Abstract
Aims
Clinical outcomes following radiofrequency ablation of ventricular tachycardias (VTs) depend on catheter tip-to-tissue contact force (CF). Left-ventricular (LV) mapping is performed via antegrade-transseptal or retrograde-transaortic approaches, and the applied CF may depend on the approach used. This study evaluated (i) the impact of antegrade-transseptal vs. retrograde-transaortic LV-mapping approaches on CF and catheter stability and (ii) the clinical value of the commonly used surrogate markers of catheter–myocardial contact—impedance, unipolar, and bipolar electrogram amplitudes.
Methods and results
An antegrade-transseptal and a retrograde-transaortic LV-mapping approach was performed in 10 patients undergoing VT ablation by using CF-sensing catheters. Operators were blinded to CF data and data were analysed according to 11 predefined LV segments. Three thousand three hundred and twenty-four mapping points (1577 antegrade, 1747 retrograde) were analysed, including 80 (2.4%) points with maximum CF > 100 g. Median antegrade and retrograde CF were 16.0 g (q1–q3; 8.4–26.2) and 15.3 g (9.8–23.4), respectively. Contact force was significantly higher antegradely in mid-anteroseptum, mid-lateral, and apical segments, and significantly higher retrogradely in basal-anteroseptum, basal-inferoseptum, basal-inferior, and basal-lateral segments. Contact force did correlate with impedance, unipolar, and bipolar electrogram amplitudes; however, there were large overlaps.
Conclusions
Antegrade vs. retrograde LV-mapping approaches result in different CF. A combined approach to the LV mapping may improve the overall LV mapping, potentially resulting in better clinical outcomes for the left VT catheter ablation. The previous surrogate markers used to assess CF do correlate with in vivo CF; however, due to a larger overlap, their clinical value is limited.
Keywords: Catheter ablation, Ventricular tachycardia, Contact force
What's new?
Either an antegrade (transseptal) or a retrograde (aortic) approach is currently used for mapping and radiofrequency (RF) ablation of the left ventricle (LV) for ventricular arrhythmia ablation, which may produce different applied contact force (CF) in different segments of the LV.
Catheter-to-myocardial tissue CF has been demonstrated as an important parameter in electroanatomical mapping and RF lesion formation, and a novel ablation catheter which measures CF has been recently developed, which may decrease ineffective lesion formation, as well as reduce complication risk from an excessively high CF.
A combined approach to LV mapping and ablation may produce improved results, potentially leading to better clinical outcomes for LV ventricular tachycardia ablation.
The clinical value of previous surrogate markers for CF, such as impedance, unipolar, and bipolar amplitudes, correlated with in vivo CF; however, due to a larger overlap, their clinical value is limited.
Introduction
In recent years, the frequency of catheter ablation for the treatment of ventricular arrhythmias has dramatically increased. Pre-clinical data have demonstrated that the catheter-to-tissue contact force (CF) is an important parameter in electroanatomical mapping and radiofrequency (RF) lesion formation.1 An excessively high CF not only increases the risk of serious complications such as cardiac perforation, steam pop, and thrombus formation, it can lead to inaccurate data acquisition and cardiac chamber geometry distortion.2,3 On the other hand, an insufficient CF can result in ineffective lesion formation, therefore poorer clinical outcomes.4 Mizuno et al.5 recently demonstrated that mapping with sufficient CF produces a better substrate characterization within the pathological areas, and that a stable CF of >8 g is required to predict good contact even during the diastolic phase of the cardiac cycle.
Today, the importance of an appropriate CF during left-ventricle (LV) mapping and ablation is widely accepted. Until recently, however, only surrogate parameters for CF have been used to guide RF ablation, such as catheter tip temperature, impedance, unipolar, and bipolar electrogram amplitudes. These surrogates can be inaccurate, and their correlation to CF in vivo has not been fully validated.6–8 Recently, CF-sensing catheters have been developed to provide real-time CF information during mapping and ablation. However, due to their novel nature, CF data during LV mapping and ablation are sparse in the literature.5
Left-ventricular mapping can be achieved either via an antegrade-transseptal approach, a retrograde-transaortic approach, or a combined approach as per the operator's preference. To our knowledge, a direct comparison of the three approaches using CF catheters has not yet been published; therefore, the optimal mapping approach is still controversial. The aim of this study was to evaluate CF during LV mapping by using the novel CF catheter, and we report the first direct comparison of the impact of the antegrade-transseptal and the retrograde-transaortic LV-mapping approaches on CF and catheter stability. We also evaluate the relationship between CF and the previously used surrogate markers for catheter–myocardial contact—impedance, unipolar, and bipolar electrogram amplitudes.
Methods
Study population
This study population consisted of 10 patients (age 55 ± 15 years, nine males) who underwent catheter ablation for recurrent, drug refractory ventricular arrhythmia. Five patients underwent ablation for ischaemic ventricular tachycardia (VT), four patients for idiopathic premature ventricular contractions and VT, and one patient for VT following myocarditis. The mean ejection fraction was 45 ± 14%, and the mean LV end-diastolic diameter was 59 ± 10 mm, respectively. Exclusion criteria included prior LV ablation procedures and paced ventricular rhythm. Written informed consent for the procedure was obtained from all the patients.
