Abstract
Introduction:
Pre-exposure prophylaxis (PrEP) may be an important safer conception strategy for HIV-1–uninfected women with HIV-1–infected partners. Understanding medication adherence in this population may inform whether PrEP is a feasible safer conception strategy.
Methods:
We evaluated predictors of pregnancy and adherence to study medication among HIV-1–uninfected women enrolled in a randomized placebo-controlled trial of PrEP among African HIV-1–serodiscordant couples. Participants were counseled on HIV-1 risk reduction, contraception, and adherence and tested for pregnancy at monthly study visits. Pill counts of dispensed drug were performed and, at a subset of visits, plasma was collected to measure active drug concentration.
Results:
Among 1785 women, pregnancy incidence was 10.2 per 100 person-years. Younger age, not using contraception, having an additional sexual partner, and reporting unprotected sex were associated with increased likelihood of pregnancy. Monthly clinic pill counts estimated that women experiencing pregnancy took 97% of prescribed doses overall, with at least 80% pill adherence for 98% of study months, and no difference in adherence in the periconception period compared with previous periods (P = 0.98). Tenofovir was detected in plasma at 71% of visits where pregnancy was discovered. By multiple measures, adherence was similar for women experiencing and not experiencing pregnancy (P ≥ 0.1).
Conclusions:
In this clinical trial of PrEP, pregnancy incidence was 10% per year despite excellent access to effective contraception. Women experiencing pregnancy had high medication adherence, suggesting that PrEP may be an acceptable and feasible safer conception strategy for HIV-1–uninfected women with HIV-1–serodiscordant partners.
Key Words: pregnancy, HIV-1 prevention, pre-exposure prophylaxis, adherence, safer conception, serodiscordant couples, sub-Saharan Africa
INTRODUCTION
For women in sub-Saharan Africa, having biologic children is important to secure a relationship, prove suitability as a spouse, maintain marriage, expand family lineage, and demonstrate health.1,2 For women at risk for HIV-1 acquisition, including women in HIV-1–serodiscordant relationships (where 1 partner is HIV-1 infected and the other is not), pregnancy desires are common3–6 but conception attempts risk sexual HIV-1 acquisition. HIV-1–uninfected women who attempt to conceive with an HIV-1–infected partner or a partner of unknown HIV-1 status need safe, feasible, and effective strategies to reduce HIV-1 acquisition risk.
Antiretroviral pre-exposure prophylaxis (PrEP) could be a key component of safer conception strategies for women in HIV-1–serodiscordant couples, particularly when the infected partner is not eligible, willing, or able to take antiretroviral treatment (ART).7–13 Oral tenofovir (TDF) and co-formulated emtricitabine (FTC)/TDF, the antiretrovirals studied in PrEP efficacy trials conducted to date, have an excellent safety profile among pregnant and breastfeeding women14,15; however, clinical trials of PrEP, like most trials of novel pharmacologic therapies, encouraged delaying pregnancy and withheld study drug during pregnancy to minimize fetal exposure. Nevertheless, pregnancy incidence has been high among HIV-1–uninfected women in trials of biomedical HIV-1 prevention interventions, including trials of PrEP.16–20 Given high pregnancy incidence and associated risks of HIV-1 acquisition, understanding correlates of pregnancy among women in HIV-1 prevention trials may inform safer conception programs.
PrEP effectiveness is highly dependent on adherence,7,8,21–23 and PrEP efficacy trials offer an early opportunity to identify populations for whom medication adherence may be challenging. Women who desire children might adhere to prevention strategies to protect a potential child from acquiring HIV-14,24–26 or, alternatively, may not adhere out of fear of side effects, including effects on the fetus. Understanding PrEP adherence in the context of conception is important given the potential of periconception PrEP as an HIV-1 risk-reduction strategy.10–12,27
We evaluated predictors of pregnancy and adherence to study medication before and during periconception among African HIV-1–uninfected women in serodiscordant partnerships enrolled in a randomized placebo-controlled trial of oral PrEP.
