A7r5 cells. cGMP concentrations in the cell lysates were elevated approximately four times above basal levels after 5, 10 and 20 minutes of stimulation with 200 μg/ml heparin and returned to near basal levels after 40 minutes. Logarithmically-growing A7r5 VSMCs (Figure 9B) exhibited higher levels of cGMP in response to heparin, and a 10 min treatment with either heparin or an anti-receptor antibody (18H6) increased the cGMP concentration. In this experiment, the increase in cGMP in response to heparin was approximately a 15-fold induction (compared to untreated control levels). 18H6 induced a more modest increase in cGMP levels and a 10 min treatment with ANP did not show a statistically significant increase in cGMP at the 10 min time point used in this experiment (Figure 9B). This time point was used to maximize the cGMP production in response to heparin, and may not represent the optimal time at which to measure ANP induced cGMP increases. Starved porcine VSMC did not yield measurable increases in cGMP. However, proliferating porcine VSMC treated with heparin or ANP exhibited modestly elevated cGMP (not shown).
Figure 9.
Heparin treatment induces increases in cGMP concentration. Panel A: A7r5 cells were cultured in 150 mm dishes, starved as described in Methods and treated with 200 μg/ml heparin for indicated times. The cGMP concentrations were determined as described in the assay protocol and are reported as fold increases. The means (± SE) represent three independent experiments. Heparin increased cGMP after 5, 10 and 20 min (p<0.0051, 0.0011, and 0.0222, respectively). Panel B: A7r5 cells were cultured in 150 mm dishes and treated with heparin (200 μg/ml), 18H6 antibody (0.8 μg/ml) or ANP (100 nM) for 10 min. The data represent triplicate repeats. Values are reported as fold increases. Heparin treatment resulted in a statistically significant increase in cGMP (p < 0.0032).
Discussion
The proliferation of VSMCs in healthy vessels is carefully regulated, and heparin’s ability to block cell signaling events in VSMCs has been well documented (e.gs. Ottlinger et al., 1993; Pukac et al., 1997). Antibodies to a heparin receptor protein were shown to mimic heparin’s effects in these cells, thereby associating heparin’s anti-proliferative effects with this receptor protein (Savage et al., 2001). An intracellular signal transduction cascade starting at the receptor presumably is involved in heparin-induced decreases in PDGF-stimulated BrdU incorporation and deactivation of ERK MAPK activity (Savage et al., 2001), expression of MKP-1 (Blaukovitch et al., 2010), and decreased Elk-1 phosphorylation (this study) in VSMCs. In fact, the changes in Elk-1 phosphorylation are consistent with reports that Elk-1 phosphorylation results in changes in gene expression. Obvious mechanisms for heparin-induced PKG-mediated modulation of VSMC proliferation involve phosphorylation of transcription factors (reviewed in Pilz and Broderick, 2005) and reported for Elk-1 (Choi et al., 2010). The results reported here provide evidence for the downstream events of heparin receptor activation involving activation of PKG. Heparin has previously been demonstrated to have an anti-proliferative effect on VSMCs in rats and in an endothelial/VSMC co-culture system, but while NO production in the heparin-treated rats played a role in the response, it did not appear to be involved in heparin-induced decreases in VSMC proliferation (Horstman et al., 2002). To our knowledge, the present study is the first to report that the effects of heparin on VSMC DNA synthesis in culture, MKP-1 synthesis, Elk-1 phosphorylation changes, and ERK activity are sensitive to PKG inhibitors.
NO is a cell permeable activator of soluble guanylyl cyclase. Treatment of cells with synthetic NO donors or activators of NO synthase causes a rapid increase in intracellular concentrations of cGMP, while particulate guanylyl cyclases often result in limited cGMP increases (Su et al., 2005). In addition, production of cGMP at the membrane does not produce cGMP increases throughout the cell (Nausch et al., 2008), consistent with the idea that limited localized signaling might lead to physiological results without significant increases in total cGMP levels. ANP treatment of VSMCs does limit their proliferation (Baldini et al., 2002), and the limited increase in cGMP induced by heparin (Figure 9) seems to be an important mechanism by which heparin interaction with the receptor induces changes in VSMC signaling and proliferation.