Left-ventricular mapping
All the patients underwent coronary angiography to exclude acute ischaemia as a trigger for ventricular arrhythmia, and transthoracic echocardiography was performed prior to each procedure to rule out LV thrombus. In patients with known atrial fibrillation, transoesophageal echocardiography was performed to rule out left atrial thrombus. No other pre-procedural imaging, such as cardiac computed tomography or intracardiac echocardiography, was performed. The procedure was performed under deep sedation with midazolam, fentanyl, and continuous infusion of propofol. For the antegrade approach, venous access was obtained via the right femoral vein, and following transseptal puncture, one 8.5 French SL1 sheath (St Jude Medical) was advanced to the left atrium. For the retrograde approach, one SR0 sheath (St Jude Medical) was inserted via the right femoral artery, and the end of the sheath was advanced near the aortic arch. Left-ventricular mapping was performed during sinus rhythm or atrial pacing by using a commercially available 7.5 French, irrigated-tip CF-sensing catheter (Thermocool® Smart Touch™, Biosense Webster, Inc.). To acquire mapping points only in stable catheter positions, the catheter was maintained at each mapping point for at least 2 s prior to point acquisition. Only points with a stable catheter position and with stable local EGMs recorded over a period of at least 2 s were acquired. A low-voltage area was defined as voltage <1.5 mV.
In this study, the LV was arbitrarily divided into 11 predefined segments: basal-anterior, mid-anterior, basal-anteroseptum, mid-anteroseptum, basal-inferoseptum, mid-inferoseptum, basal-inferior, mid-inferior, basal-lateral, mid-lateral, and apex (Figures 1 and 2). For each patient, two separate three-dimensional (3D) electroanatomical maps (CARTO 3™, Biosense Webster) were acquired, each with a minimum of 100 mapping points: one map was performed by using an antegrade (transseptal) approach and the other by using a retrograde (transaortic) approach.
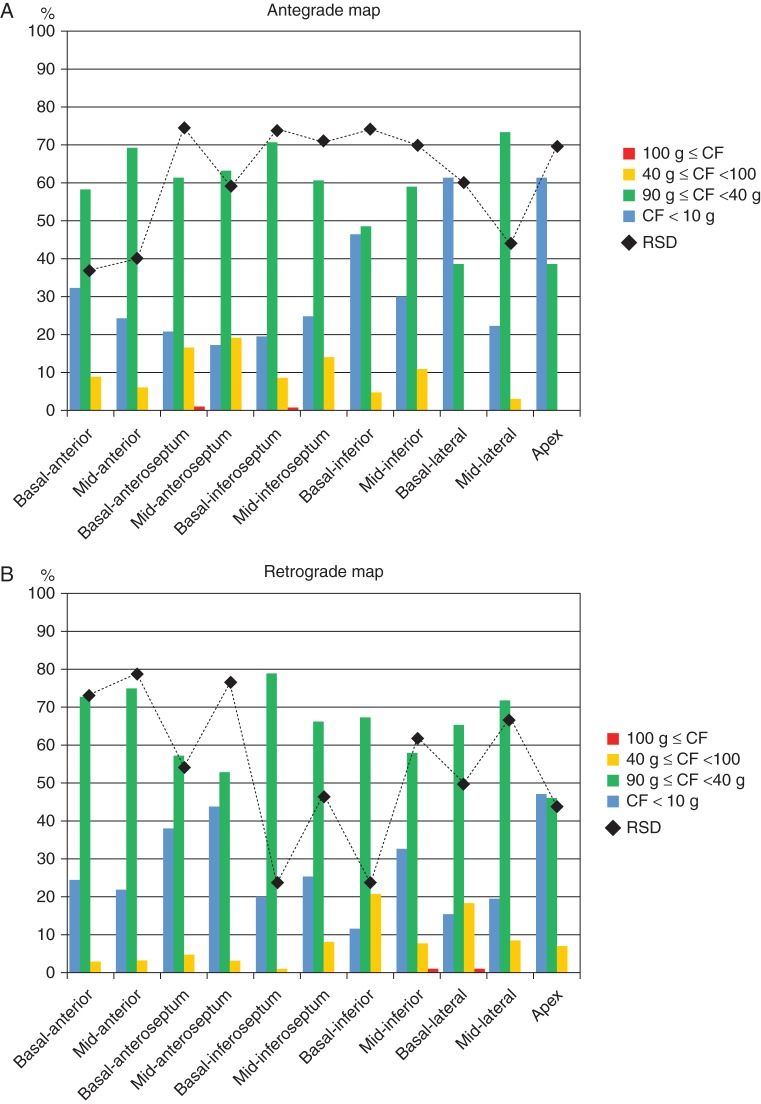
Figure 1.
Comparison of CF and RSD of CF during antegrade and retrograde mapping. Distribution of CF varied widely according to predefined segments and approaches. The bars are colour-coded, indicating the percentage of CF recorded in the predefined areas (Y-axis). Relative standard deviation values also varied widely according to the predefined segments and approaches (black dots, Y-axis). See the text for details. (A) Antegrade approach. (B) Retrograde approach.
Figure 2.
