Structured Abstract
Study Design
In vivo study defining expression of the neurotrophins, brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), in cervical intervertebral discs following painful whole body vibration.
Objective
The goal of this study is to determine if BDNF and NGF are expressed in cervical discs after painful whole body vibration in a rat model.
Summary of Background Data
Whole body vibration is a possible source of neck pain and has been implicated as increasing the risk for disc disorders. Typically anneural regions of painful human lumbar discs exhibit hyper-innervation, suggesting nerve in-growth as potentially contributing to disc degeneration and pain. BDNF and NGF are upregulated in painfully degenerate lumbar discs and hypothesized to contribute to this pathology.
Methods
Male Holtzman rats underwent seven days of repeated whole body vibration (15Hz, 30 minutes/day) or sham exposures, followed by seven days of rest. Cervical discs were collected for analysis of BDNF and NGF expression through RT-qPCR and western blot analysis. Immunohistochemistry also evaluated their regional expression in the disc.
Results
Vibration significantly increases BDNF mRNA levels (p=0.036), as well as total NGF mRNA (p=0.035). Protein expression of both BDNF (p=0.006) and the 75kDa NGF (p=0.045) increase by nearly 4- and 10-fold, respectively. Both BDNF mRNA (R2=0.396 p=0.012) and protein (R2=0.280; p=0.035) levels are significantly correlated with the degree of behavioral sensitivity (i.e. pain) at day 14. Total-NGF mRNA is also significantly correlated with the extent of behavioral sensitivity (p=0.044, R2=0.276). Both neurotrophins are most increased in the inner annulus fibrosus and nucleus pulposus.
Conclusion
The increases in BDNF and NGF in the cervical discs after painful vibration are observed in typically anneural regions of the disc, consistent with reports of its hyper-innervation. Yet, the induction of nerve in-growth into the disc was not explicitly investigated. Neurotrophin expression also correlates with behavioral sensitivity, suggesting a role for both neurotrophins in the development of disc pain.
Keywords: whole body vibration, intervertebral disc, pain, neurotrophin, nerve growth factor, brain-derived neurotrophic factor
Introduction
Whole body vibration (WBV) is increasingly identified as a source of spinal pain. Epidemiological studies suggest that long-term WBV exposure increases risks for low back pain, spinal degeneration, and lumbar intervertebral disc (IVD) herniation [1-3]. Occupational exposure to WBV through frequent operation of vibrating vehicles is linked to higher incidences of neck pain [4-6]. For example, 33.5% of tractor drivers report neck pain at least once a week, with the frequency of episodes increasing with use [6]. Despite strong suggestions that WBV exposure is linked to chronic neck pain, the mechanism(s) involved remains unknown.
IVD damage and/or its degeneration are sources of pain, supported largely through work on the pathogenesis of chronic low back pain and disorders of the lumbar spine [7-9]. In healthy discs, the outer one-third of the annulus fibrosus is innervated; the remainder of the annulus fibrosus and the nucleus pulposus are both aneural and avascular [10]. However, in degenerated human discs, nerve in-growth is also evident in the inner annulus fibrosus and nucleus pulposus [11,12]. The extent of innervation is greatest at those spinal levels with pain [11], and asymptomatic degenerated discs do not exhibit innervation [12]. Although hyper-innervation is evident in painful lumbar discs, it is unknown if these same develops in the cervical discs.
The neurotrophins, brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), increase in painful degenerated human IVDs, and are thought to relate to its hyper-innervation [13,14]. Both neurotrophins are essential in neuronal development and maintenance, and modulate pain [15]. NGF is a peripheral pain mediator, with increased release by dorsal root ganglia neurons in response to lumbar disc herniation [16]. Similarly, BDNF modulates nociceptive processing in the spinal dorsal horn in models of disc herniation [17]. Both BDNF and NGF are expressed in the annulus fibrosus and nucleus pulposus of non-degenerate human discs [13], suggesting that neurotrophins in resident disc cells may promote nerve in-growth. Furthermore, BDNF increases in severely degenerated discs relative to non-degenerate and moderately degenerated discs [13]. Despite their speculated role in disc degeneration and pain, no study has investigated the role of neurotrophins and innervation in discs after WBV in the context of neck pain.
