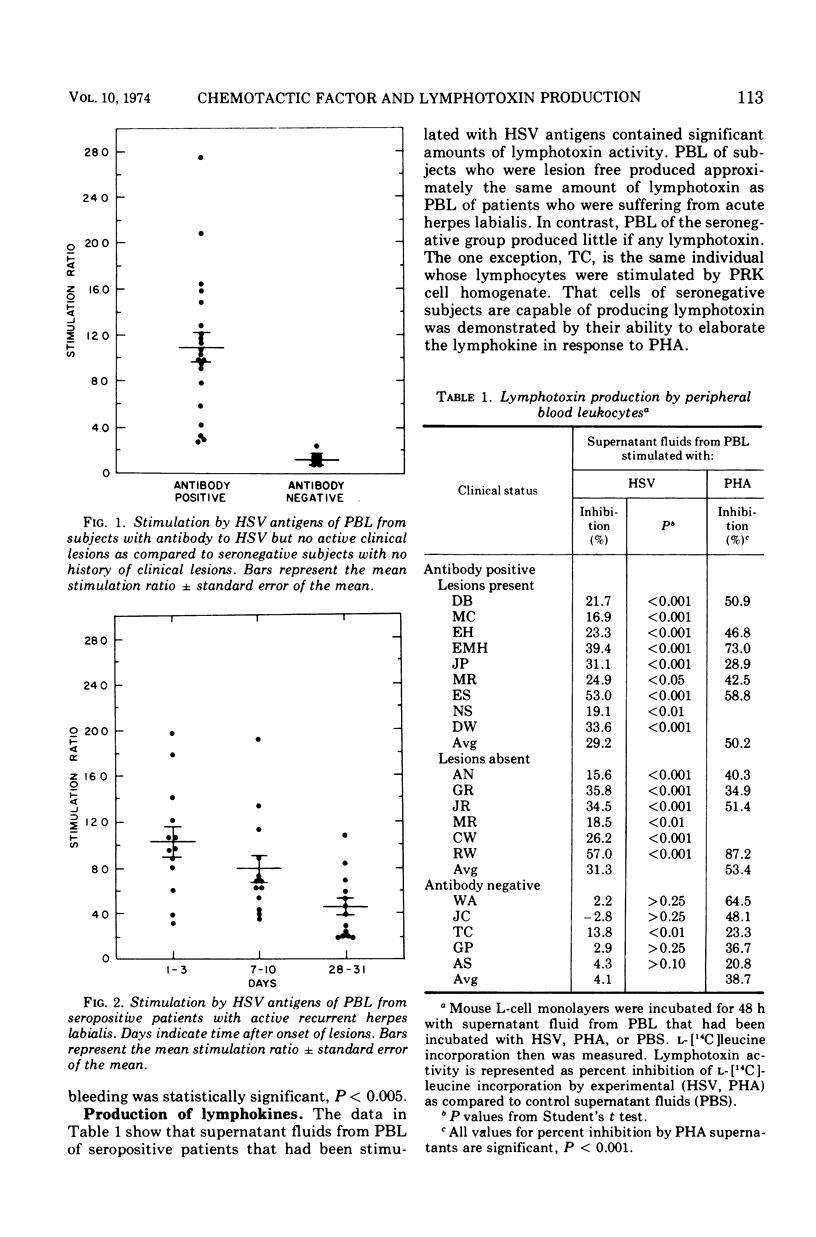
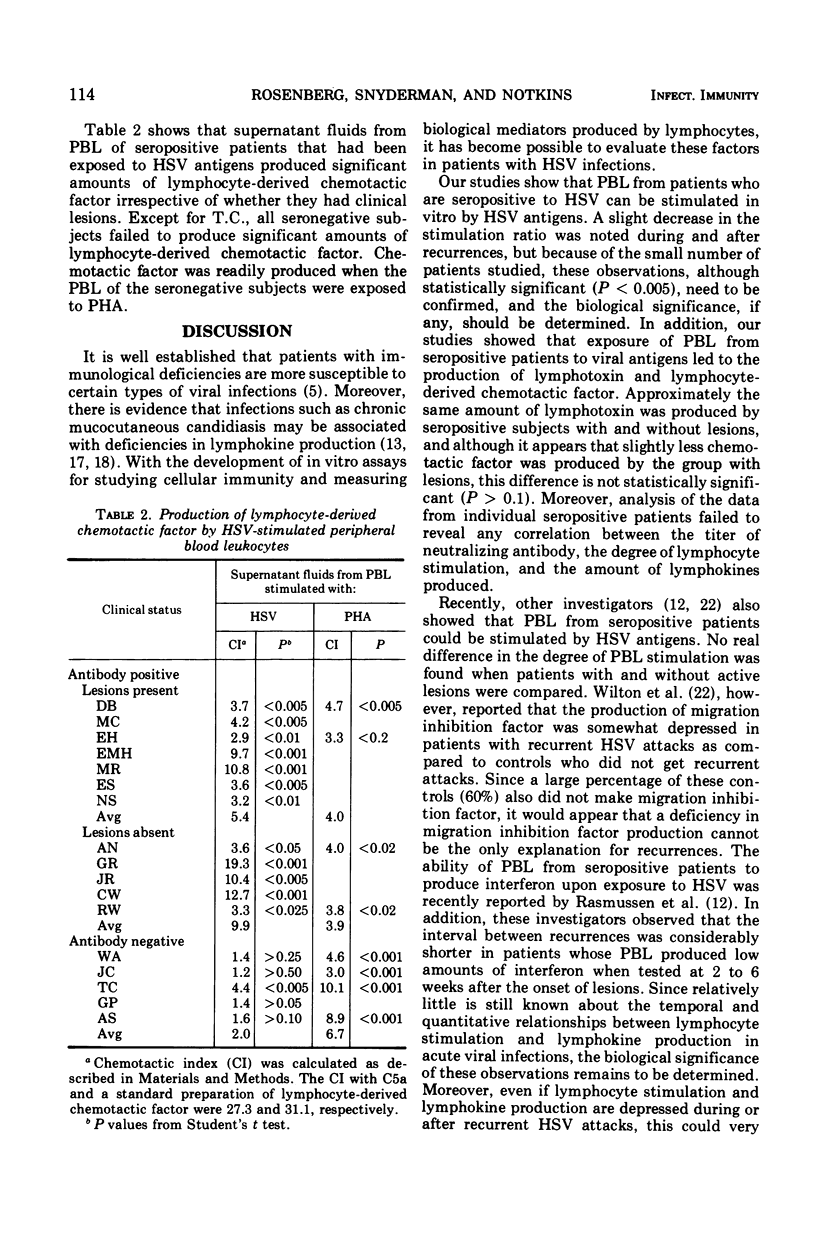
Abstract
Peripheral blood leukocytes (PBL) of 41 subjects were tested for their ability to be stimulated by herpes simplex virus antigens as measured by [3H]thymidine incorporation and lymphokine production (i.e., lymphocyte-derived chemotactic factor and lymphotoxin). The PBL of seronegative subjects failed to respond to viral antigens as measured by stimulation or lymphokine production. In contrast, PBL from seropositive subjects without clinical lesions of herpes labialis were stimulated by herpes simplex virus antigens and produced lymphokines. PBL from seropositive patients with clinical lesions of herpes labialis also produced lymphokines, but [3H]thymidine incorporation was slightly depressed. These findings suggest that lymphocytes from patients with a recent herpes simplex virus infection may respond less vigorously to in vitro stimulation by herpes antigens, but our study fails to show any basic defect in the responsiveness of the lymphocytes to account for recurrent herpetic episodes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- David J. R. Lymphocyte mediators and cellular hypersensitivity. N Engl J Med. 1973 Jan 18;288(3):143–149. doi: 10.1056/NEJM197301182880311. [DOI] [PubMed] [Google Scholar]
- Elfenbein G. J., Rosenberg G. L. In vitro proliferation of rabbit bone marrow-derived and thymus-derived lymphocytes in response to vaccinia virus. Cell Immunol. 1973 Jun;7(3):516–521. doi: 10.1016/0008-8749(73)90216-5. [DOI] [PubMed] [Google Scholar]