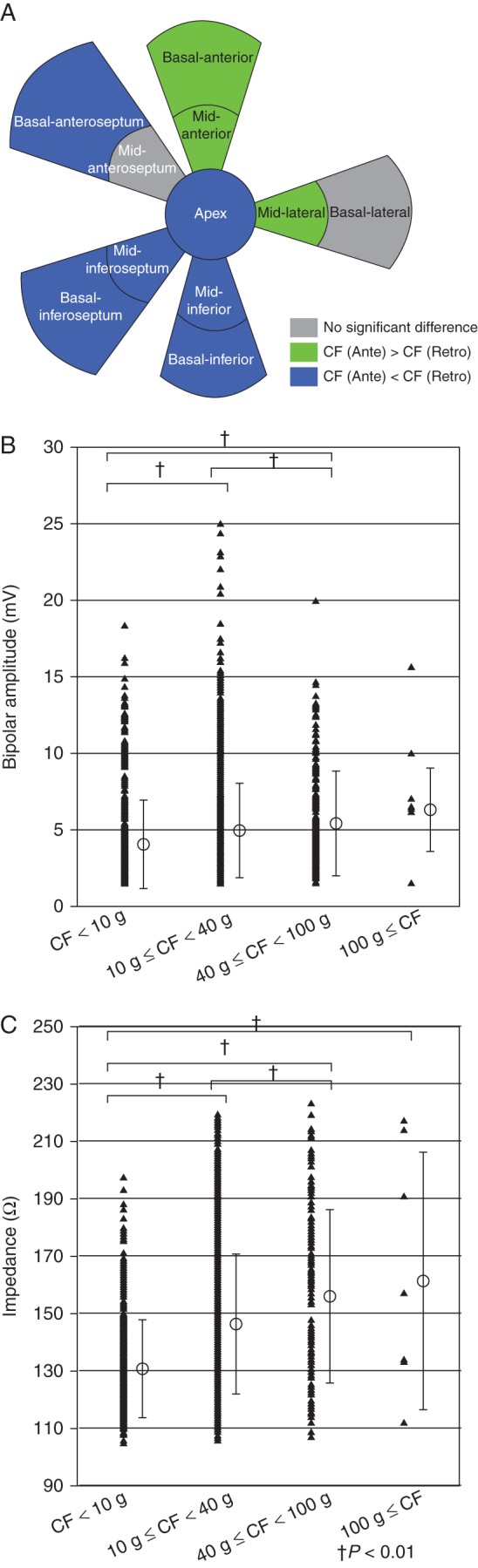
Contact force distribution and CF relationship with bipolar amplitude and tissue impedance. (A) Comparison of median CF between antegrade and retrograde approaches. Green colour indicates CF antegrade > CF retrograde, blue colour indicates CF antegrade < CF retrograde, and grey colour indicates no difference. (B) Relationship between CF and bipolar amplitude. Contact force and local bipolar amplitude showed a significant association; however, there were large overlaps for a given CF. (C) Relationship between CF and tissue impedance. Contact force and tissue impedance showed a significant association; however, there were large overlaps for a given CF.
Contact force measurement during mapping
Ablation was performed by using a CF-sensing catheter with a 3.5 mm irrigated-tip connected by a tiny spring to the shaft of the catheter. The catheter tip CF and direction are obtained with a resolution of <1 g every 50 ms by measuring the degree of spring deformation. This is then transmitted via a magnetic transmitter at the catheter tip to three location sensors in the catheter shaft. After a 20 min warmup period where the catheter was positioned in the heart, calibration of the CF-sensing catheter when the catheter tip is free in the LV was performed prior to 3D electroanatomical mapping, as assessed on fluoroscopy and local EGMs. Recalibration was performed every 30 min, or when the catheter was moved into the sheath or removed from the patient. Operators performing the procedure as well as at the electroanatomical mapping system were blinded to CF and contact information during the procedure; however, data were registered by using the 3D electroanatomical system (CARTO® 3, Biosense Webster Inc.) with CARTO® 3 SMARTTOUCH™ Software Module.
Contact force values were measured every 50 ms. For each mapping point, CF data were analysed over a 1.0 s period to cover at least one ventricular beat. The mean CF at each mapping point was arbitrarily classified as low CF (<10 g), moderate CF (10–39 g), high CF (40–99 g), or excessively high CF (≥100 g). Mean CF values, unipolar, and bipolar electrogram amplitudes were then recorded and analysed.
As a parameter of catheter stability during mapping, the relative standard deviation (RSD) was calculated at each mapped point (duration 1.0 s) as:
Lower RSD, or lower variation, is presumed to reflect a more stable catheter placement. This parameter has been previously validated in the left atrium in our institution, and further data have been submitted for publication.9
Statistical analysis
For a statistical analysis of our data, Wilcoxon's test, the χ2 test, Student's t-test, or one-way analysis of variance were performed as appropriate, and a P value of <0.05 was considered statistically significant. Continuous data were shown as mean ± SD for normally distributed data, and otherwise as median values (first quartile–third quartile). No sample size calculation was performed. All authors had full access to the collected data and have read and concur with the manuscript as written. Statistical analysis was performed by using JMP 9.0 software package (SAS Institute, Inc.).
Results
Contact force during left-ventricular mapping
Mean heart rate during mapping was 71 ± 9 b.p.m. No complications occurred during mapping or ablation in this study. A total of 3324 LV-mapping points were acquired and analysed in 10 patients (Tables 1–4). In five patients, a pathological, low-voltage area was identified. Mean CF during LV mapping was 15.6 g (25.0 − 9.3), and ranged from 10.1 g (5.6–17.2) in the basal-anteroseptal segment to 18.9 g (12.6–32.4), respectively, in the basal-inferior segment. An excessively high mean CF (≥100 g) was noted in four mapping points (0.1%) and located in the mid-inferior (n = 2), and mid-lateral (n = 2) segments. A transient excessively high CF was noted in 66 mapping points (1.9%). These were located in the mid-lateral (n = 19), basal-lateral (n = 16), apical (n = 10), mid-inferior (n = 8), basal-inferior (n = 6), mid-anterior (n = 4), basal-inferoseptal (n = 2), and basal-anteroseptal (n = 1) segments.