METHODS
Study Population and Procedures
The Partners PrEP Study was a phase III, randomized, double-blind, placebo-controlled, 3-arm clinical trial of daily oral TDF and FTC/TDF PrEP or placebo provided to HIV-1–uninfected members of HIV-1–serodiscordant couples. Beginning in July 2008, 4747 HIV-1–serodiscordant couples were enrolled and followed at 9 research sites in Kenya and Uganda. Eligible couples were sexually active and planned to remain in the relationship for the duration of the study. HIV-1–uninfected participants had normal renal function and were not infected with Hepatitis B virus. HIV-1–uninfected women were neither pregnant nor immediately planning pregnancy at the time of enrollment, counseled to delay pregnancy until the end of the study, and offered contraception, provided at no cost on-site. HIV-1–infected partners were not receiving and did not meet Kenyan or Ugandan guidelines for initiation of ART at enrollment and were monitored and actively referred for ART initiation if they became eligible during study follow-up. At each study visit, couples received a package of HIV-1 prevention services, including risk-reduction counseling, couples counseling, and condoms.8,28
At monthly follow-up visits for up to 36 months, HIV-1–uninfected partners underwent rapid HIV-1 testing, dispensation of study medication, and adherence counseling. For HIV-1–uninfected women, monthly visits included pregnancy testing with urine β-HCG and contraceptive counseling and provision; pregnant women were referred to local antenatal clinics, and study drug was held during pregnancy and breastfeeding. Testing for sexually transmitted infections (Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis) was conducted at baseline and annually. Interviewer-administered questionnaires captured demographic, partnership characteristics, sexual behavior, contraceptive use, and medical history data.
In July 2011, the trial's independent Data and Safety Monitoring Board recommended discontinuation of the trial placebo arm and public report of the results due to demonstration of PrEP efficacy for HIV-1 protection.8
The study protocol was reviewed by human subjects committees at the University of Washington and all study sites. Participants provided written informed consent.
Statistical Methods
This analysis includes data collected up to July 2011, when primary efficacy results were disseminated and the placebo arm was discontinued, and is limited to 1785 couples with HIV-1–uninfected female partners. Follow-up time from women who seroconverted to HIV-1 was censored at the time of seroconversion.
Pregnancy Incidence and Correlates
Pregnancy incidence was calculated as the number of new pregnancies divided by the total person-years of follow-up (excluding pregnant follow-up time). Baseline and time-dependent factors associated with incident pregnancy were examined using an Andersen–Gill29 extension to the Cox proportional hazards model to allow for multiple pregnancies per woman. Adjusted models included all factors associated with incident pregnancy in univariate analysis at P < 0.05.
Study Medication Adherence Among Women With and Without Pregnancy
We used several approaches to assess adherence to study medication. Pill count adherence was calculated from monthly clinic counts of dispensed and returned study pills, based on date of study dispensation and days since last visit. Missed visits were assigned an adherence value of zero because a missed visit corresponded with no pills dispensed. Previous work conducted in a subset of Partner PrEP Study participants showed high correlation between clinic-based pill counts, unannounced home pill counts, and electronic monitoring of pill bottle opening.30
We used log-binomial regression with generalized estimating equations to compare the relative risk of adhering to at least 80% of study drug doses among HIV-1–uninfected women who experienced pregnancy compared with women who did not experience pregnancy.31 High adherence was defined as taking at least 80% of doses based on biologic plausibility,32 prior definitions of low and high adherence to antiretroviral PrEP,7 and prior adherence data from this study.30 A priori specified covariates included age, unprotected sex, sex with an additional partner, use of an effective contraceptive method, and time in the study.
To examine the relationship between periconception periods of follow-up and adherence to study drug restricted to women who became pregnant (thus removing differences between women who did and did not experience pregnancy), we evaluated adherence during the periconception period, defined as the 3 months before the visit at which the first pregnancy was discovered, compared with follow-up before the periconception period. To minimize confounding by enrollment characteristics that could be associated with both adherence and becoming pregnant, we used conditional logistic regression33 with adjustment for time-dependent confounders (any unprotected sex, sex with an additional partner, use of an effective contraceptive method, and time in the study), selected a priori based on factors with strong associations with pregnancy.
