Abstract
A purified elastase from Pseudomonas aeruginosa was highly destructive for fluid-phase and cell-bound C1 and C3 and fluid-phase C5, C8, and C9. Inactivation of C4, C2, C6, and C7 by the enzyme varied from 0 to 67%. Low concentrations of elastase generated, then inactivated, a chemotactic factor from human C5 but not from C3. Higher enzyme concentrations inactivated the C5 chemotactic activity at a faster rate. Elastase treatment of sensitized pseudomonads containing cell-bound C3 reduced the phagocytic indexes of polymorphonuclear leukocytes. The data support the proposed chemopathogenic role of the elastase in generation of the characteristic non-inflammatory Pseudomonas vasculitis.
Full text
PDF



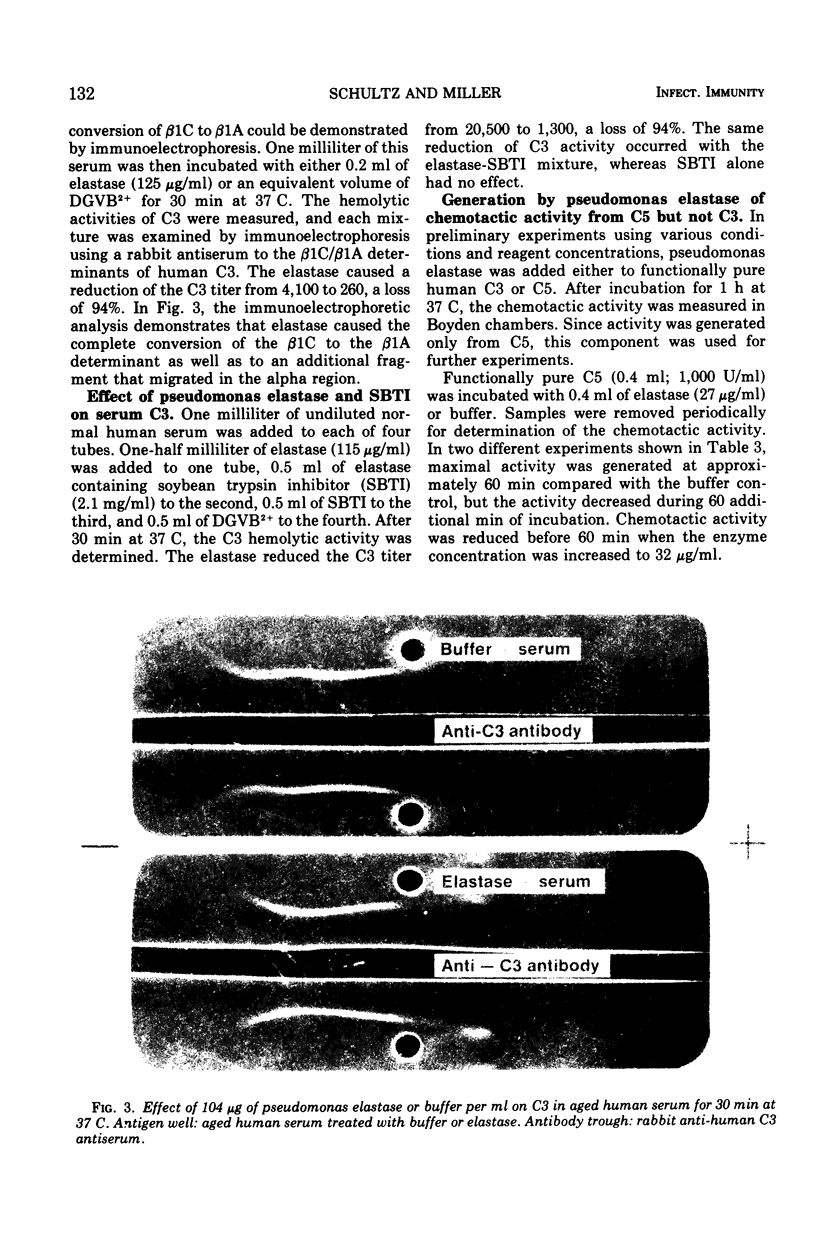
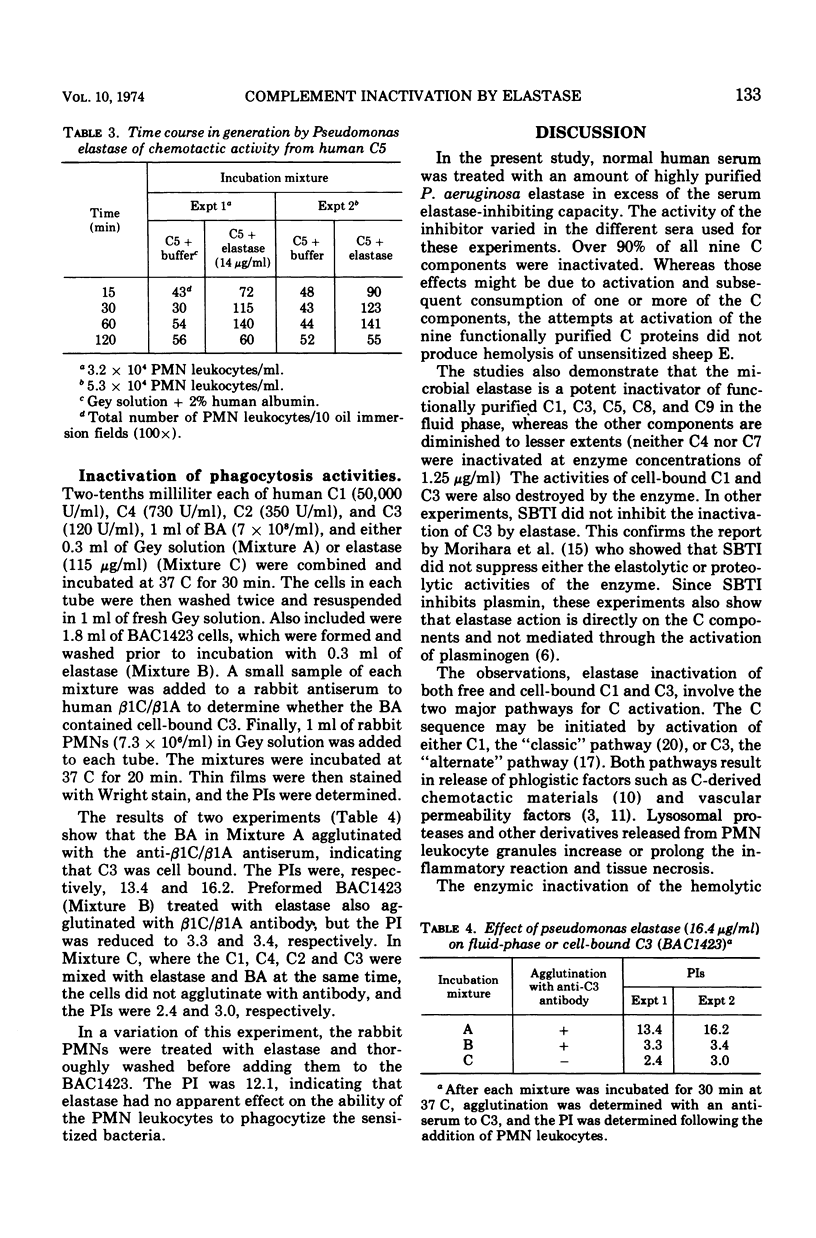


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen K. F. Inborn and acquired abnormalities of the complement system of man. Johns Hopkins Med J. 1971 Feb;128(2):57–74. [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J., Cochrane C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–1130. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., ROSEN F. S. ACTION OF COMPLEMENT IN HEREDITARY ANGIONEUROTIC EDEMA: THE ROLE OF C'1-ESTERASE. J Clin Invest. 1964 Nov;43:2204–2213. doi: 10.1172/JCI105094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORKNER C. E., Jr, FREI E., 3rd, EDGCOMB J. H., UTZ J. P. Pseudomonas septicemia; observations on twenty-three cases. Am J Med. 1958 Dec;25(6):877–889. doi: 10.1016/0002-9343(58)90060-3. [DOI] [PubMed] [Google Scholar]
- Gigli I., Nelson R. A., Jr Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp Cell Res. 1968 Jul;51(1):45–67. doi: 10.1016/0014-4827(68)90158-4. [DOI] [PubMed] [Google Scholar]