Table 1.
Patient characteristics
| Patient characteristics | N = 10 |
|---|---|
| Age | 55 ± 15 |
| Male | 9 (90%) |
| Old myocardial infarction | 5 (50%) |
| Idiopathic VT/VES | 4 (40%) |
| After myocarditis | 1 (10%) |
| Left ventricular ejection fraction (%) | 45 ± 14 |
| Left ventricular end-diastolic diameter (mm) | 59 ± 10 |
| Electrical storm | 5 (50%) |
Table 4.
Catheter stability data according to the predefined segments
| Antegrade median RSD (%) | Retrograde median RSD (%) | P value | Antegrade continuous positive CF %Points | Retrograde continuous positive CF %Points | P value | |
|---|---|---|---|---|---|---|
| Basal-anterior | 37.0 | 73.4 | <0.0001 | 44.0 | 26.5 | 0.004 |
| Mid-anterior | 39.8 | 78.9 | <0.0001 | 50.0 | 26.3 | <0.0001 |
| Basal-anteroseptum | 74.6 | 54.2 | 0.0051 | 24.7 | 21.7 | 0.63 |
| Mid-anteroseptum | 59.1 | 76.6 | 0.15 | 40.2 | 25.8 | 0.013 |
| Basal-inferoseptum | 74.1 | 23.6 | <0.0001 | 26.4 | 43.6 | 0.0087 |
| Mid-inferoseptum | 71.0 | 46.6 | <0.0001 | 35.1 | 34.5 | 0.92 |
| Basal-inferior | 74.1 | 23.6 | <0.0001 | 42.5 | 67.9 | <0.0001 |
| Mid-inferior | 70.0 | 62.0 | <0.0001 | 38.8 | 34.6 | 0.44 |
| Basal-lateral | 60.1 | 49.9 | 0.089 | 38.0 | 70.6 | <0.0001 |
| Mid-lateral | 43.9 | 66.8 | 0.035 | 55.1 | 37.7 | 0.0003 |
| Apex | 69.7 | 43.6 | <0.0001 | 56.6 | 41.2 | 0.0084 |
| Total | 61.9 | 54.3 | <0.0001 | 42.5 | 41.4 | 0.52 |
Comparison of antegrade and retrograde left-ventricular mapping
For LV mapping in this study, a median of 156 points was acquired by using the antegrade approach, and 169 points by using the retrograde approach (P = 0.23) (Tables 2 and 3). Median antegrade and retrograde CF were 20.2 ± 16.0 and 19.1 ± 14.0 g, respectively (P = 0.048) (Figure 1). There was no statistically significant difference between the antegrade and retrograde LV maps for the parameters of LV volume, LV surface area, and LV low-voltage area (Table 3). Mapping duration also showed no significant difference (Table 3).
Table 2.
Contact force according to the predefined segments
| Antegrade median No. of points | Retrograde median No. of points | Antegrade median CF (g) | Retrograde median CF (g) | P value | Antegrade %Points ≥10 g | Retrograde %Points ≥10 g | P value | |
|---|---|---|---|---|---|---|---|---|
| Basal-anterior | 10 | 16 | 13.7 (7.1–18.0) | 13.2 (9.8–18.0) | 0.94 | 70.0 | 75.5 | 0.34 |
| Mid-anterior | 10 | 14 | 15.3 (9.3–28.1) | 14.4 (9.8–18.7) | 0.083 | 75.0 | 78.3 | 0.51 |
| Basal-anteroseptum | 3 | 9 | 7.7 (5.1–13.8) | 13.8 (7.3–17.7) | 0.0022 | 38.7 | 62.0 | 0.0016 |
| Mid-anteroseptum | 16 | 16 | 14.6 (9.5–20.5) | 10.8 (7.0–16.1) | <0.0001 | 76.6 | 56.1 | <0.0001 |
| Basal-inferoseptum | 9 | 11 | 12.0 (6.0–16.1) | 15.3 (10.8–20.8) | 0.0015 | 63.2 | 80.0 | 0.0087 |
| Mid-inferoseptum | 12 | 10 | 10.4 (6.3–19.7) | 13.1 (9.4–21.3) | 0.0072 | 53.4 | 74.3 | 0.0005 |
| Basal-inferior | 12 | 19 | 16.8 (7.6–25.1) | 18.7 (13.1–34.6) | 0.0007 | 75.6 | 88.2 | 0.0033 |
| Mid-inferior | 14 | 19 | 16.9 (10.5–27.1) | 14.5 (7.6–25.3) | 0.083 | 80.3 | 67.0 | 0.0074 |
| Basal-lateral | 21 | 21 | 15.3 (7.5–22.5) | 19.9 (12.4–35.6) | <0.0001 | 67.5 | 84.4 | <0.0001 |
| Mid-lateral | 20 | 22 | 18.1 (10.9–28.8) | 15.7 (10.6–24.0) | 0.029 | 79.0 | 80.3 | 0.75 |
| Ape): | 16 | 11 | 19.8 (13.7–32.8) | 10.6 (6.6–17.2) | <0.0001 | 82.5 | 52.7 | <0.0001 |
| Total | 156 | 169 | 16.0 (8.4–26.2) | 15.3 (9.8–23.4) | 0.44 | 71.0 | 74.1 | 0.041 |
Table 3.