We previously developed a rat model of repeated WBV exposure that produces cervical spinal compression and extension, and pain in the forepaw that is sustained for 7 days after the last WBV exposure [18]. We use that model to examine if repeated WBV alters expression of BDNF and NGF in the cervical discs at day 7 after WBV. Neurotrophin mRNA transcript and protein expression were quantified in the cervical discs using RT-qPCR and western blot and correlated with the mechanical withdrawal thresholds on the day of harvest to examine if there is a relationship to behavioral sensitivity. Immunohistochemistry localized both neurotrophins in the outer annulus fibrosus, inner annulus fibrosus, and nucleus pulposus to determine if there are regional changes.
Materials and Methods
Procedures were approved by our IACUC and performed according to the guidelines of the Committee for Research and Ethical Issues of the IASP [19]. Male Holtzman rats (250-275g) were housed under AAALAC-compliant conditions with a 12-12 hour light-dark cycle and free access to food and water.
Rats underwent either a repeated WBV or sham exposure, using published methods [18]. WBV was performed under isoflurane inhalation anesthesia (4% induction; 2.5% maintenance) for 30 minutes daily for 7 days in a prone position on a vibrating plate. The plate was cyclically vibrated along the long-axis of the rat's spine, with a peak-to-peak magnitude of 1.5mm at 15Hz and 0.55g. A separate group of sham control rats received the same daily anesthesia but no vibration. All rats were allowed to rest for an additional 7 days after the final WBV. Behavioral hypersensitivity was measured in the forepaws prior to the start of the study (baseline) and daily until tissue harvest (day 14) by quantifying the mechanical withdrawal threshold [18,20,21]. Withdrawal thresholds were compared using a one-way ANOVA.
At day 14, cervical intervertebral discs (C5-C8) were harvested to quantify BDNF and NGF mRNA levels using RT-qPCR. Rats (WBV n=8; sham n=7) were anesthetized with sodium pentobarbital (65mg/kg) and transcardially perfused with 300mL of ice-cold 0.1M phosphate buffered saline (PBS; pH 7.4). Discs were dissected, snap frozen on dry ice, and stored at -80°C before tissue processing. RNA was isolated with Trizol (Life Technologies; Carlsbad, CA) or the Allprep kit (Qiagen; Germantown, MD) and cDNA was prepared using SupersciptIII Reverse Transcriptase (Life Technologies). Cervical discs from naïve unoperated rats (n=2) were included as controls. Power Sybr Green (Applied Biosystems; Carlsbad, CA) was used for RT-qPCR on the 7300 System (Applied Biosystems). Cycle conditions were 50°C for 2 minutes, 95°C or 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds, followed by melting curve analysis. Reactions were performed in duplicate, and primers confirmed to amplify a single band by 2% agarose gel electrophoresis. Efficiency was between 90-110%, and CyA was selected as the most stably expressed from a panel of housekeeping genes for relative quantification [21]. RT-qPCR was carried out using published BDNF primers [22] and primers specific to pro-NGF (forward5′-CATCCACCCACCCAGTCTTC-3′, reverse5′-CACCTCCTTGCCCTTGATGT-3′), total-NGF (forward5′-TACTGCACCACGACTCACAC-3′ and reverse5′-CCTGATGAACCTCCAGGCAG-3′). Quantification was performed using the comparative ΔΔCt method [23].