Finally, for women randomized to the trial's active arms (TDF or FTC/TDF), stored plasma from selected study visits was tested for tenofovir drug concentrations, using methods previously described (assay limit of quantitation = 0.3 ng/mL).8 For the present analysis, we tested samples from the visit at which pregnancy was first discovered among women who became pregnant and had a sample available from this study visit. Detection of tenofovir was compared between these samples and specimens from a randomly selected cohort of women who did not become pregnant; samples from the random cohort were tested from across the study follow-up (months 1, 3, 6, 12, 18, 24, and 36, as available depending on the length of follow-up). For this analysis, a priori specified covariates included age, any unprotected sex, sex with an additional partner, use of an effective contraceptive method, and time in the study. This analysis was conducted in R version 2.12.2 using the Lumley survey package (version 3.26 http://faculty.washington.edu/tlumley/survey/).34 All other analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
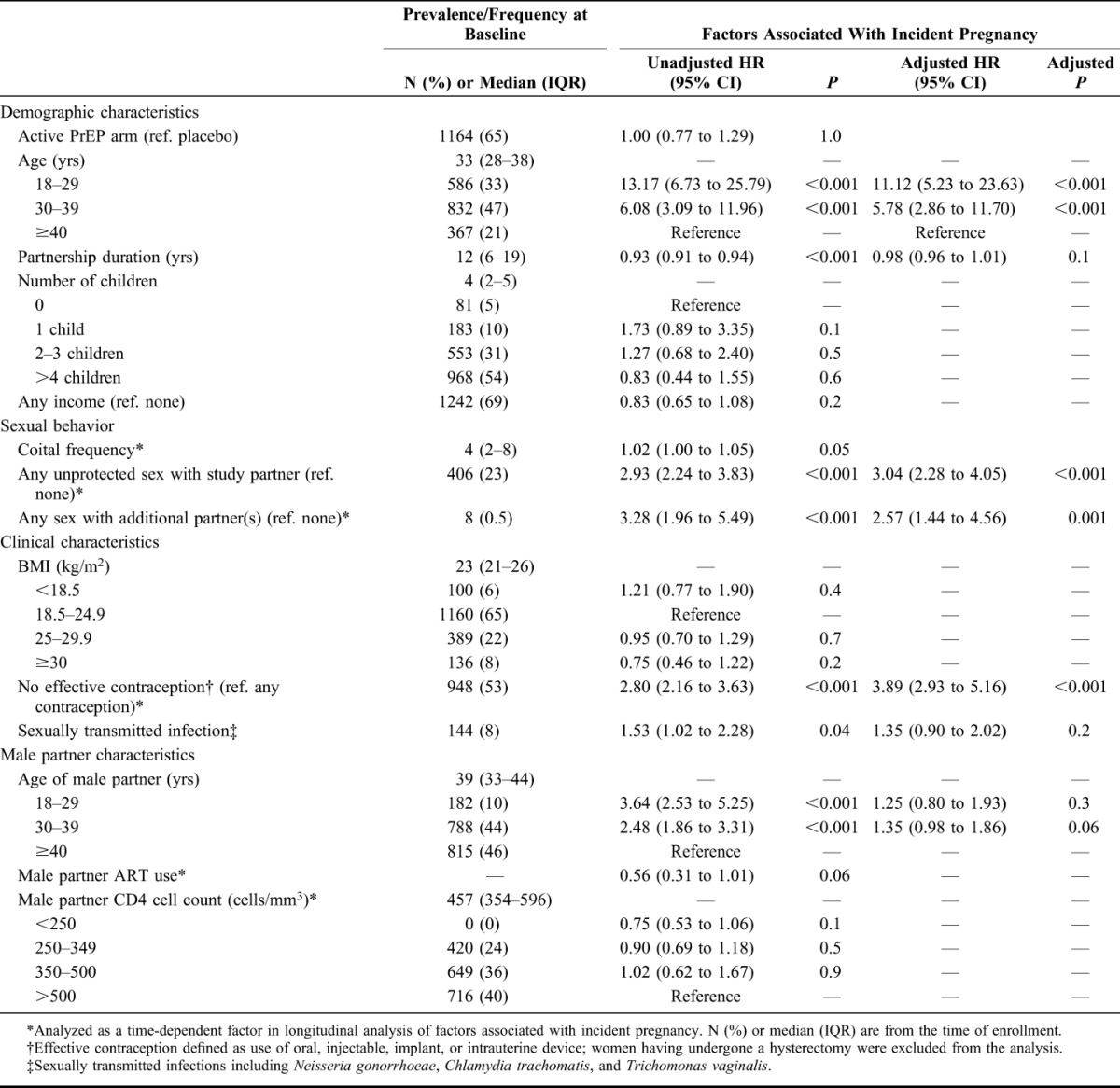
The median age of the 1785 HIV-1–uninfected women included in this analysis was 33 years [interquartile range (IQR), 28–38], median partnership duration was 12 years (IQR, 6–19), and median number of children was 4 (2–5) with 5% of women reporting no children (Table 1). Twenty-three percent of women reported sex without condoms in the month before enrollment and 53% were not using effective contraception.