Three-dimensional mapping data of the two approaches
| Antegrade map | Retrograde map | P value | |
|---|---|---|---|
| Acquired points | 158 ± 33 | 175 ± 24 | 0.21 |
| LV volume (cm3) | 150 ± 53 | 165 ± 63 | 0.79 |
| LV surface (cm2) | 151 ± 31 | 151 ± 36 | 0.98 |
| Low-voltage area (cm2) | 30.9 ± 36.0 | 30.3 ± 37.4 | 0.98 |
| Duration of mapping (min) | 24 ± 9.2 | 30 ± 9.1 | 0.16 |
LV, left ventricle.
A significant variability in CF was observed among the different predefined segments (Table 2, Figure 1). Via the antegrade approach, mean CF ranged from 7.7 g (5.1–13.8) at the basal-anteroseptum to 19.8 g (13.7–32.8) at the apex (Figure 1), respectively. Via the retrograde approach, mean CF ranged from 10.6 (6.6–17.2) at the apex to 19.9 g (12.4–35.6) at the basal-lateral segment, respectively (Figure 1). Mean CF was significantly higher when using the antegrade approach in the mid-anteroseptum, mid-lateral, and apex, and significantly higher when using the retrograde approach in the basal-anteroseptum, basal-inferoseptum, basal-inferior, and basal-lateral segments (Figure 2A).
As shown in Table 2, low CF < 10 g was noted with a significantly higher incidence via the antegrade approach compared with the retrograde approach in the basal-anteroseptum, basal-inferoseptum, basal-inferior, and basal-lateral segments. In contrast, the retrograde approach led to significantly higher incidences of low CF compared with the antegrade approach in the mid-anteroseptum, mid-inferior, and apex segments.
Relative standard deviation of contact force
The RSD of CF for each predefined segment is presented in Table 4. Median antegrade and retrograde RSD were 61.9 and 54.3%, respectively (P < 0.0001). This suggests that, as a whole, better catheter stability was achieved during the retrograde approach. Relative standard deviation was similar in the mid-anteroseptum and significantly smaller antegradely than retrogradely in the anterior segment, mid-anteroseptum, and mid-lateral segment. There was a trend towards smaller RSDs retrogradely than antegradely in the basal-lateral segment, and was significantly smaller in the remaining areas (Figure 3).
Figure 3.
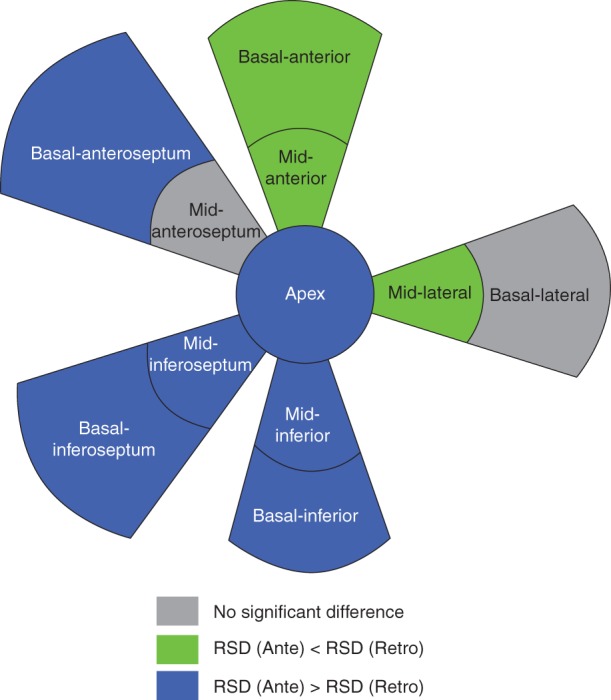
Comparison of catheter stability between antegrade and retrograde approaches. Green indicates RSD antegrade < RSD retrograde, blue indicates RSD antegrade > RSD retrograde, and grey indicates no difference. Smaller RSD was considered to be associated with a more stable catheter placement. RSD, relative standard deviation.
Relationship between contact force, unipolar and bipolar amplitudes, contact force, and impedance
Out of 3324 mapping points, 2486 points (74.8%) had a voltage of >1.5 mV and were consequently included in this section of analysis. The relationship between CF and bipolar amplitude is shown in Figure 2B, and between CF and tissue impedance in Figure 2C. Contact force values did correlate with unipolar (P < 0.001, R = 0.095) and bipolar (P < 0.001, R = 0.11) amplitudes and tissue impedance (P = 0.001, R = 0.30), respectively. However, unipolar and bipolar amplitudes, and tissue impedance for any given CF varied widely, and there were large overlaps.