Discs were also analyzed to quantify protein levels by western blot after WBV (n=8) and sham (n=8). Samples were homogenized in lysis buffer (150mM NaCl, 50mM Tris-Cl pH 8.0, 1mM EDTA, 1% triton X-100) with protease and phosphatase inhibitors (Sigma); protein was measured by BCA assay (Thermo Scientific; Waltham, MA). Equal amounts of protein were loaded on gradient gels and transferred to PVDF membranes. Membranes were incubated overnight at 4°C with mouse anti-GAPDH (1:1000; Cell Signaling; Danvers, MA), rabbit anti-NGF (1:200; Santa Cruz Biotechnology), and rabbit anti-BDNF (1:200; Abcam; Carlsbad, CA) and then incubated for two hours with goat anti-rabbit IRDye 800(1:15,000; Li-Cor; Lincoln, NE). Membranes were imaged using the Odyssey Infrared Imaging System and quantified using the Odyssey Application Software (Li-Cor). Expression was normalized to GAPDH, which served as a loading control for each sample. Normalized BDNF and NGF mRNA and protein levels were each compared between groups using separate Student's t-tests. Separate linear regressions evaluated if the percent reduction in withdrawal thresholds from baseline correlated with transcript or protein levels for each of BDNF and NGF; significance was determined by separate ANOVA's.
Spatial localization of neurotrophin expression was analyzed using immunohistochemistry from separate group of rats (WBV n=4; sham n=3). Rats were anesthetized on day 14 with sodium pentobarbital (65mg/kg) and perfused with 300mL PBS followed by 250mL of 4% paraformaldehyde in PBS and post-fixed in 4% paraformaldehyde overnight at 4°C. Spinal columns were harvested en bloc and stored in 30% sucrose in PBS at 4°C and then decalcified using 10% EDTA (pH 7.2-7.4) for two weeks. Samples were embedded in Tissue-Tek® OCT Compound (Saukura Finetek; Torrance, CA) and serially sectioned (20μm) at the sagittal midline and thaw-mounted onto slides at room-temperature. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in 0.01M PBS. Antigen retrieval was performed by incubating slides in DeCal Antigen Retrieval (BioGenex; Fremont, CA) solution for 30 minutes. Slides were washed, blocked with normal horse serum (Vector) for 90 minutes and incubated in primary antibody against either NGF or BDNF (1:500, Santa Cruz Biotechnology) overnight at 4°C. After washing, sections were incubated with biotinylated donkey-anti rabbit secondary antibody (1:1000; Vector) for 30 minutes and developed using 3,3-diamimobenzidine (Vector). Slides were imaged using an Olympus BX51 microscope.
At least two representative images of each disc were taken at 20x magnification. Images were analyzed using semi-quantitative measures to grade the extent of NGF and BDNF expression in different regions of the disc: the outer annulus fibrosus (OAF), inner annulus fibrosus (IAF) and nucleus pulposus (NP) (Figure 1). Each region was identified through landmarks in the extracellular matrix, endplate and NP; the OAF and IAF were distinguished using cell morphology [24], and graded by two blinded evaluators. Each region of the disc was separately graded using an adapted scoring technique previously used to grade human discs [25]. Scoring used a 4-point scale, based on the distribution of immunopositive cells: no staining (-), mild staining (-/+), moderate staining (+) and robust staining (++). Scores from each observer were averaged by region in each disc. Because the scoring data were not normally distributed a non-parametric Mann Whitney U-Test determined significance between groups. Significance between regions within the same group was determined using a non-parametric Wilcoxon Signed-Rank Test.
Figure 1.
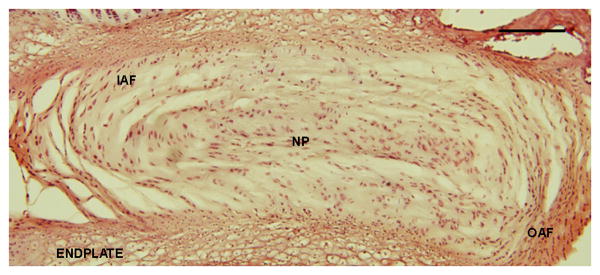
Hematoxylin (blue) and eosin (pink) stain schematic of cervical disc in the sagittal plane showing the IAF, OAF, NP and endplate regions of the IVD. Scale bar is 100μm.