There are PKG-independent effects of cGMP in VSMCs, but PKG is involved in the maintenance of the contractile phenotype characteristic of healthy VSMCs in vitro (Lincoln et al., 2006). Cells of this contractile phenotype are not highly proliferative. Therefore, many agents that can prevent a proliferative response to mitogenic signals might require PKG to be effective. In fact, a recent report directly links cGMP increasing agents to decreases in cell proliferation that result in therapeutic responses (Schwappacher et al., 2013). In addition, PKG levels typically decrease as VSMCs are sub-cultured (Cornwell and Lincoln, 1989; Lin et al., 2004) a result that coincides with reports of a decrease in heparin sensitivity with subculture (e.gs. Ottlinger et al., 1993; Pukac et al., 1997). In this regard, it is interesting that our PKG knockdown cells appear to maximally incorporate BrdU into DNA without further PDGF activation. Our data indicating the involvement of PKG in heparin-induced changes in signaling and proliferation are consistent with these results. Interestingly, it has been shown that activation of PKG results in its down-regulation, indicating a mechanism by which the response to cGMP-inducing signals can also be limited (Dey et al., 2009) and both NO and PDGF induced PKG decreases appear to require protein tyrosine phosphatase 1B (Zhuang et al., 2011). Although studies with genetically modified mice do not confirm decreased VSMC proliferation by PKG signaling pathways (Feil et al., 2003), a review of this system indicates complicated changes in gene regulation in these mice, making conclusions regarding cGMP/PKG signaling drawn from studies with these mice less clear (Hofmann et al., 2006).
ANP, 8-Br-cGMP, heparin, and anti-heparin receptor antibodies reduced PDGF-stimulated BrdU incorporation into VSMCs. Treatment of the cells with PKG inhibitor released the inhibitory effects of these agents. 8-Br-cAMP and IBMX also attenuated BrdU incorporation under these conditions, but their effects were insensitive to the PKG inhibitor, Rp-8-pCPT-cGMS. cAMP has anti-proliferative effects on VSMCs (e.g. Wu et al., 1993); however our results indicate that while both cGMP- and cAMP-dependent mechanisms induce anti-proliferative effects on VSMCs, the heparin-induced response is not operating through a cAMP-dependent mechanism. Heparin treatment caused an elevation in cGMP concentrations further supporting a role for PKG in heparin’s anti-proliferative effects in VSMCs.
One possible mechanism whereby heparin could affect VSMCs while inducing modest elevations of cGMP levels is through localization in caveolae. Peterson et al. (1999) reported that heparin affects the expression of caveolin and that caveolin expression influences VSMC growth, but whether these changes measured at 24 h explain ERK activity differences observed in less than an hour is unclear. Such a mechanism could also explain other heparin effects identified as involving caveolae (Liu et al., 2007). If the heparin receptor is localized in caveolae, binding could result in localized signaling through PKG by activating a NOS protein in VSMC (Cheah et al., 2002) or caveolae might modulate PKG activity adjacent to the caveolae.
Different PKG locations result in different cellular responses (Nausch et al., 2008; Schlossmann and Desch, 2011; Su et al., 2005). For example, PKG modulates calcium levels through several mechanisms including: decreasing the frequency of agonist-induced Ca2+ oscillations (Perez-Zoghbi et al., 2010), decreasing channel activity of TRPC1/TRPC3 and TRPC6 channels (Chen et al., 2009; Takahashi et al., 2008), and modulation of Ca2+-dependent big potassium channels (Fellner and Arendshorst, 2010).
Whatever the mechanism and down-stream signaling from PKG, our data suggest that heparin treatment causes the elevation of cGMP levels and indicate a role for PKG in heparin-induced decreased VSMC proliferation. The existence of a heparin receptor on the cell surface of VSMCs and vascular endothelial cells has been reported by our laboratory (Blaukovitch et al., 2010; Patton et al., 1995; Savage et al., 2001). Here we provide evidence for a mechanism whereby heparin interacts with vascular smooth muscle cells and either directly or indirectly activates a guanylyl cyclase that causes a rapid elevation of cGMP which, in turn, activates PKG. This idea is supported by 1) the rapid elevation of intracellular cGMP in heparin-treated cells, 2) the sensitivity of the heparin effects to PKG inhibitors, and 3) the sensitivity to partial knock down of PKG protein. Identification of the heparin receptor recognized by the anti-receptor antibodies produced by this lab will provide important information regarding the link(s) between the heparin receptor and cGMP/PKG.