- Ironside P. N. Production of anti-human-globulin in goats. Immunology. 1968 Oct;15(4):503–507. [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- MARGARETTEN W., NAKAI H., LANDING B. H. Significance of selective vasculitis and the "bone-marrow" syndrome in Pseudomonas septicemia. N Engl J Med. 1961 Oct 19;265:773–776. doi: 10.1056/NEJM196110192651603. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- PENSKY J., WURZ L., PILLEMER L., LEPOW I. H. The properdin system and immunity. XII. Assay, properties and partial purification of a hydrazine-sensitive serum factor (factor A) in the properdin system. Z Immun exp ther. 1959 Oct-Nov;118:329–348. [PubMed] [Google Scholar]
- RABIN E. R., GRABER C. D., VOGEL E. H., Jr, FINKELSTEIN R. A., TUMBUSCH W. A. Fatal pseudomonas infection in burned patients. A clinical, bacteriologic and anatomic study. N Engl J Med. 1961 Dec 21;265:1225–1231. doi: 10.1056/NEJM196112212652501. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- TEPLITZ C., DAVIS D., MASON A. D., Jr, MONCRIEF J. A. PSEUDOMONAS BURN WOUND SEPSIS. I PATHOGENESIS OF EXPERIMENTAL PSEUDOMONAS BURN WOUND SEPSIS. J Surg Res. 1964 May;4:200–216. doi: 10.1016/s0022-4804(64)80026-3. [DOI] [PubMed] [Google Scholar]
- TEPLITZ C. PATHOGENESIS OF PSEUDOMONAS VASCULITIS AND SEPTIC LEGIONS. Arch Pathol. 1965 Sep;80:297–307. [PubMed] [Google Scholar]
- Uss R. H., Fenton J. W., 2nd, Muraschi T. F., Miller K. D. Two distinct elastases from different strains of Pseudomonas aeruginosa. Biochim Biophys Acta. 1969 Sep 30;191(1):179–181. doi: 10.1016/0005-2744(69)90332-5. [DOI] [PubMed] [Google Scholar]
- Vroon D. H., Schultz D. R., Zarco R. M. The separation of nine components and two inactivators of components of complement in humansserum. Immunochemistry. 1970 Jan;7(1):43–61. doi: 10.1016/0019-2791(70)90029-7. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]