Results
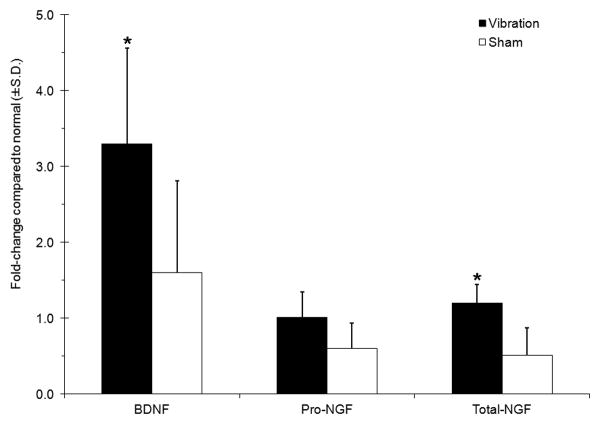
WBV induced behavioral sensitivity that was sustained throughout the exposure and rest periods. The mechanical withdrawal thresholds for the forepaw were significantly lower after WBV than sham exposures (p=0.018). Both BDNF and NGF mRNA were detected in all groups (Figure 2). BDNF levels were greater than normal in both groups, with a nearly double and significant increase in mRNA after vibration over sham (p=0.036) (Figure 2). No differences were detected for the pro-domain of NGF mRNA. Yet, total-NGF mRNA was significantly elevated after vibration over sham (p=0.035).
Figure 2.
Transcript levels of BDNF and NGF are upregulated in the cervical disc following WBV. BDNF and total-NGF remain significantly (*p<0.036) higher in vibrated discs compared to shams.
Protein levels for both neurotrophins exhibited similar trends as mRNA levels, with increases after WBV (Figure 3). BDNF protein increased significantly (p=0.006) more than four-fold after WBV over sham. Two forms of NGF protein were detected: one at 28kDa and one at 75kDa (Figure 3). The 28kDa form was not different between groups; but, 75kDa NGF was over 10-fold greater after WBV than sham (p=0.045). Increased neurotrophin expression was also confirmed by immunopositive staining in the IVDs after vibration (Figure 4 & Table 1). Immunoreactivity was detected in fibrobrast-like cells in the OAF and rounded, chondrocyte-like cells in the NP and IAF for both neurotrophins (Figure 4). BDNF expression after WBV was present in all samples, but exhibited variable expression, ranging from mild to robust. BDNF in the sham discs was generally absent or only mild (Figure 4 & Table 1). NGF exhibited a similar trend, with two samples with no expression and only mild expression in a single control rat. NGF labeling was evident in all samples that underwent vibration, with either moderate or robust expression (Figure 4 & Table 1). Overall, expression of both BDNF and NGF was significantly increased in the discs after vibration (p=0.003 for BDNF; p=0.001 for NGF).
Figure 3.
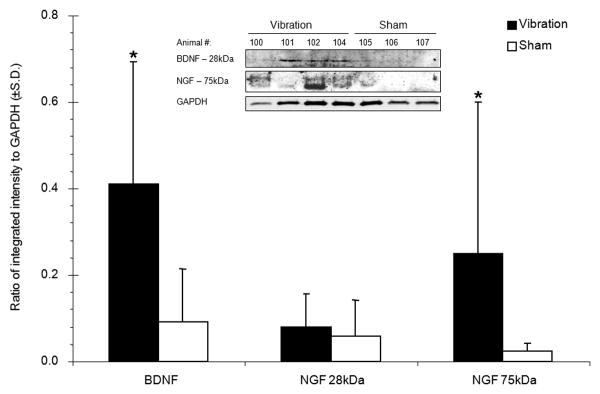
Expression of BDNF and NGF protein as measured by western blot and normalized to GAPDH levels. Following WBV, BDNF and the 75kDa isoform of NGF are each significantly upregulated (*p<0.045) compared to levels in the sham discs. The inset shows representative bands labeled for both neurotrophins and GAPDH.
Figure 4.