Acknowledgments
The authors acknowledge preliminary PKG knock-down studies by Kristen Cornell and the technical help of Sara Lynn N Farwell, Matthew M. Miller and Traci Rickert.
Abbreviations
- VSMC
Vascular smooth muscle cell
- MKP-1
MAPK phosphatase-1
- IGF
Insulin-related growth factor
- iNOS
inducible NOS
- PKG
cGMP-dependent protein kinase
- ANF or ANP
atrial natriuretic factor or peptide
- PMA
phorbal myristic acid
- pElk
phospho Elk-1
- IBMX
3-isobutyl-1-methylxanthine
- DAB
3,3′-diaminobenzidine
- TBST
TBS with 0.1% tween-20
- TRITC
Tetramethyl Rhodamine Isothiocyanate
- PKA
cAMP-dependent protein kinase
Footnotes
Contract grant sponsor: Public Health Service; Contract grant number: HL54269.
References
- Baldini P, De Vito P, Fraziano M, Mattioli P, Luly P, Di Nardo P. Atrial natriuretic factor inhibits mitogen-induced growth in aortic smooth muscle cells. J Cell Physiol. 2002;193:103–109. doi: 10.1002/jcp.10155. [DOI] [PubMed] [Google Scholar]
- Begum N, Song Y, Rienzie J, Ragolia L. Vascular smooth muscle cell growth and insulin regulation of mitogen-activated protein kinase in hypertension. The American journal of physiology. 1998;275(1 Pt 1):C42–49. doi: 10.1152/ajpcell.1998.275.1.C42. [DOI] [PubMed] [Google Scholar]
- Blaukovitch CI, Pugh R, Gilotti AC, Kanyi D, Lowe-Krentz LJ. Heparin treatment of vascular smooth muscle cells results in the synthesis of the dual-specificity phosphatase MKP-1. J Cell Biochem. 2010;110(2):382–391. doi: 10.1002/jcb.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Roux D, Lenomand P, Dowd S, Keyse S, Pouysségur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot JJ, Jr, Addonizio ML, Rosenberg R, Karnovsky MJ. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. The Journal of cell biology. 1981;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot JJJ, Pukac LA, Caleb BL, Wright TC, Karnovsky MJ. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J Cell Biol. 1989;109:3147–3155. doi: 10.1083/jcb.109.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot JJJ, Wong K, Herman B, Hoover RL, Albertini DF, Wright TC, Caleb BL, Karnovsky MJ. Binding and internalization of heparin by vascular smooth muscle cells. J Cell Physiol. 1985;124:13–20. doi: 10.1002/jcp.1041240104. [DOI] [PubMed] [Google Scholar]
- Cheah LS, Gwee M, Das R, Ballard H, Yang YF, Daniel EE, Kwan CY. Evidence for the existence of a constitutive nitric oxide synthase in vascular smooth muscle. Clinical and experimental pharmacology & physiology. 2002;29(8):725–727. doi: 10.1046/j.1440-1681.2002.03707.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Crossland RF, Noorani MM, Marrelli SP. Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. American journal of physiology Heart and circulatory physiology. 2009;297(1):H417–424. doi: 10.1152/ajpheart.01130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Sellak H, Brown FM, Lincoln TM. cGMP-dependent protein kinase and the regulation of vascular smooth muscle cell gene expression: possible involvement of Elk-1 sumoylation. Am J Physiol Heart Circ Physiol. 2010;299(5):H1660–1670. doi: 10.1152/ajpheart.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes AW, Karnovsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Cornwell T, Lincoln T. Regulation of intracellular Ca2+ levels by atriopeptin and 8-bromo-cyclic GMP is mediated by cGMP-dependent protein kinase. J Biological Chemistry. 1989;264:1146–1155. [PubMed] [Google Scholar]