TABLE 1.
Participant Characteristics and Associations With Incident Pregnancy
Pregnancy Incidence and Predictors
During 2827.5 person-years of follow-up, 267 women had 288 pregnancies for an incidence of 10.2 pregnancies per 100 person-years of follow-up [95% confidence interval (CI): 9.1 to 11.3]. Of the 267 women who became pregnant, 247 had 1 pregnancy and 20 had 2 pregnancies. Pregnancies occurred steadily throughout the follow-up period (data not shown). In multivariate analysis, multiple factors were independently associated with increased likelihood of pregnancy (Table 1): unprotected sex with the study partner [adjusted hazard ratio (aHR), 3.04; 95% CI: 2.28 to 4.05], having an additional sexual partner during follow-up (aHR, 2.57; 95% CI: 1.44 to 4.56), younger age (aHR, 11.12, 95% CI: 5.23 to 23.63, for age 18–29 and aHR 5.78, 95% CI: 2.86 to 11.70, for age 30–39, each compared with age ≥40 years), and not using effective contraception (aHR 3.89, 95% CI: 2.93 to 5.16). Effective contraceptive use was reported at 57.6% of follow-up visits, a proportion that was relatively consistent throughout follow-up (ranging from 54.1% to 62.2%).
Pregnancy and Adherence to Study Drug
Clinic-based pill count adherence was high, with 97.0% (SD, 6.9) of dispensed pills taken by women who experienced a pregnancy and 97.9% (SD, 6.0) taken by women without pregnancies. High adherence (defined as ≥80% of dispensed pills taken) was present at 97.7% of visits among women who became pregnant, which was not statistically different than among women who did not become pregnant, for whom ≥80% adherence was present for 98.7% of visits [adjusted relative risk (aRR), 0.99; 95% CI: 0.99 to 1.0; Table 2].
TABLE 2.