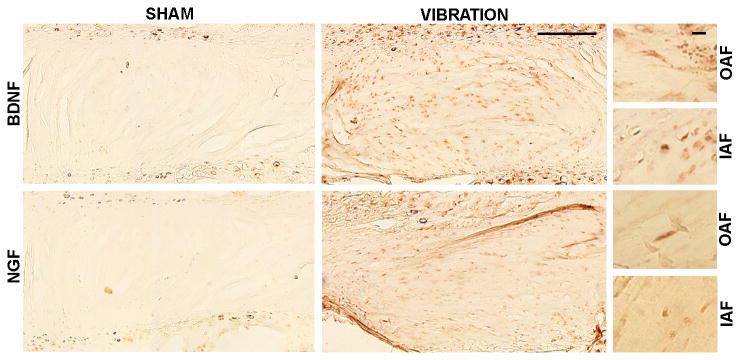
Increased BDNF and NGF labeling is evident in discs after WBV compared to sham discs. Both neurotrophins are expressed in rounded cells of the OAF and fibroblast-like cells of the IAF and NP. Scale bars are 200μm in full panel and 100μm in the panels on the right.
Table I. Scoring on NGF and BDNF labeling in the OAF, IAF and NP.
| BDNF | NGF | ||||||
|---|---|---|---|---|---|---|---|
| Rat ID | OAF | IAF | NP | OAF | IAF | NP | |
| WBV | 20 | −/+ | −/+ | + | + | + | ++ |
| 21 | + | - | −/+ | + | + | + | |
| 23 | + | ++ | ++ | ++ | ++ | ++ | |
| 24 | ++ | ++ | ++ | + | ++ | ++ | |
|
| |||||||
| SHAM | 32 | - | - | - | −/+ | −/+ | −/+ |
| 33 | - | −/+ | * | - | - | - | |
| 34 | −/+ | + | −/+ | - | - | - | |
Key: (-) no staining, (−/+) mild staining, (+) moderate staining, (++) robust staining
Expression of both neurotrophins was increased in all regions of the disc following WBV (Figure 5). The distribution of BDNF and NGF positive labeling varied within the regions of discs exposed to WBV (Figure 5), with greater labeling for both neurotrophins in the NP and IAF than the OAF (Table 1). BDNF expression was greatest in the IAF and NP, and significantly increased in the IAF (p=0.003) and the NP (p=0.002) over expression in the OAF (Figure 5). However, there was no difference in BDNF between the IAF and the NP (Table 1). NGF labeling was also greatest in the NP and IAF; but, this increase was only significant in the NP compared to the OAF (p=0.007). There were no regional differences detected in the sham discs.
Figure 5.
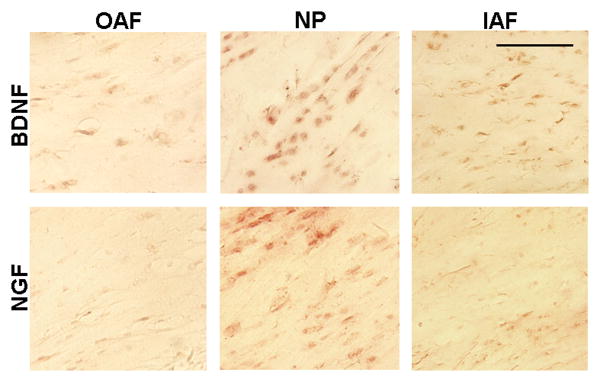
Positive labeling for both neurotrophins is evident in all regions of the disc. BDNF labeling in the IAF and NP is greater than in the OAF. NGF expression is greater in the NP than the OAF.
Neurotrophin responses correlated with behavioral sensitivity, although weakly. Both BDNF and total-NGF mRNA levels were significantly correlated (pBDNF=0.012; ptNGF=0.044) with the percent reduction in withdrawal thresholds (Figure 6), though both were weak (R2=0.396 BDNF; R2=0.276 total-NGF). Neither form of NGF showed any relationship to behavioral sensitivity; BDNF protein was significantly correlated (R2=0.280; p=0.035) with behavioral sensitivity.
Figure 6.