- Granger G. A., Kolb W. P. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Horton J. E., Oppenheim J. J., Mergenhagen S. E. Elaboration of lymphotoxin by cultured human peripheral blood leucocytes stimulated with dental-plaque deposits. Clin Exp Immunol. 1973 Mar;13(3):383–393. [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers R. L., Pettit T. H. Corneal immune response to Herpes simplex virus antigens. J Immunol. 1973 Jun;110(6):1575–1590. [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. II. N Engl J Med. 1973 Oct 4;289(14):719–725. doi: 10.1056/NEJM197310042891404. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. E., Jordan G. W., Stevens D. A., Merigan T. C. Lymphocyte interferon production and transformation after Herpes simplex infections in humans. J Immunol. 1974 Feb;112(2):728–736. [PubMed] [Google Scholar]
- Rocklin R. E., Chilgren R. A., Hong R., David J. R. Transfer of cellular hypersensitivity in chronic mucocutaneous candidiasis monitored in vivo and in vitro. Cell Immunol. 1970 Sep;1(3):290–299. doi: 10.1016/0008-8749(70)90050-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Notkins A. L. Induction of cellular immunity to herpes simplex virus: relationship to the humoral immune response. J Immunol. 1974 Mar;112(3):1019–1025. [PubMed] [Google Scholar]
- Rosenberg G. L., Wohlenberg C., Nahmias A. J., Notkins A. L. Differentiation of type 1 and type 2 herpes simplex virus by in vitro stimulation of immune lymphocytes. J Immunol. 1972 Aug;109(2):413–414. [PubMed] [Google Scholar]
- Schulking M. L., Adler W. H., 3rd, Altemeier W. A., 3rd, Ayoub E. M. Transfer factor in the treatment of a case of chronic mucocutaneous candidiasis. Cell Immunol. 1972 Apr;3(4):606–615. doi: 10.1016/0008-8749(72)90122-0. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Frankel A., Blaese R. M. Defective mononuclear leukocyte chemotaxis: a previously unrecognized immune dysfunction. Studies in a patient with chronic mucocutaneous candidiasis. Ann Intern Med. 1973 Apr;78(4):509–513. doi: 10.7326/0003-4819-78-4-509. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]



