Abstract
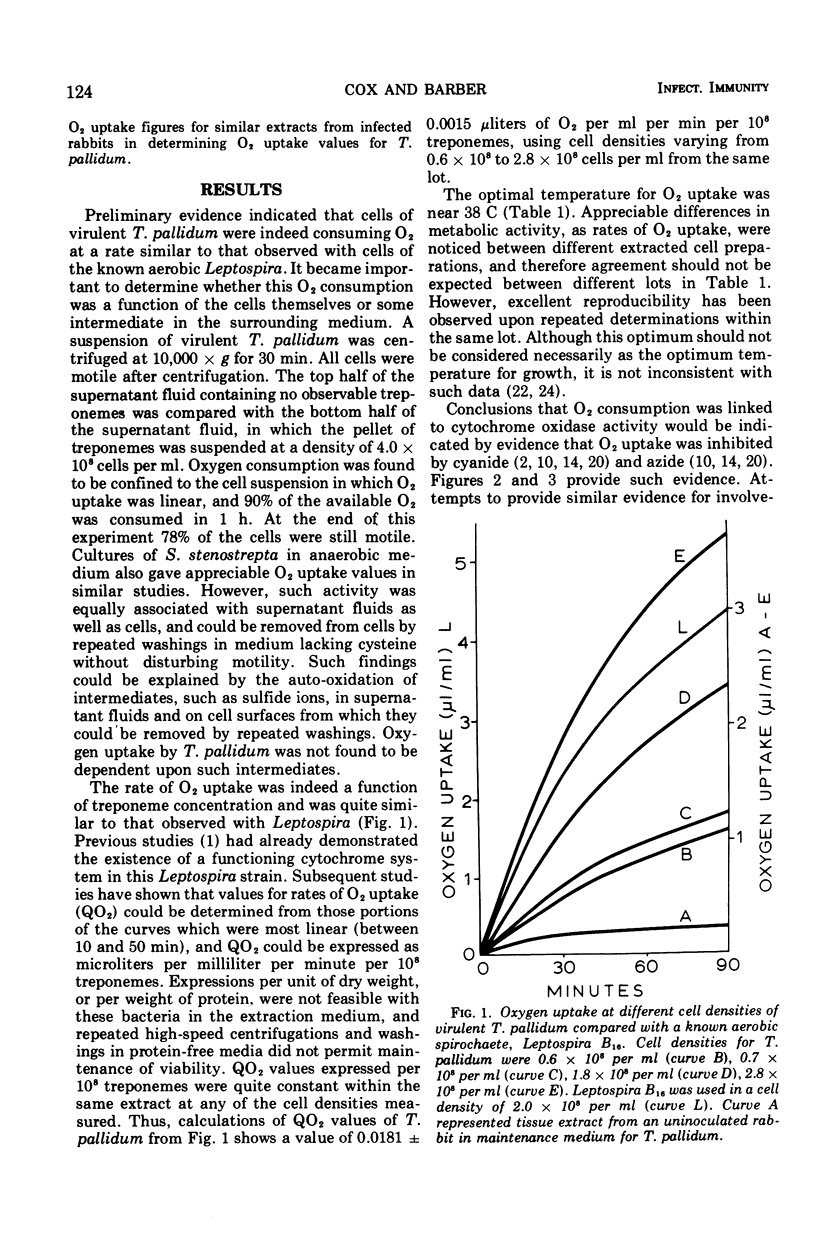
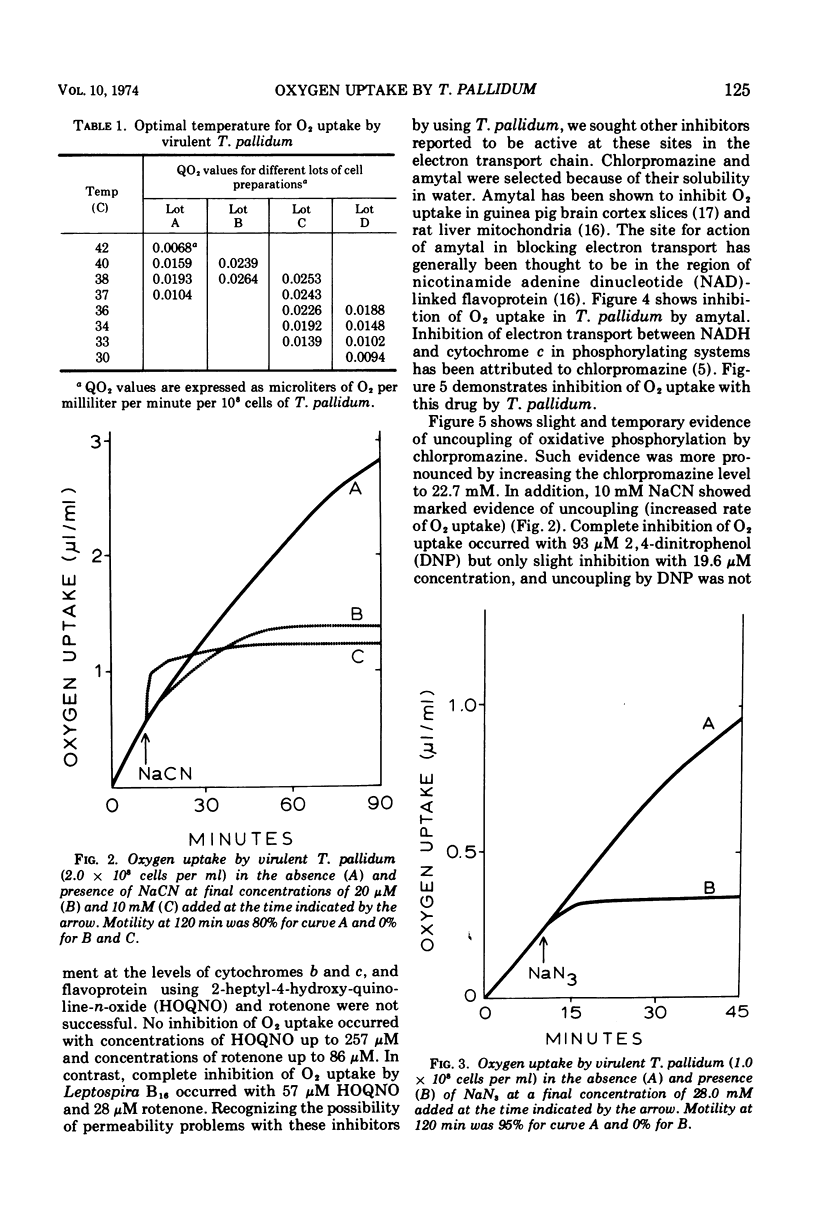
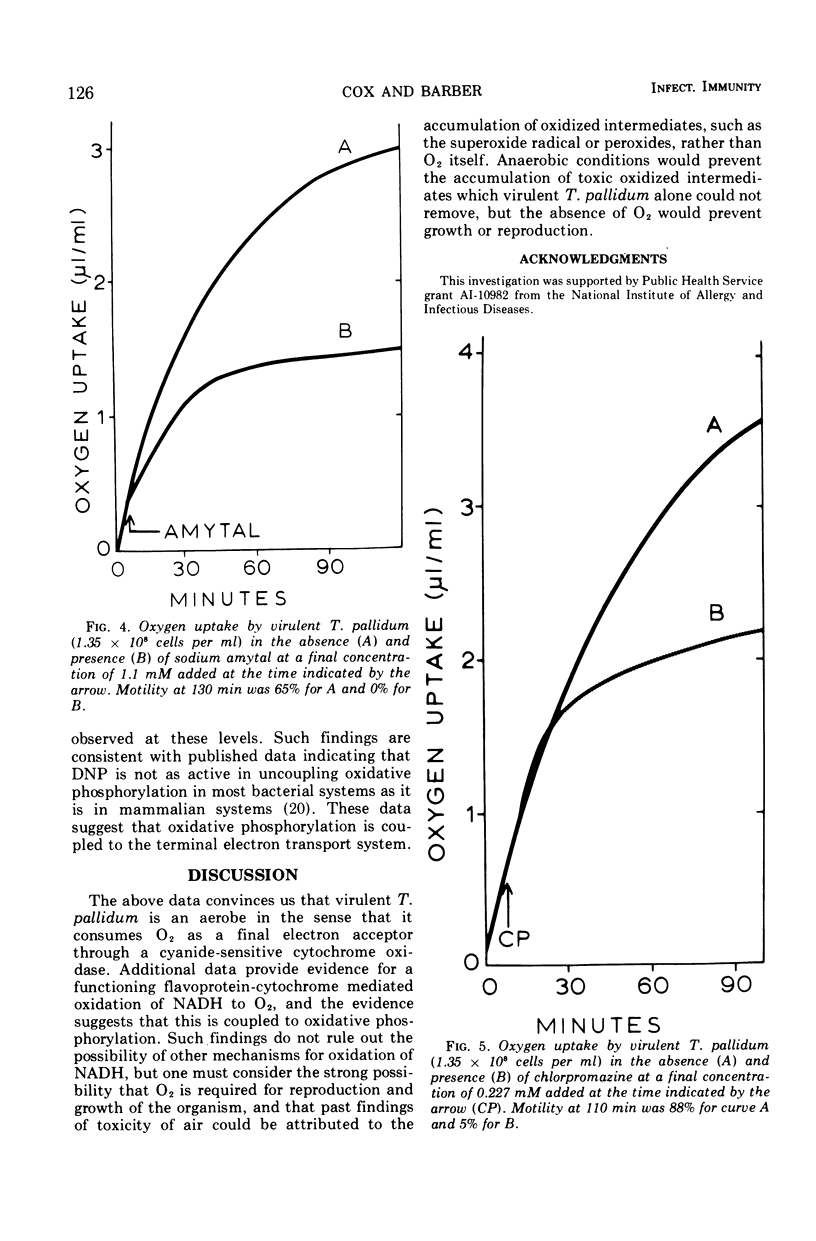
Virulent Treponema pallidum has been shown to consume O2 at a rate similar to that of the known aerobic spirochaete, Leptospira. Such O2 uptake is cyanide sensitive, indicating a functioning cytochrome oxidase. Inhibition of O2 uptake by azide, chlorpromazine, and amytal further suggests a functioning electron transport system for the oxidation of nicotinamide adenine dinucleotide (reduced) to O2. Evidence is consistent with the probability that this terminal electron-transport system is coupled to oxidative phosphorylation. The potential significance of these findings is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Cox C. D. Terminal electron transport in Leptospira. J Bacteriol. 1969 Mar;97(3):1001–1004. doi: 10.1128/jb.97.3.1001-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J., Dietrich W. E., Jr Respiratory mechanisms in the Flexibacteriaceae. I. Studies on the terminal oxidase system of Leucothrix mucor. Arch Biochem Biophys. 1968 Oct;128(1):40–50. doi: 10.1016/0003-9861(68)90007-6. [DOI] [PubMed] [Google Scholar]
