Abstract
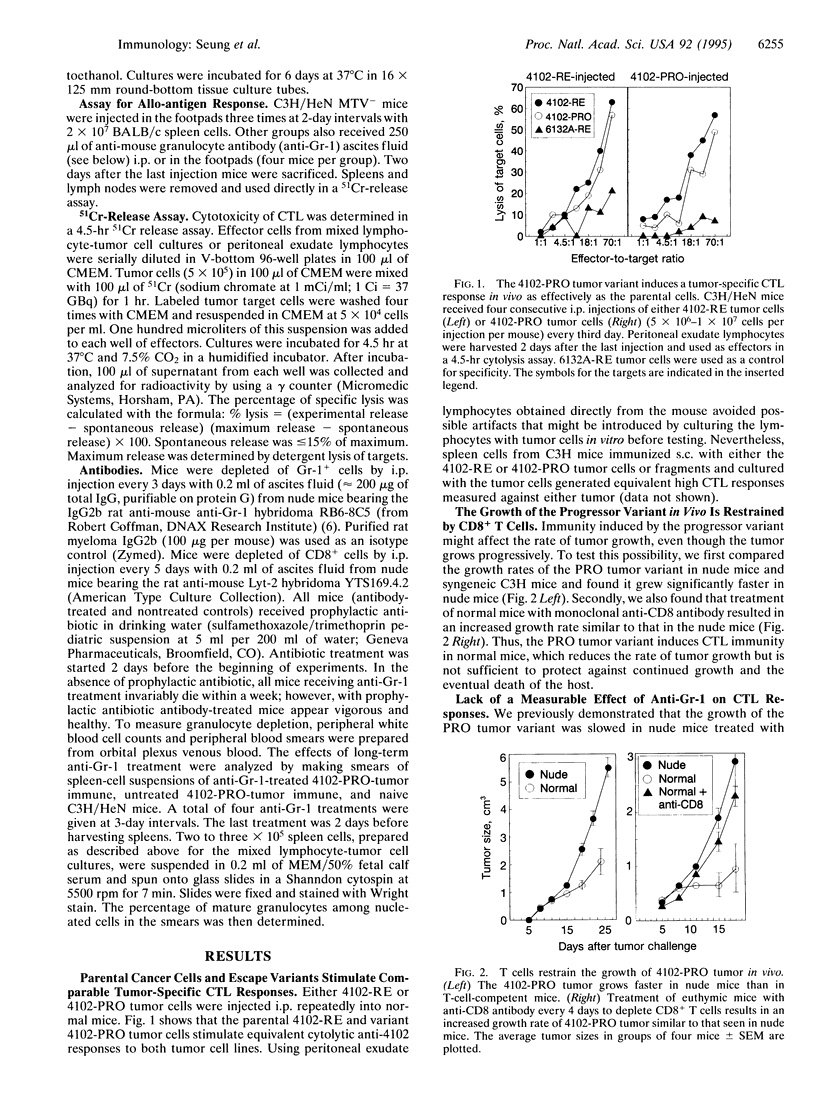
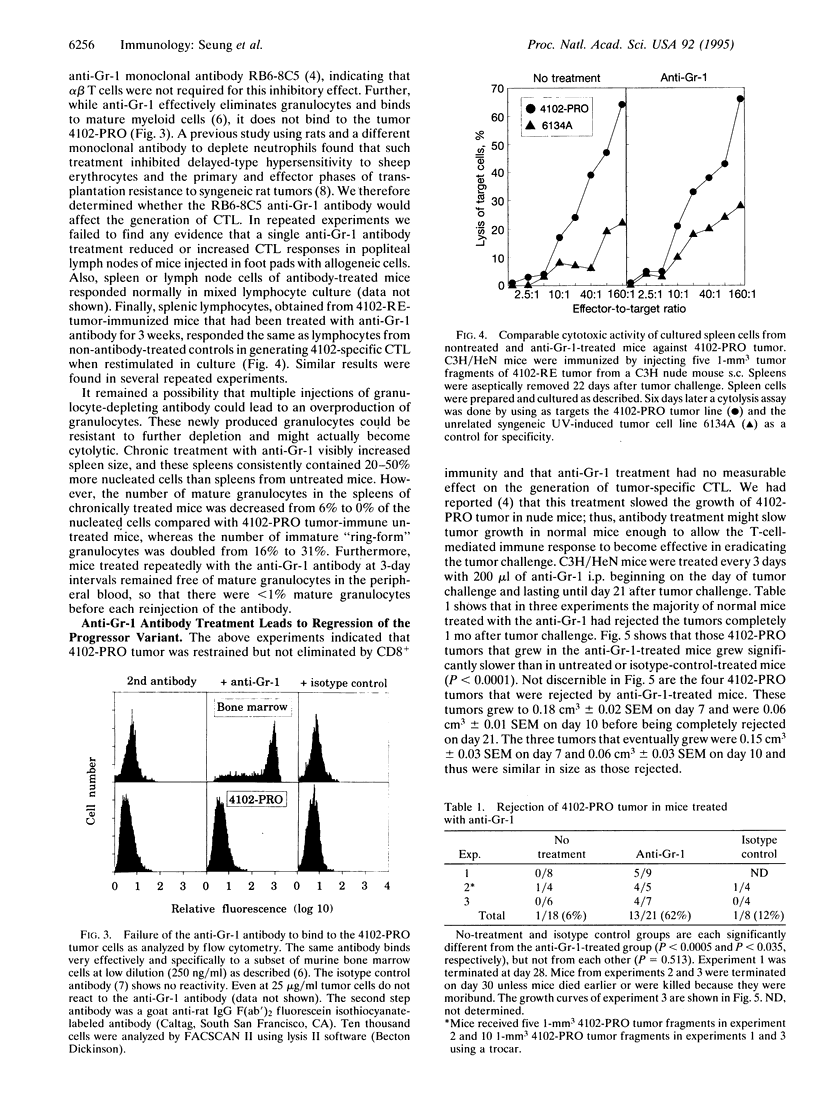
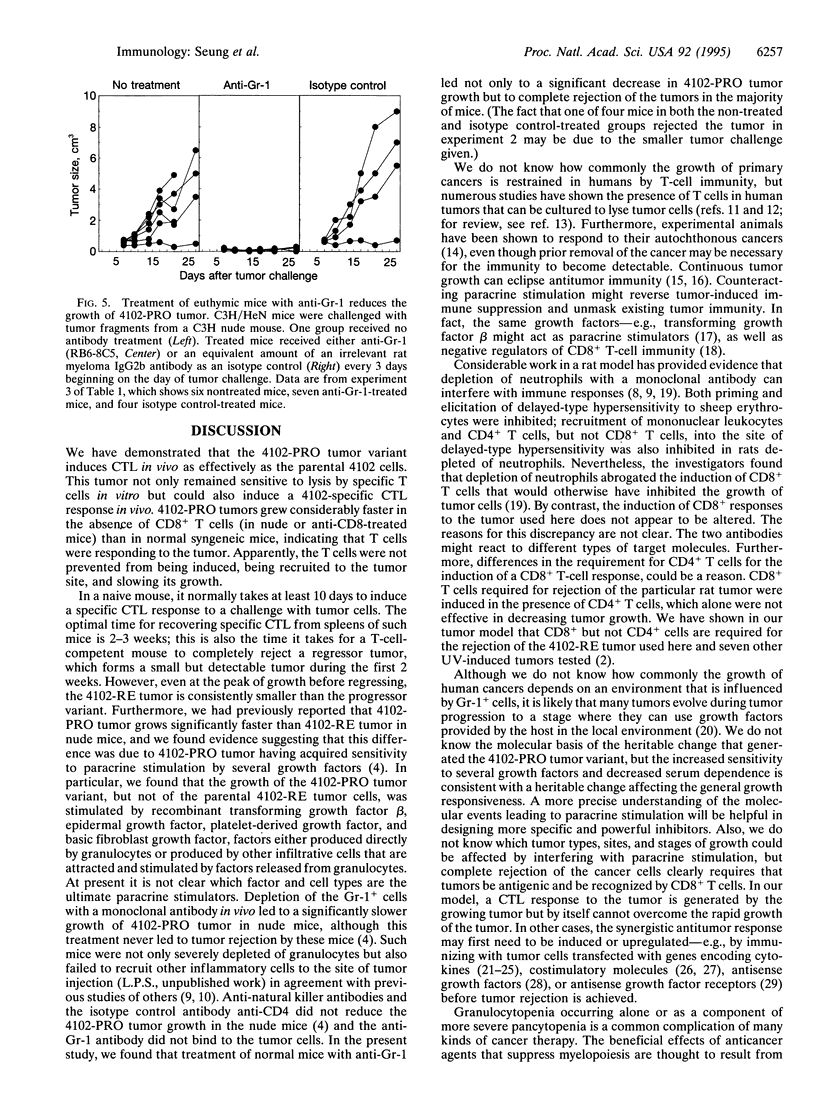
During tumor progression, variants may arise that grow more vigorously. The fate of such variants depends upon the balance between aggressiveness of the variant and the strength of the host immunity. Although enhancing host immunity to cancer is a logical objective, eliminating host factors necessary for aggressive growth of the variant should also be considered. The present study illustrates this concept in the model of a spontaneously occurring, progressively growing variant of an ultraviolet light-induced tumor. The variant produces chemotactic factors that attract host leukocytes and is stimulated in vitro by defined growth factors that can be produced or induced by leukocytes. This study also shows that CD8+ T-cell immunity reduces the rate of tumor growth; however, the variant continues to grow and kills the host. Treatment with a monoclonal anti-granulocyte antibody that counteracts the infiltration of the tumor cell inoculum by non-T-cell leukocytes did not interfere with the CD8+ T-cell-mediated immune response but resulted in rejection of the tumor challenge, indicating a synergy between CD8+ T-cell-mediated immunity and the inhibition of paracrine stimulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosco M., Giovarelli M., Forni M., Modesti A., Scarpa S., Masuelli L., Forni G. Low doses of IL-4 injected perilymphatically in tumor-bearing mice inhibit the growth of poorly and apparently nonimmunogenic tumors and induce a tumor-specific immune memory. J Immunol. 1990 Nov 1;145(9):3136–3143. [PubMed] [Google Scholar]
- Bubeník J., Símová J., Jandlová T. Immunotherapy of cancer using local administration of lymphoid cells transformed by IL-2 cDNA and constitutively producing IL-2. Immunol Lett. 1990 Feb;23(4):287–292. doi: 10.1016/0165-2478(90)90074-z. [DOI] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994 Jan 1;179(1):259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., Allison J. P. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993 Jun 1;177(6):1791–1796. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Klein E., Vánky F., Galili U., Vose B. M., Fopp M. Separation and characteristics of tumor-infiltrating lymphocytes in man. Contemp Top Immunobiol. 1980;10:79–107. doi: 10.1007/978-1-4684-3677-8_4. [DOI] [PubMed] [Google Scholar]
- Kripke M. L. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974 Nov;53(5):1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Budmen M. B., Fidler I. J. Production of specific macrophage activating factor by lymphocytes from tumor-bearing mice. Cell Immunol. 1977 May;30(2):341–352. doi: 10.1016/0008-8749(77)90077-6. [DOI] [PubMed] [Google Scholar]
- Kudo C., Yamashita T., Araki A., Terashita M., Watanabe T., Atsumi M., Tamura M., Sendo F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. I. Inhibition by RP-3 treatment of the priming and effector phases of delayed type hypersensitivity to sheep red blood cells in rats. J Immunol. 1993 May 1;150(9):3728–3738. [PubMed] [Google Scholar]
- Kudo C., Yamashita T., Terashita M., Sendo F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. II. Inhibition by RP-3 treatment of mononuclear leukocyte recruitment in delayed-type hypersensitivity to sheep red blood cells in rats. J Immunol. 1993 May 1;150(9):3739–3746. [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages. Curr Opin Immunol. 1989;2(5):689–692. doi: 10.1016/0952-7915(90)90031-b. [DOI] [PubMed] [Google Scholar]
- McKearn T. J., Fitch F. W., Smilek D. E., Sarmiento M., Stuart F. P. Properties of rat anti-MHC antibodies produced by cloned rat-mouse hybridomas. Immunol Rev. 1979;47:91–115. doi: 10.1111/j.1600-065x.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- North R. J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984 May 1;159(5):1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarek L. A., Starr B. A., Toledano A. Y., Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995 Jan 1;181(1):435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicoff M., Sell C., Rubini M., Coppola D., Ambrose D., Baserga R., Rubin R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994 Apr 15;54(8):2218–2222. [PubMed] [Google Scholar]