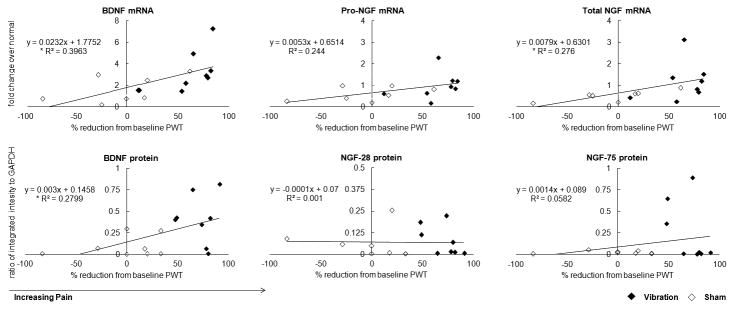
Correlations of BDNF and NGF mRNA and protein levels with percent reduction in paw withdrawal threshold (PWT) at day 14. BDNF (*p=0.012) and total-NGF (*p=0.044) mRNA are significantly correlated with greater decreases in PWT Protein levels for both neurotrophins are weakly correlated with PWT, with only BDNF being significant (*p=0.035).
Discussion
Painful WBV is associated with upregulation of both BDNF and NGF, especially in the inner regions of the cervical disc, even after a period of rest after the last exposure (Figures 2-5). These neurotrophins also increase in painful and degenerated lumbar IVDs and have been associated with innervation of typically annerual regions of the disc [9,13]. Despite such reports, this is the first study to note changes in these neurotrophins associated with pain due to WBV. Although the cervical spine undergoes significant mechanical deformation in this painful WBV model [18], the physiological mechanisms responsible for pain onset and/or maintenance are not defined. Increased disc innervation has been reported in ovine models of accelerated degeneration due to annular lesions or tears and in humans with painful IVD [12,26,27]. The known role of BNDF and NGF in promoting nerve growth in the disc [9,13], together with the significant correlations between their expression and pain in our model (Figure 6), support their potential role in regulating disc pain from WBV.
Both BDNF transcript and protein levels are increased in discs exposed to WBV and are significantly correlated with increased behavioral sensitivity at day 14 (Figures 2, 3 & 6), suggesting a role for BDNF in the pathomechanisms governing cervical neck pain. In addition to regulating the growth and survival of neural cells, BDNF modulates nociception via its anterograde transport from the DRG and release from primary afferents, increasing neuronal hyperexcitability in the spinal dorsal horn [15,28-32]. In fact, sequestering spinal BDNF using its TrkB receptor attenuates pain after spinal joint injury [33]. Orita et al (2011) also showed that intradiscal inhibition of BDNF in a puncture model decreases expression of the neuropeptide, calcitonin gene-related peptide, in the neurons that innervate the disc [34]. Since BDNF expression in the disc increases with degenerative grade, BDNF signaling has been implicated in discogenic pain [13,35]. The findings of our study add further support to such hypothesis, particularly related to WBV.
Although gene expression for total-NGF significantly increases after painful WBV, the pro-NGF gene does not (Figure 2). Yet, total-NGF accounts for both the pro- and mature transcripts of NGF. Pro-NGF (∼30kDa), a precursor of NGF, is active in several inflammatory conditions [36,37]. Intraplantar injection of pro-NGF, which binds the p75NTR NGF receptor, can induce hyperalgesia [38]; pro-NGF levels do not correlate with pain after WBV (Figure 6). Since only total-NGF levels correlate with behavioral sensitivity, the mature transcript may be crucial in the development of disc pain. Further, pro-NGF protein increases only slightly after WBV, while, the high molecular weight 75kDa NGF species exhibits a robust (10-fold) (Figure 3). Although this higher molecular weight species has not been previously reported in the disc, it is prevalent in extracerebral blood vessels and the external carotid artery [39]. Freemont et al (2002) reported microvascular blood vessels expressing NGF adjacent to ingrown nerves in painful human lumbar IVDs [9]. Regardless, increases in both of the NGF isoforms indicate its active production following WBV, despite not being directly related to pain. NGF in all regions of non-degenerate discs [13,40], together with evidence in discs following WBV in our study (Figure 4 & Table 1), indicate that NGF may support disc health and may be increased in response to pain or injury.