Adherence to Study Medication (PrEP/Placebo), by Multiple Measures
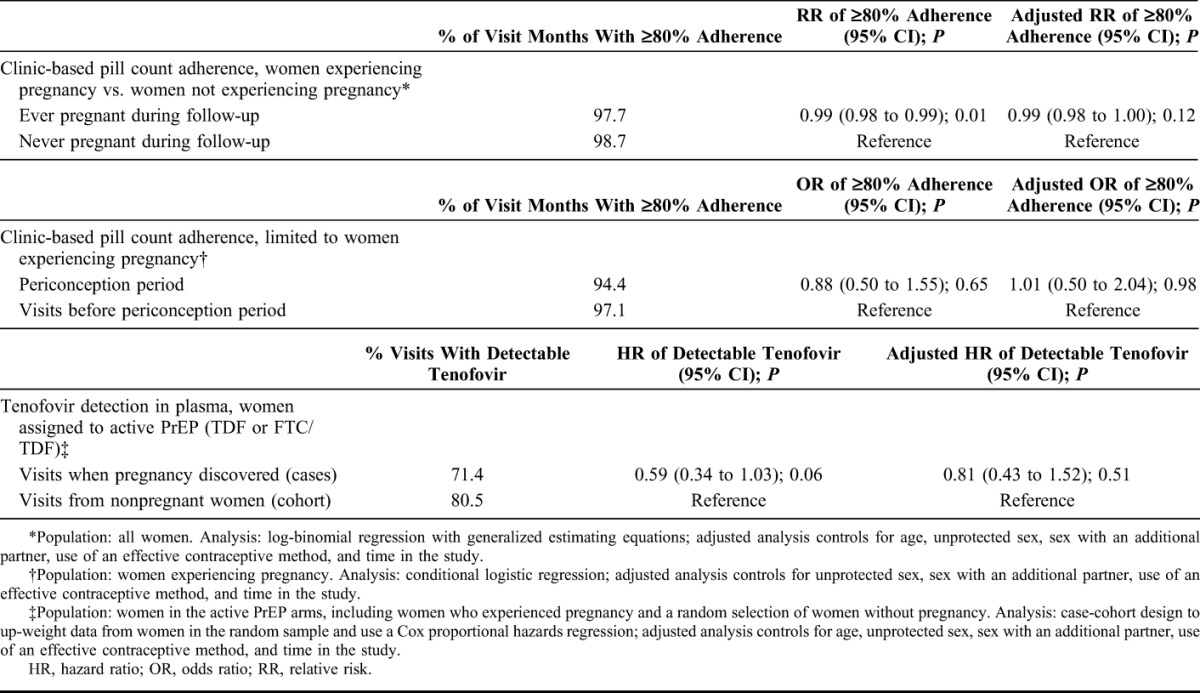
To discern whether adherence differed relative to the time of pregnancy, we conducted an analysis of adherence among the subset of women who became pregnant. In these women, the likelihood of adhering to at least 80% of pills dispensed during the 3-month periconception period was similar to other time points before pregnancy in unadjusted analysis (odds ratio, 0.88; 95% CI: 0.50 to 1.55) and after adjustment for effective contraceptive use, unprotected sex, additional partners, and time in study (adjusted odds ratio = 1.01; 95% CI: 0.50 to 2.04; P = 0.98) (Fig. 1 and Table 2).
FIGURE 1.
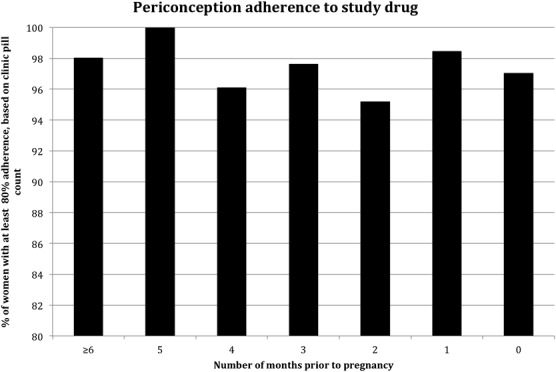
Adherence relative to pregnancy among 267 women with pregnancies. Percentage of women taking at least 80% of pills prescribed (by clinic-based pill count) is graphed on the y axis. Number of months before pregnancy is on the x axis, month 0 is the month where pregnancy was first detected. The percentage of women with high adherence did not differ by month.
We also evaluated tenofovir concentrations among pregnant and nonpregnant follow-up periods. Tenofovir plasma concentrations were available from 76 women who became pregnant at the visit when their pregnancy was first discovered (77 specimens, with 1 woman having 2 pregnancies) and 103 women who did not become pregnant (329 specimens across their follow-up, with a median of 4 samples per woman). Tenofovir was detectable (consistent with dosing in the previous week) in 71% (55/77) of specimens from women who became pregnant and 81% (252/313) of specimens from women not experiencing pregnancy (aHR, 0.81; 95% CI: 0.43 to 1.52). There was also no statistically significant difference when a higher concentration cutoff was used (≥40 ng/mL of tenofovir, suggesting steady-state dosing, data not shown).