- Schwarz L. C., Gingras M. C., Goldberg G., Greenberg A. H., Wright J. A. Loss of growth factor dependence and conversion of transforming growth factor-beta 1 inhibition to stimulation in metastatic H-ras-transformed murine fibroblasts. Cancer Res. 1988 Dec 15;48(24 Pt 1):6999–7003. [PubMed] [Google Scholar]
- Seung S., Urban J. L., Schreiber H. A tumor escape variant that has lost one major histocompatibility complex class I restriction element induces specific CD8+ T cells to an antigen that no longer serves as a target. J Exp Med. 1993 Sep 1;178(3):933–940. doi: 10.1084/jem.178.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E., Sendo F. Abrogation of tumor-inhibitory MRC-OX8+ (CD8+) effector T-cell generation in rats by selective depletion of neutrophils in vivo using a monoclonal antibody. Int J Cancer. 1993 Apr 22;54(1):131–136. doi: 10.1002/ijc.2910540121. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G., Beauchamp R. D., Koeppen H., Park B. H., Schreiber H., Moses H. L., Rowley D. A. A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1486–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Vanky F., Klein E. Lymphocyte cytotoxicity against autologous tumour biopsy cells in humans. Int J Cancer. 1977 Oct 15;20(4):512–519. doi: 10.1002/ijc.2910200407. [DOI] [PubMed] [Google Scholar]
- Ward P. L., Koeppen H. K., Hurteau T., Rowley D. A., Schreiber H. Major histocompatibility complex class I and unique antigen expression by murine tumors that escaped from CD8+ T-cell-dependent surveillance. Cancer Res. 1990 Jul 1;50(13):3851–3858. [PubMed] [Google Scholar]
- Ward P. L., Koeppen H., Hurteau T., Schreiber H. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologous normal cells. J Exp Med. 1989 Jul 1;170(1):217–232. doi: 10.1084/jem.170.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kuribayashi K., Miyatake S., Nishihara K., Nakayama E., Taniyama T., Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]