- Dey N, Busch J, Frnacis S, Corbin J, Lincoln T. Cyclic GMP specifically suppresses type-1α cGMP-dependent protein kinase expression by ubiquitination. Cellular Signalling. 2009;21:859–866. doi: 10.1016/j.cellsig.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasciano S, Patel R, Handy I, Patel C. Regulation of vascular smooth muscle proliferation by heparin. Inhibition of cyclin-dependent kinase 2 activity by p27kip1. J Biol Chem. 2005;280:15682–15689. doi: 10.1074/jbc.M411458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Lohmann S, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system. Insights from genetically modified mice. Circ Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- Fellner SK, Arendshorst WJ. Complex interactions of NO/cGMP/PKG systems on Ca2+ signaling in afferent arteriolar vascular smooth muscle. American journal of physiology Heart and circulatory physiology. 2010;298(1):H144–151. doi: 10.1152/ajpheart.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst R, Brueckl C, Kuebler W, Zahler S, Krotz F, Gorlach A, Vollmar A, Kiemer A. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- Horstman D, Fischer L, Kouretas P, Hannan R, Rich G. Role of nitric oxide in heparin-induced atenuation of hypoxic pulmonary vascular remodeling. J Applied Physiology. 2002;92:2012–2018. doi: 10.1152/japplphysiol.00664.2001. [DOI] [PubMed] [Google Scholar]
- Jacob A, Molkentin JD, Smolenski A, Lohmann SM, Begum N. Insulin inhibits PDGF-directed VSMC migration via NO/cGMP increase of MKP-1 and its inactivation of MAPKs. American journal of physiology Cell physiology. 2002;283(3):C704–713. doi: 10.1152/ajpcell.00110.2002. [DOI] [PubMed] [Google Scholar]
- Lin G, Chow S, Lin J, Wang G, Lue T, Lin C-S. Effect of cell passage and density on protein kinase G expression and activation in vascular smooth muscle cells. Journal of Cellular Biochemistry. 2004;92:104–112. doi: 10.1002/jcb.20043. [DOI] [PubMed] [Google Scholar]
- Lincoln T, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Frontiers in Bioscience. 2006;11:356–367. doi: 10.2741/1803. [DOI] [PubMed] [Google Scholar]
- Liu YT, Song L, Templeton DM. Heparin suppresses lipid raft-mediated signaling and ligand-independent EGF receptor activation. J Cell Physiol. 2007;211(1):205–212. doi: 10.1002/jcp.20924. [DOI] [PubMed] [Google Scholar]
- Melichar VO, Behr-Roussel D, Zabel U, Uttenthal LO, Rodrigo J, Rupin A, Verbeuren TJ, Kumar HSA, Schmidt HH. Reduced cGMP signaling associated with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16671–16676. doi: 10.1073/pnas.0405509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch LW, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):365–370. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottlinger ME, Pukac LA, Karnovsky MJ. Heparin inhibits mitogen-activated protein kinase activation in intact rat vascular smooth muscle cells. J Biol Chem. 1993;268:19173–19176. [PubMed] [Google Scholar]
- Patton WAI, Granzow CA, Getts LA, Thomas SC, Zotter LM, Gunzel KA, Lowe-Krentz LJ. Identification of a heparin-binding protein using monoclonal antibodies that block heparin binding to porcine aortic endothelial cells. Biochem J. 1995;311:461–469. doi: 10.1042/bj3110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zoghbi J, Bai Y, Sanderson M. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. Journal of General Physiology. 2010;135(3):247–259. doi: 10.1085/jgp.200910365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T, Kleppe L, Caplice N, Pan S, Mueske C, Simari R. The regulation of caveolin expression and localization by serum and heparin in vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;265:722–727. doi: 10.1006/bbrc.1999.1738. [DOI] [PubMed] [Google Scholar]
- Pilz R, Broderick K. Role of cyclic GMP in gene regulation. Frontiers in Bioscience. 2005;10:1239–1268. doi: 10.2741/1616. [DOI] [PubMed] [Google Scholar]
- Pukac LA, Carter JE, Ottlinger ME, Karnovsky MJ. Mechanisms of inhibition by heparin of PDGF stimulated MAP kinase activation in vascular smooth muscle cells. J Cell Physiol. 1997;172(1):69–78. doi: 10.1002/(SICI)1097-4652(199707)172:1<69::AID-JCP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Reilly C, Kindy M, Brown K, Rosenberg R, Sonenshein G. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989;264:6990–6995. [PubMed] [Google Scholar]