Although both BDNF and 75kDa NGF increase following WBV, there is a high degree of variability (Figure 3). This variability may be due to fact that tissue processing techniques for RT-qPCR and western blot assay the entire disc and regional analyses were not performed, as with the IHC (Figures 4 & 5). In fact, regional analysis of both neurotrophins indicates that there is an overall difference and regionally (Figures 4 & 5 & Table 1). The upregulation in the inner disc regions after WBV is also observed in patients with disc pain [9,13], suggesting that localization of these neurotrophins in the nucleus may induce subsequent innervation of the disc.
Increased expression of NGF in the inner regions of the disc is seen exclusively in patients with painful discs and hypothesized to occur simultaneously with hyper-innervation, suggesting that nerve fibers only grew into discs with local production of NGF [9]. NGF production by human degenerate NP cells has been reported to promote nerve in-growth in-vitro [41]. In our study, NGF is greatest in the NP (Figure 5 & Table 1), suggesting that repeated painful WBV may induce its production. Recently, BDNF has been purported to promote nerve in-growth into degenerate disc tissue [13,35]. Both neurite outgrowth and the percent of neurite expressing cells decrease with anti-BDNF treatment in cultured degenerate human NP cells [35]. Because BDNF expression is greater in the IAF and NP than the OAF (Figure 5 & Table 1), BDNF may also promote innervation into the disc. However, this study did not investigate whether such subsequent innervation was induced; additional studies are needed to evaluate if there is indeed a causal relationship between vibration, expression of these neuorotrophins in the disc and pain..
This is the first study demonstrating that painful WBV is not only associated with increased neurotrophins in the cervical disc, but those increases are in the disc regions where similar upregulation is observed in painful lumbar IVDs in humans. Moreover, BDNF transcript and protein, and NGF transcript levels correlate with the extent of behavioral sensitivity. This study provides additional support for these neurotrophins as having roles in disc pain, possibly via regulating nerve growth. Although additional studies are needed to define the specific temporal relationships between neurotrophins, nerve growth and pain, these findings provide encouraging data supporting the notion that neck pain from WBV may result from direct biochemical changes in the disc.
Acknowledgments
This project was supported through funding provided by grants from the Department of Defense (W81XWH-10-2-0140) and the National Institutes of Health (NIAMS) (AR064157).
The Manuscript submitted does not contain information about medical device(s)/drug(s). Department of Defense (W81XWH-10-2-0140) and the National Institutes of Health (NIAMS) (AR064157) grant funds were received to support this work. Relevant financial activities outside the submitted work: grants.
References
- 1.Bovenzi M, Hulshof C. An updated review of epidemiological studies on the relationship between exposure to whole-body vibration and low back pain. J Sound Vib. 1998;215:595–613. [Google Scholar]
- 2.Pope M, Magnusson M, Lundstrom R, et al. Guidelines for whole body vibration health surveillance. J Sound Vib. 2002;253:131–67. [Google Scholar]
- 3.Gregory DE, Callaghan JP. Does vibration influence the initiation of intervertebral disc herniation? Spine. 36:E225–31. doi: 10.1097/BRS.0b013e3181d89094. [DOI] [PubMed] [Google Scholar]
- 4.Milosavljevic S, Bagheri N, Vasiljev RM, et al. Does daily exposure to whole-body vibration and mechanical shock relate to the prevalence of low back and neck pain in a rural workforce? Ann Occup Hyg. 2012;56:10–7. doi: 10.1093/annhyg/mer068. [DOI] [PubMed] [Google Scholar]
- 5.Rehn B, Nilsson T, Lundström R, et al. Neck pain combined with arm pain among professional drivers of forest machines and the association with whole-body vibration exposure. Ergonomics. 2009;52:1240–7. doi: 10.1080/00140130902939889. [DOI] [PubMed] [Google Scholar]
- 6.Scutter S, Türker KS, Hall R. Headaches and neck pain in farmers. Aust J Rural Health. 1997;5:2–5. doi: 10.1111/j.1440-1584.1997.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 7.Luoma K, Riihimäki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 8.Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25:853–7. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 9.Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–92. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa H, O'Brien JP, Smith WT, et al. The neuropathology of intervertebral discs removed for low-back pain. J Pathol. 1980;132:95–104. doi: 10.1002/path.1711320202. [DOI] [PubMed] [Google Scholar]