Discussion
This is the first clinical study that directly compares the antegrade-transseptal LV-mapping strategy with the retrograde-transaortic approach, by using a novel CF-sensing catheter. We demonstrated that (i) the range of CF observed during the LV catheter mapping and the ablation varies significantly, from a low CF (<10 g) to a high CF (>40 g) and excessively high CF (>100 g), respectively, which may result in complications such as cardiac tamponade; (ii) previously used surrogate markers such as electrogram amplitudes and impedance values for assessment of good or adequate catheter-to-myocardial tissue contact correlated well with CF values obtained by using the novel CF catheters; however, large variations in the surrogate values for any given CF indicate that these markers were inferior compared with CF and thus of limited use in clinical practice; and (iii) both CF and RSD showed a large variation with respect to the predefined segments between the two different approaches.
Until recently, one important parameter for lesion formation that could not be assessed in vivo was CF. The previous studies have demonstrated that a low CF results in inadequate lesion formation.3,10 For example, Di Biase et al. demonstrated in an animal model that regardless of the power used, no RF ablation points formed with a force of <10 g translated to a transmural lesion. Our data have identified that there is a significant difference in the distribution of areas of low CF depending on the mapping approach used.
More importantly, an inappropriately high CF is associated with potentially life-threatening complications including steam pops and pericardial tamponade secondary to myocardial perforation.1–4,11–13 A recent study found that a majority of lesions placed by using high power (45 W) and high pressures (>40 g), respectively, were associated with char and crater formation (66.7%).3 Shah et al.13 also showed that maximum CF exceeding 100 g during catheter manipulation and ablation is associated with a significant risk of cardiac perforation.
By using the novel CF-sensing catheter, we demonstrated that during an ablation procedure, significant variations in CF occur, ranging from a low CF (<10 g) to a transient excessively high CF (>100 g). Contact force-sensing catheters allow operators to avoid the formation of insufficient ablation lesions during a poor catheter-to-myocardial contact, thus potentially improving the success rates and clinical outcomes. More importantly, by avoiding inappropriately high CF, this technology has the potential to reduce complications.1–4,11–13
Many indirect surrogate markers of good or adequate catheter-to-myocardial contact have been described, including direct catheter visualization as well as temperature, impedance, unipolar, and bipolar electrogram amplitudes.6–8,14,15 The previous literature have shown that these markers are influenced by several parameters such as temperature, body size, and cabling.16,17 In this study, we assessed the relationship between CF and the surrogate markers of impedance, unipolar, and bipolar electrode amplitudes. We found that there was a statistically significant association between the CF and these surrogate markers; however, at any given CF point recorded, there was a large overlap in surrogate marker values. This indicates that the accuracy of these surrogate markers is inferior to CF, and their use is thus of limited value in clinical practice.
Our study is the first to directly compare the antegrade-transseptal LV-mapping approach to the retrograde-transaortic approach. In 2008, the first studies using the novel irrigated-tip CF-sensing catheters were performed in animal models. These studies have qualified the usefulness of CF to predict lesion size during catheter ablation, and to predict the likelihood of complications such as steam pop and myocardial perforation from excessive force.2,4,11,12 The first human studies, published in 2012, looked at the ablation of atrial tachyarrhythmias, and confirmed the effectiveness of CF for improved lesion formation, as well as its safety profile and capacity to reduce complication rates.18–26
In our study, we demonstrated that the overall median CF showed no significant differences between the antegrade and retrograde approach; however, median CF varied widely with respect to the specific predefined segments between the antegrade and retrograde approaches. Moreover, catheter stability also showed wide variation with respect to the predefined segments between the two approaches. In addition, we found that some LV segments can be better reached with an antegrade strategy while other segments with a retrograde strategy, and that the two approaches can compliment each other. This suggests that a combined transaortic and transseptal approach to LV mapping and ablation may improve the accuracy of the LV map, as well as improving the adequacy of the ablation lesions.
Although not statistically significant, the mean mapping duration was ∼6 min longer via the retrograde approach. One explanation for longer mapping durations is that via the retrograde approach, it may sometimes be difficult to pass through the aortic valve, particularly in patients with arteriosclerosis.
To date, Mizuno et al. have described the only other study in the literature assessing the use of CF-sensing catheters in LV tachyarrhythmia mapping and ablation.5 Their study compared a combined antegrade and retrograde LV strategy with a retrograde-only approach, and demonstrated that the clinical outcomes were superior with a combined approach. They also showed that mapping with adequate and stable CF produces a better substrate characterization within the pathological areas, and that a CF > 8 g is required to predict adequate contact throughout the whole cardiac cycle. They proposed that the reason for poorer CF values during the retrograde approach may be related to the requirement of two curves in the mapping catheter to reach the anterior and basal-septal walls of the LV. However, their study did not directly compare an antegrade with a retrograde mapping approach.
Mizuno et al.5 have also demonstrated that a ‘continuous positive’ CF predicted a stable catheter placement. We evaluated the relationship between RSD values and mapped points with continuous positive CF values (Table 4). Relative standard deviation, which we adopted as a parameter of catheter stability, showed a significant association with the positive CF value. Diagnostic performance of RSD for continuous positive CF values was evaluated by receiver operator characteristic (ROC) analysis. Area under the ROC curve was 0.82. When the cutoff value was defined as the nearest point to the top left corner of the ROC curve, the cutoff value of RSD was 58.7%. On using this cutoff value, specificity and sensitivity were 82.7 and 74.0%, respectively.