DISCUSSION
As in many clinical trials of biomedical interventions, women were eligible for enrollment into the Partners PrEP Study if they were not pregnant and reported no plans for pregnancy. The trial protocol included counseling to avoid pregnancy, contraceptive counseling, and provision of free contraception, without a requirement for contraception. In these circumstances, just over half of women used effective contraception, and pregnancy incidence was 10% per year among HIV-1–uninfected women with HIV-1–infected partners. By multiple measures, adherence to study drug (blinded PrEP or placebo) was high among women experiencing pregnancy.
Medication adherence has been low in some studies of PrEP for women.22,23 However, in the Partners PrEP Study, adherence was high, by multiple measures.8,30 In the present analysis, we found that women with and without pregnancies had high adherence to study drug, as measured both by pill counts and detection of tenofovir in plasma. Because women in PrEP trials have been encouraged to delay pregnancy and counseled about unclear safety data for tenofovir use in early pregnancy, we explored whether women with pregnancy were less likely to take study drug during the periconception period, but found no difference. These data suggest that women were willing to use PrEP around the time of conception, even in the absence of data regarding the safety and efficacy of PrEP for HIV-1 prevention.
In contrast to these results, among women with pregnancy in CAPRISA 004, a median of 50% of sex acts were protected according to the dosing schedule of 2 gel applications per sex act. Women with pregnancy were about half as likely to adhere to at least 80% of study gel doses compared with women without pregnancy.35 Several differences in study populations may explain these differences. The CAPRISA 004 study required participants to start contraception at trial entry—use was 100% at baseline and 97% at 18 months36; thus, women with pregnancies in that study may have been less likely to adhere to study gel because pregnancies also likely reflected nonadherence to contraception. In addition, CAPRISA 004 enrolled individual younger women (median age 22) at high risk for HIV-1 acquisition, whereas the Partners PrEP Study enrolled older women (median age, 33) in known HIV-1–serodiscordant couples. Qualitative data suggest that higher medication adherence in the Partners PrEP Study may have been partially because of partner involvement.37 In addition, although neither trial reported prospective pregnancy intention data, qualitative interviews with 36 couples experiencing pregnancy while enrolled in the Partners PrEP Study suggested that most intended to become pregnant or were pleased when they discovered they were pregnant.38 Thus, it is possible that most of the pregnancies in the Partners PrEP Study were intended or planned.
HIV-1 prevention studies enrolling women from sub-Saharan Africa have reported pregnancy incidence ranging from 3.95/100 person-years in the CAPRISA 004 trial of tenofovir vaginal gel in South Africa36 to 52/100 person-years in a phase 2 trial of oral tenofovir in West Africa.19 High pregnancy incidence in studies providing access to and counseling about contraception highlights the importance of HIV-1 prevention for women who may want to conceive with an infected or high-risk partner.39,40 Factors associated with incident pregnancy in this study included younger age, unprotected sex, having an outside partner, and not being on effective contraception. These associations are intuitive and consistent with previous reports of pregnancy predictors among women enrolled in HIV-1 prevention trials.16–18,41 Interestingly, our data did not show an association with partner CD4 cell count or ART use, suggesting that women were not making decisions to conceive based on markers of HIV-1 transmission risk from their partners. Our results do not point to a specific group to target in safer conception interventions but highlight that sexually active women of reproductive age who are not on contraception may benefit from routine discussions of fertility goals and counseling for the best HIV-1 risk-reduction strategies given her goals. Women who enroll in a clinical trial without plans for pregnancy merit ongoing counseling sensitive to the fact that her goals may change over time.40
Limitations to this study include interpreting pregnancy incidence without prospective data around fertility intention. Second, studying adherence to an intervention with unknown efficacy (at the time of the study) with blinded randomization to placebo makes both the measure of adherence and the significance of the findings an imperfect reflection of what delivery of effective PrEP might find. Although this analysis focuses on the periconception period, women having children within serodiscordant partnerships remain at risk after conception. Couples who achieve pregnancy are at particular risk for transmitting and acquiring HIV with associated risks of perinatal transmission.42–44 Risk-reduction interventions, including PrEP, should continue to be evaluated for women during pregnancy and postpartum periods.