- Rodríguez A, Gómez-Ambrosi J, Catalán V, Fortuno A, Frühbeck G. Leptin inhibits the proliferation of vascular smooth muscle cells induced by angiotensin II through nitric oxide-dependent mechanisms. Mediators of Inflammation. 2010 doi: 10.1155/2010/105489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan PJ, Davis P, Moskaluk CA, Kearns M, Krutzsch H, Siebenlist U, Kelly K. PAC-1: a mitogen-induced nuclear protein-tyrosine phosphatase. Science. 1993;259:1763–1769. doi: 10.1126/science.7681221. [DOI] [PubMed] [Google Scholar]
- Sato J, Nair K, Hiddinga J, Eberhardt N, Fitzpatrick L, Katusic Z, O’Brien T. eNOS gene transfer to vascular smooth muscle cells inhibits cell proliferation via upregulation of p27 and p21 and not apoptosis. Cardiovasc Res. 2000;47:697–706. doi: 10.1016/s0008-6363(00)00137-1. [DOI] [PubMed] [Google Scholar]
- Savage JM, Gilotti AC, Granzow CA, Molina F, Lowe-Krentz LJ. Antibodies against a heparin receptor slow cell proliferation and decrease MAPK activation in vascular smooth muscle cells. J Cell Physiol. 2001;187:283–293. doi: 10.1002/jcp.1076. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Desch M. IRAG and novel PKG targeting in the cardiovascular system. American journal of physiology Heart and circulatory physiology. 2011;301(3):H672–682. doi: 10.1152/ajpheart.00198.2011. [DOI] [PubMed] [Google Scholar]
- Schwappacher R, Kilic A, Kojonazarov B, Lang M, Diep T, Zhuang S, Gawlowski T, Schermuly RT, Pfeifer A, Boss GR, Pilz RB. A Molecular Mechanism for Therapeutic Effects of cGMP-elevating Agents in Pulmonary Arterial Hypertension. The Journal of biological chemistry. 2013;288(23):16557–16566. doi: 10.1074/jbc.M113.458729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HS, Lee HJ, Nishida M, Lee MS, Tamura R, Yamashita S, Matsuzawa Y, Lee IK, Koh GY. Betacellulin and amphiregulin induce upregulation of cyclin D1 and DNA synthesis activity through differential signaling pathways in vascular smooth muscle cells. Circ Res. 2003;93(4):302–310. doi: 10.1161/01.RES.0000086803.64109.9E. [DOI] [PubMed] [Google Scholar]
- Su J, Scholz P, Weiss H. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Experimental Biology and Medicine. 2005;230:242–250. doi: 10.1177/153537020523000403. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Haneda M, Togawa M, Isono M, Shikano T, Araki S, Nakagawa T, Kashiwagi A, Guan KL, Kikkawa R. Atrial natriuretic peptide induces the expression of MKP-1, a mitogen-activated protein kinase phosphatase, in glomerular mesangial cells. J Biol Chem. 1996;271:544–547. doi: 10.1074/jbc.271.1.544. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, Mori M, Honda A, Inoue R. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. Journal of Physiology. 2008;586(17):4209–4223. doi: 10.1113/jphysiol.2008.156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantini B, Manes A, Fiumana E, Pignatti C, Guarnieri C, Zannoli R, Branzi A, Galie N. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol. 2005;100:131–138. doi: 10.1007/s00395-004-0504-5. [DOI] [PubMed] [Google Scholar]
- Tete H, Schroeder R, Stahl R, Wolf G. Atrial natriuretic peptide attenuates ANG II-induced hypertrophy of renal tubular cells. American J Physiology Renal Physiology. 2001;281:F81–F90. doi: 10.1152/ajprenal.2001.281.1.F81. [DOI] [PubMed] [Google Scholar]
- Wang S, Li Y. Expression of constitutively active cGMP-dependent protein kinase inhibits glucose-induced vascular smooth muscle cell proliferation. American journal of physiology Heart and circulatory physiology. 2009;297(6):H2075–2083. doi: 10.1152/ajpheart.00521.2009. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Dent P, Jelinek T, Wolfman A, Weber M, Sturgill T. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19(55):6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- Zhuang D, Balani P, Pu Q, Thakran S, Hassid A. Suppression of PKG by PDGF or nitric oxide in differentiated aortic smooth muscle cells: obligatory role of protein tyrosine phosphatase 1B. American journal of physiology Heart and circulatory physiology. 2011;300(1):H57–63. doi: 10.1152/ajpheart.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]