It is important to mention that in different studies, different CF-sensing technologies were used and different CF values were measured. In this study, we used the Thermocool® Smart Touch™ catheter (Biosense Webster). The tip of this catheter is connected to the proximal ring electrode via a tiny spring. The force applied to the tissue results in the spring compressing and/or stretching, and its magnitude and direction are monitored by the tracking of a magnetic signal between a transmitter located in the tip and three receiving sensors in the shaft of the catheter. In general, the mean CF is displayed. In contrast, the study by Shah et al.13 used a CF catheter (Tacticath, Endosense SA) with an optical fibre-based force sensor that measures the maximum CF mounted at the tip electrode. Di Biase et al.3 used the Intellisense™ system (Hansen Medical) that measures the resistance of the moving catheter within a robotic sheath. At the present time, no direct comparison of these different technologies have been made, hence we do not know the impact of these CF-sensing technologies on the overall results.
Limitations
According to our institutional standards, non-steerable sheaths were used during antegrade-transseptal LV mapping in our study. The steerable sheaths may have impact on CF and catheter stability. Shah et al.13 demonstrated in an animal study that the utilization of steerable sheaths during an antegrade approach may have a significant impact on catheter CF. In addition, according to our institutional standards we adopted the SR0 sheath for the retrograde approach, which may have affected catheter manipulation compared with the other sheaths.
During LV mapping, multiple factors in addition to CF may influence unipolar and bipolar signal amplitudes, such as myocardial thickness, myocardial surface (e.g. papillary muscle, pouch, smooth surface), myocardial scar, and catheter position (parallel vs. perpendicular). However, in vivo, it is not feasible to evaluate the impact of each of these factors on the signal amplitude in thousands of mapping points.
The number of patients in the study population is small, as in many published VT pilot studies, and no sample size calculation was performed to determine the study population. However, we aimed to evaluate the correlation of the CF with the previous surrogate markers by using the novel CF catheter, and assess its potential feasibility and safety in improving the success rate in VT ablation.27–30 In addition, the patients in our study were highly selected with preserved LV functions and small LV volumes, compared with the majority of VT patients who have structural heart disease, which may influence the CF values and catheter stability.
Finally, there is to date no data to support the improvement of mapping accuracy and clinical outcomes by using CF information.
Conclusions
A large range of CF can be observed during LV mapping, varying from low CF (<10 g) to high (>40 g) and transient excessively high CF (>100 g). The previous surrogate markers used to assess CF do correlate with in vivo CF; however, due to a larger overlap, their clinical value is therefore limited. Thus, the CF-guided LV mapping and ablation may have the potential to decrease the rates of complications due to an excessively high CF, and improve the clinical outcomes by allowing operators to avoid very low CF.
In addition, CF values as well as RSD values, which indicate catheter stability, varied widely with respect to the different segments of the LV between the antegrade and retrograde approaches. Our study suggests that a combined antegrade and retrograde approach may improve the accuracy of LV mapping, and potentially improve clinical outcomes of catheter ablation for left VT.
Funding
Funding to pay the Open Access publication charges for this article was provided by Biosense Webster Inc.
Acknowledgments
Conflict of interest: R.R.T. received travel grants, research grants, and speaker honoraria from Biosense Webster and St Jude Medical. H.M. received travel grants from Biosense Webster. A. R. received travel grants from St Jude Medical and Biosense Webster. K.-H.K. received research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, Cryocath, and Biotronik, he is a consultant to St Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis, and scientific advisor and shareholder of Endosense. T.L. received a fellowship grant from St Jude Medical.
References
- 1.Okumura Y, Johnson SB, Bunch TJ, Henz BD, O'Brien CJ, Packer DL. A systematical analysis of in vivo contact forces on virtual catheter tip/tissue surface contact during cardiac mapping and intervention. J Cardiovasc Electrophysiol. 2008;19:632–40. doi: 10.1111/j.1540-8167.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–62. doi: 10.1161/CIRCEP.108.803650. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L, Natale A, Barrett C, Tan C, Elayi CS, Ching CK, et al. Relationship between catheter forces, lesion characteristics, ‘Popping’, and char formation: experience with robotic navigation system. J Cardiovasc Electrophysiol. 2009;20:436–40. doi: 10.1111/j.1540-8167.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- 4.Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real-time contact force curve (force-time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol. 2010;21:1038–43. doi: 10.1111/j.1540-8167.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno H, Vergara P, Maccabelli G, Trevisi N, Eng SC, Brombin C, et al. Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol. 2013;24:519–24. doi: 10.1111/jce.12080. [DOI] [PubMed] [Google Scholar]
- 6.Wittkampf FHM, Nakagawa H. RF catheter ablation: lessons on lesions. Pacing Clin Electrophysiol. 2006;29:1285–97. doi: 10.1111/j.1540-8159.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 7.Avitall B, Mughal K, Hare J, Helms R, Krum D. The effects of electrode-tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol. 1997;20:2899–910. doi: 10.1111/j.1540-8159.1997.tb05458.x. [DOI] [PubMed] [Google Scholar]