In conclusion, these data show that women at risk for HIV-1 acquisition within stable, mutually disclosed, HIV-1–serodiscordant partnerships, with understanding of that risk,45 and with ready access to condoms, contraception, and counseling still have a high pregnancy rate. In addition, they remained highly adherent to PrEP, both overall and around the time of conception. Now that FTC/TDF is approved and recommended for use as oral PrEP46–48 and there is enthusiasm for PrEP as a safer conception strategy,10–12 implementation and demonstration projects should include women with pregnancy and/or plans for pregnancy to understand the risks, the benefits, and challenges to biomedical prevention in this high priority group.
ACKNOWLEDGMENTS
The authors thank the couples who participated in this study, the teams at the study sites, and the University of Washington for work on data collection and management.
APPENDIX. Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Kenya Medical Research Institute, Nairobi, Kenya: Nelly Rwamba Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, WA) and site laboratory oversight was provided by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
The Partners PrEP study was supported by research grant (OOP47674) from the Bill & Melinda Gates Foundation; Gilead Sciences provided medication. This analysis was also supported by the National Institute of Mental Health and the Eunice Kennedy Shriver National Institute of Child Health and Development of the National Institutes of Health under award numbers K23MH095655, K24MH87227, R21HD074439, K99HD076679, and R01MH095507.
C.H. has a contract with Gilead Sciences for partial support of a clinical study managed by Johns Hopkins University. The remaining authors have no conflicts of interest to disclose.
The content is the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Members of the Partners PrEP Study Team are listed in Appendix.
REFERENCES
- 1.Dyer SJ, Abrahams N, Hoffman M, et al. “Men leave me as I cannot have children”: women's experiences with involuntary childlessness. Hum Reprod. 2002;17:1663–1668 [DOI] [PubMed] [Google Scholar]
- 2.Rutstein SO, Shah IH. Infecundity, Infertility and Childlessness in Developing Countries. Calverton, MD: ORC Macro and the World Health Organization; 2004 [Google Scholar]
- 3.Guthrie BL, Choi RY, Bosire R, et al. Predicting pregnancy in HIV-1-discordant couples. AIDS Behav. 2010;14:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews LT, Crankshaw T, Giddy J, et al. Reproductive decision-making and periconception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav. 2013;17:461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyeza-Kashesya J, Ekstrom AM, Kaharuza F, et al. My partner wants a child: a cross-sectional study of the determinants of the desire for children among mutually disclosed sero-discordant couples receiving care in Uganda. BMC Public Health. 2010;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyeza-Kashesya J, Kaharuza F, Mirembe F, et al. The dilemma of safe sex and having children: challenges facing HIV sero-discordant couples in Uganda. Afr Health Sci. 2009;9:2–12 [PMC free article] [PubMed] [Google Scholar]
- 7.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434 [DOI] [PubMed] [Google Scholar]
- 10.Vernazza PL, Graf I, Sonnenberg-Schwan U, et al. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS. 2011;25:2005–2008 [DOI] [PubMed] [Google Scholar]
- 11.Lampe MA, Smith DK, Anderson GJ, et al. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol. 2011;204:488:e1–e8 [DOI] [PubMed] [Google Scholar]
- 12.Matthews LT, Baeten JM, Celum C, et al. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS. 2010;24:1975–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews LT, Smit JA, Cu-Uvin S, et al. Antiretrovirals and safer conception for HIV-serodiscordant couples. Curr Opin HIV AIDS. 2012;7:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mugo N, Hong T, Celum C, et al. Pregnancy incidence and birth outcomes among African women in a clinical trial of pre-exposure prophylaxis: the Partners PrEP Study. Kuala Lampur, Malaysia: International AIDS Society; 2013. Abstract WEAC0101 [Google Scholar]
- 15.The Antiretorival Pregnancy Registry. Available at: www.apregistry.com. Accessed May 15, 2012