- DAWKINS M. J., JUDAH J. D., REES K. R. The mechanism of action of chlorpromazine. Reduced diphosphopyridine nucleotidecytochrome c reductase and coupled phosphorylation. Biochem J. 1959 Sep;73:16–23. doi: 10.1042/bj0730016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H., Steinman H. G. The Nutritional Requirements of Treponemata: I. Arginine, Acetic Acid, Sulfur-containing Compounds, and Serum Albumin as Essential Growth-promoting Factors for the Reiter Treponeme. J Bacteriol. 1948 Aug;56(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Specific agglutination of Treponema pallidum by sera from rabbits and human beings with treponemal infections. J Exp Med. 1955 Apr 1;101(4):367–382. doi: 10.1084/jem.101.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLANDER D. H., NELL E. E. Improved preservation of Treponema pallidum and other bacteria by freezing with glycerol. Appl Microbiol. 1954 May;2(3):164–170. doi: 10.1128/am.2.3.164-170.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Nell E. E. A study of the properties of sorbent, the reagent employed in the fluorescent treponemal antibody-absorption test. Am J Epidemiol. 1972 Aug;96(2):141–152. doi: 10.1093/oxfordjournals.aje.a121440. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Canale-Parola E. Carbohydrate metabolism in Spirochaeta stenostrepta. J Bacteriol. 1970 Jul;103(1):216–226. doi: 10.1128/jb.103.1.216-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr The use of freeze-preserved treponemes in the Treponema pallidum immobilization test. Cryobiology. 1972 Oct;9(5):404–410. doi: 10.1016/0011-2240(72)90157-5. [DOI] [PubMed] [Google Scholar]
- PORTNOY J., BREWER J. H., HARRIS A. Rapid plasma reagin card test for syphilis and other treponematoses. Public Health Rep. 1962 Aug;77:645–652. [PMC free article] [PubMed] [Google Scholar]
- PUMPHREY A. M., REDFEARN E. R. INHIBITION OF SUCCINATE OXIDATION BY BARBITURATES IN TIGHTLY COUPLED MITOCHONDRIA. Biochim Biophys Acta. 1963 Aug 13;74:317–327. doi: 10.1016/0006-3002(63)91375-1. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Staneck J. L., Henneberry R. C., Cox C. D. Growth requirements of pathogenic Leptospira. Infect Immun. 1973 Jun;7(6):886–897. doi: 10.1128/iai.7.6.886-897.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER M. M. Factors influencing the in vitro survival of Treponema pallidum. Am J Hyg. 1960 May;71:401–417. doi: 10.1093/oxfordjournals.aje.a120123. [DOI] [PubMed] [Google Scholar]