- 8.Thiagalingam A, D'Avila A, McPherson C, Malchano Z, Ruskin J, Reddy VY. Impedance and temperature monitoring improve the safety of closed-loop irrigated-tip radiofrequency ablation. J Cardiovasc Electrophysiol. 2007;18:318–25. doi: 10.1111/j.1540-8167.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 9.Makimoto H, Metzner A, Lin T, Rillig A, Wohlmuth P, Arya A, et al. In vivo contact force analysis and correlation with tissue impedance during left atrial mapping and catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013 doi: 10.1161/CIRCEP.113.000556. doi:10.1161/CIRCEP.113.000556. [DOI] [PubMed] [Google Scholar]
- 10.Matía Francés R, Hernández Madrid A, Delgado A, Carrizo L, Pindado C, Moro Serrano C, et al. Characterization of the impact of catheter-tissue contact force in lesion formation during cavo-tricuspid isthmus ablation in an experimental swine model. Europace. 2013 doi: 10.1093/europace/eut351. doi:10.1093/europace/eut351. [DOI] [PubMed] [Google Scholar]
- 11.Thiagalingam A, D'Avila A, Foley L, Guerrero JL, Lambert H, Leo G, et al. Importance of catheter contact force during irrigated radiofrequency ablation: evaluation in a porcine ex vivo model using a force-sensing catheter. J Cardiovasc Electrophysiol. 2010;21:806–11. doi: 10.1111/j.1540-8167.2009.01693.x. [DOI] [PubMed] [Google Scholar]
- 12.Perna F, Heist EK, Danik SB, Barrett CD, Ruskin JN, Mansour M. Assessment of catheter tip contact force resulting in cardiac perforation in swine atria using force sensing technology. Circ Arrhythm Electrophysiol. 2011;4:218–24. doi: 10.1161/CIRCEP.110.959429. [DOI] [PubMed] [Google Scholar]
- 13.Shah D, Lambert H, Langenkamp A, Vanenkov Y, Leo G, Gentil-Baron P, et al. Catheter tip force required for mechanical perforation of porcine cardiac chambers. Europace. 2011;13:277–83. doi: 10.1093/europace/euq403. [DOI] [PubMed] [Google Scholar]
- 14.Strickberger SA, Vorperian VR, Man KC, Williamson BD, Kalbfleisch SJ, Hasse C, et al. Relation between impedance and endocardial contact during radiofrequency catheter ablation. Am Heart J. 1994;128:226–9. doi: 10.1016/0002-8703(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Walcott GP, Hall JA, Rollins DL, Smith WM, Kay GN, et al. Electrode impedance: an indicator of electrode-tissue contact and lesion dimensions during linear ablation. J Interv Card Electrophysiol. 2000;4:645–54. doi: 10.1023/a:1026586119600. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Hulse JE, Walsh EP, Saul JP. Factors influencing impedance during radiofrequency ablation in humans. Chin Med J. 1995;108:450–5. [PubMed] [Google Scholar]
- 17.Kumar S, Haqqani HM, Chan M, Lee J, Yudi M, Wong MCG, et al. Predictive value of impedance changes for real-time contact force measurements during catheter ablation of atrial arrhythmias in humans. Heart Rhythm. 2013;10:962–9. doi: 10.1016/j.hrthm.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Kuck K-H, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, et al. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm. 2012;9:18–23. doi: 10.1016/j.hrthm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–95. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Morton JB, Halloran K, Spence SJ, Lee G, Wong MCG, et al. Effect of respiration on catheter-tissue contact force during ablation of atrial arrhythmias. Heart Rhythm. 2012;9:1041–7. doi: 10.1016/j.hrthm.2012.02.015. e1041. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Morton JB, Lee J, Halloran K, Spence SJ, Gorelik A, et al. Prospective characterization of catheter-tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2012;5:1124–9. doi: 10.1161/CIRCEP.112.972208. [DOI] [PubMed] [Google Scholar]
- 22.Haldar S, Jarman JWE, Panikker S, Jones DG, Salukhe T, Gupta D, et al. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. Int J Cardiol. 2013;168:1160–6. doi: 10.1016/j.ijcard.2012.11.072. [DOI] [PubMed] [Google Scholar]
- 23.Kerst G, Weig H-J, Weretka S, Seizer P, Hofbeck M, Gawaz M, et al. Contact force-controlled zero-fluoroscopy catheter ablation of right-sided and left atrial arrhythmia substrates. Heart Rhythm. 2012;9:709–14. doi: 10.1016/j.hrthm.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment. Circ Arrhythm Electrophysiol. 2013;6:327–33. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- 25.Sacher F, Wright M, Derval N, Denis A, Ramoul K, Roten L, et al. Endocardial versus epicardial ventricular radiofrequency ablation: utility of in vivo contact force assessment. Circ Arrhythm Electrophysiol. 2013;6:144–50. doi: 10.1161/CIRCEP.111.974501. [DOI] [PubMed] [Google Scholar]
- 26.Stabile G, Solimene F, Calò L, Anselmino M, Castro A, Pratola C, et al. Catheter-tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace. 2014;16:335–40. doi: 10.1093/europace/eut262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy VY, Neuzil P, Taborsky M, Ruskin JN. Short-term results of substrate mapping and radiofrequency ablation of ischemic ventricular tachycardia using a Saline-Irrigated Catheter. J Am Coll Cardiol. 2003;41:2228–36. doi: 10.1016/s0735-1097(03)00492-3. [DOI] [PubMed] [Google Scholar]
- 28.Berruezo A, Fernandez-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, et al. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–21. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 29.Cesario DA, Vaseghi M, Boyle NG, Fishbein MC, Valderrábano M, Narasimhan C, et al. Value of high-density endocardial and epicardial mapping for catheter ablation of hemodynamically unstable ventricular tachycardia. Heart Rhythm. 2006;3:1–10. doi: 10.1016/j.hrthm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Berruezo A, Mont L, Nava S, Chueca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–7. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]