- 11.Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–81. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 12.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 13.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber HE, Ingram JA, Hoelscher G, et al. Brain-derived neurotrophic factor and its receptor in the human and the sand rat intervertebral disc. Arthritis Res Ther. 2008;10:R82. doi: 10.1186/ar2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 16.Onda A, Murata Y, Rydevik B, et al. Nerve growth factor content in dorsal root ganglion as related to changes in pain behavior in a rat model of experimental lumbar disc herniation. Spine. 2005;15:188–93. doi: 10.1097/01.brs.0000150830.12518.26. [DOI] [PubMed] [Google Scholar]
- 17.Onda A, Murata Y, Rydevik B, et al. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine. 2004;29:1857–61. doi: 10.1097/01.brs.0000137054.08788.b2. [DOI] [PubMed] [Google Scholar]
- 18.Baig HA, Guarino BB, Lipschutz D, et al. Whole body vibration induces forepaw and hind paw behavioral sensitivity in the rat. J Orthop Res. 2013;31:1739–44. doi: 10.1002/jor.22432. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30:1924–32. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 21.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: Behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–93. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–87. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2008;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Pattappa G, Li Z, Peroglio M, Wismer N, et al. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221:480–96. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokubo Y, Uchida K, Kobayashi S, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9:285–95. doi: 10.3171/SPI/2008/9/9/285. [DOI] [PubMed] [Google Scholar]
- 26.Melrose J, Roberts S, Smith S, et al. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27:1278–85. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 27.Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14:513–21. doi: 10.1016/j.spinee.2013.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho HJ, Kim JK, Zhou XF, et al. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res. 1997;76:269–72. doi: 10.1016/s0006-8993(97)00597-0. [DOI] [PubMed] [Google Scholar]
- 29.Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485–88. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 30.Li CQ, Xu JM, Liu D, et al. Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Mol Pain. 2008;4:27. doi: 10.1186/1744-8069-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael GJ, Averill S, Nitkunan A, et al. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–90. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Kras JV, Weisshaar CL, Quindlen J, et al. Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res. 2013;91:1312–21. doi: 10.1002/jnr.23254. [DOI] [PubMed] [Google Scholar]
- 34.Orita S, Eguchi Y, Kamoda H, et al. Brain-derived neurotrophic factor inhibition at the punctured intervertebral disc downregulates the production of calcitonin gene-related peptide in dorsal root ganglia in rats. Spine. 2011;36:1737–43. doi: 10.1097/BRS.0b013e31821d7b9f. [DOI] [PubMed] [Google Scholar]
- 35.Richardson SM, Purmessur D, Baird P, et al. Degenerate human nucleus pulposus cells promote neurite outgrowth in neural cells. PLoS One. 2012;7:e47735. doi: 10.1371/journal.pone.0047735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dicou E. Multiple biological activities for two peptides derived from the nerve growth factor precursor. Biochem Biophys Res Commun. 2006;343:833–7. doi: 10.1016/j.bbrc.2006.06.171. [DOI] [PubMed] [Google Scholar]
- 37.Dicou E. High levels of the proNGF peptides LIP1 and LIP2 in the serum and synovial fluid of rheumatoid arthritis patients: evidence for two new cytokines. J Neuroimmunol. 2008;194:143–6. doi: 10.1016/j.jneuroim.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Ito T, Inoue G, et al. The p75 receptor is associated with inflammatory thermal hypersensitivity. J Neurosci Res. 2008;86:3566–74. doi: 10.1002/jnr.21808. [DOI] [PubMed] [Google Scholar]
- 39.Randolph CL, Bierl MA, Isaacson LG. Regulation of NGF and NT-3 protein expression in peripheral targets by sympathetic input. Brain Res. 2007;1144:59–69. doi: 10.1016/j.brainres.2007.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–42. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi K, Inoue G, Koshi T, et al. Nerve growth factor of cultured medium extracted from human degenerative nucleus pulposus promotes sensory nerve growth and induces substance p in vitro. Spine. 2009;34:2263–9. doi: 10.1097/BRS.0b013e3181a5521d. [DOI] [PubMed] [Google Scholar]