- 16.Ngure K, Heffron R, Mugo NR, et al. Contraceptive method and pregnancy incidence among women in HIV-1-serodiscordant partnerships. AIDS. 2012;26:513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odutola A, Baisley K, Hayes RJ, et al. Pregnancy and contraceptive use among women participating in an HIV prevention trial in Tanzania. Sex Transm Infect. 2012;88:436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid SE, Dai JY, Wang J, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 21.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). Paper 26LB. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections, March 2013, Atlanta, GA
- 24.Mindry D, Maman S, Chirowodza A, et al. Looking to the future: South African men and women negotiating HIV risk and relationship intimacy. Cult Health Sex. 2011;13:589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardeguez AD, Lindsey JC, Shannon M, et al. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr. 2008;48:408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sha BE, Tierney C, Cohn SE, et al. Postpartum viral load rebound in HIV-1-infected women treated with highly active antiretroviral therapy: AIDS Clinical Trials Group Protocol A5150. HIV Clin Trials. 2011;12:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whetham J, Taylor S, Charlwood L, et al. Pre-exposure prophylaxis for conception (PrEP-C) as a risk reduction strategy in HIV-positive men and HIV-negative women in the UK. AIDS Care. 2014;26:332–336 [DOI] [PubMed] [Google Scholar]
- 28.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6:e25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120 [Google Scholar]
- 30.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East africa. PLoS Med. 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153 [DOI] [PubMed] [Google Scholar]
- 34.Breslow NE, Lumley T, Ballantyne CM, et al. Improved Horvitz-Thompson estimation of model parameters from two-phase stratified samples: applications in Epidemiology. Stat Biosci. 2009;1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews LT, Sibeko S, Mansoor LE, et al. Women with pregnancies had lower adherence to 1% tenofovir vaginal gel as HIV preexposure prophylaxis in CAPRISA 004, a phase IIB randomized-controlled trial. PLoS One. 2013;8:e56400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibeko S, Baxter C, Yende N, et al. Contraceptive choices, pregnancy rates, and outcomes in a microbicide trial. Obstet Gynecol. 2011;118:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngure K, Baeten JM, Mugo N, et al. I feared: My intention was a child but I was very afraid: fertility intentions and HIV-1 risk perception among HIV-1 serodiscordant couples in Kenya. Abstract MOPE313. Paper presented at: AIDS 20122012; Washington, DC
- 39.The World Bank. Fertility rate (total births per woman), Table. 2013; word development indicators. Available at: http://data.worldbank.org/indicator/SP.DYN.TFRT.IN. Accessed September 12, 2013
- 40.Ssali A, Namukwaya S, Bufumbo L, et al. Pregnancy in HIV clinical trials in sub Saharan Africa: failure of consent or contraception? PLoS One. 2013;8:e73556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halpern V, Lie CC, Feldblum P, et al. Predictors of pregnancy in microbicide trials. Contraception. 2011;83:436–440 [DOI] [PubMed] [Google Scholar]
- 42.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharsany AB, Hancock N, Frohlich JA, et al. Screening for “window-period” acute HIV infection among pregnant women in rural South Africa. HIV Med. 2010;11:661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moodley D, Esterhuizen TM, Pather T, et al. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23:1255–1259 [DOI] [PubMed] [Google Scholar]
- 45.Heffron R, Ngure K, Mugo N, et al. Willingness of Kenyan HIV-1 serodiscordant couples to use antiretroviral-based HIV-1 prevention strategies. J Acquir Immune Defic Syndr. 2012;61:116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FDA. Press release: FDA approves first drug for reducing the risk of sexually acquired HIV infection. July 16, 2012. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm312210.htm. Accessed June 23, 2014 [Google Scholar]
- 47.CDC. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586–589 [PubMed] [Google Scholar]
- 48.WHO. Guidance on Pre-exposure Oral Prophylaxis (PrEP) for Serodiscordant Couples, Men and Transgender Women Who Have Sex With Men at High Risk of HIV: Recommendations for Use in the Context of Demonstration Projects. Geneva, Switzerland: WHO; 2012 [PubMed] [Google Scholar]


