Abstract
Implementation of Einstein's law connecting charge, diffusion coefficient, and mobility to interpret experimental data on proteins from single molecule electrophoresis through nanopores faces serious difficulties. The protein charge and diffusion coefficient, inferred with the Einstein law, can be orders of magnitude smaller than their bare values depending on the electrolyte concentration, pore diameter, chemical nature of the pore wall, and the externally applied voltage. The main contributors to the discrepancies are the coupled dynamics of the protein and its counterion cloud, confinement effects inside the pore, and the protein-pore-surface interaction. We have addressed these ingredients by harking on classical theories of electrophoresis of macroions and hydrodynamics inside pores, and deriving new results for pore-protein interactions. Putting together various components, we present approximate analytical formulas for the effective charge, diffusion coefficient, and mobility of a protein in the context of single molecule electrophoresis experiments. For the omnipresent pore-protein interactions, nonlinear dependence of the velocity of protein on voltage sets in readily and analytical formulas for this effect are presented. The derived formulas enable the determination of the bare charge and size of a protein from the experimentally measured apparent values.
I. INTRODUCTION
The fundamental law relating the charge Q, diffusion coefficient D, and electrophoretic mobility μ of an ion in a solution is the Einstein equation
| (1) |
where kBT is the Boltzmann constant times the absolute temperature, and μ = U/E is the ratio of the velocity U of the ion to the externally applied electric field E. Although this law is derived from equilibrium considerations and in the linear response regime, it is routinely used to characterize proteins in single molecule electrophoresis experiments with nanopores,1–14 where non-equilibrium effects are omnipresent. By fitting the experimentally determined histograms of the transit times of the protein molecule in a nanopore with a one-dimensional diffusion-drift model, D and U of the protein molecule are obtained. These values of D and U are then used to get the protein charge Q by using Eq. (1) and the protein radius Rg by additionally using the Stokes-Einstein relation for a sphere D = kBT/(6πηRg) (η is viscosity of the electrolyte solution). It turns out that Eq. (1) is dramatically inadequate in inferring the fundamental characteristics of the protein molecules from the single-molecule electrophoresis through nanopores.13,14
The failure of Eq. (1) arises from many factors working together. As the charged molecule moves down the electric potential gradient along the pore, it faces an opposing drag from its counterion cloud. The drags of the protein and the counterion cloud are mediated by hydrodynamic and electrostatic forces inside the pore. The finite size of the pore restricts the translational degree of freedom in directions orthogonal to the pore axis. In addition, the protein molecules can adsorb at the pore wall resulting in a reduced mobility. Although a complete resolution of all of these issues is an insurmountable task, we present here an approximate generalization of Eq. (1) by allowing for protein-counterion-cloud dynamics, confinement effects from the pore, and protein-pore-wall interaction as
| (2) |
where Qeff and Deff are the effective charge and effective diffusion coefficient depending on the electrolyte concentration, pore radius, and pore-protein interaction energy. In addition, we give analytical formulas for the nonlinear dependence of μ on E and the mean translocation time. Equation (2) may be taken as a generalization of Qeff and Deff in bulk electrophoresis16–18 to the presence of the pore.
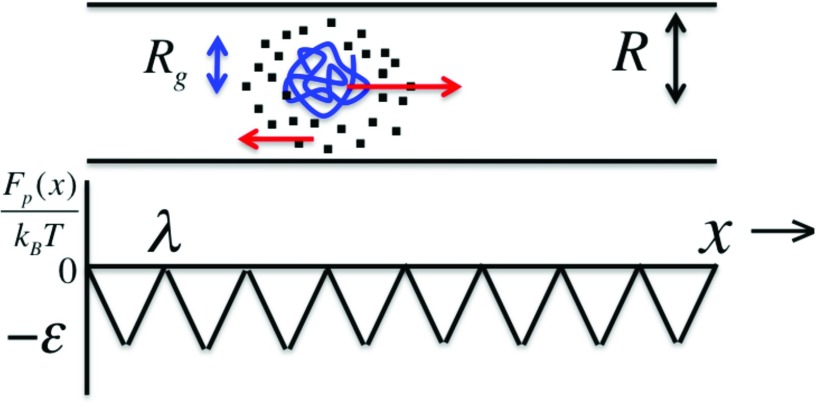
We consider the mobility of a protein molecule of radius of gyration Rg and net surface charge Q inside a cylindrical pore of uniform radius R and length L containing an electrolyte solution (with monovalent salt concentration cs and viscosity η) under a constant electric field E along the pore axis (Figure 1). The potential interaction Fp between the protein and the pore wall is modeled as a saw-tooth periodic potential of period λ (comparable to 2Rg) and depth εkBT. Each barrier is associated with the protein migration by a distance of 2Rg. Ignoring the inertial force, the Langevin equation for the instantaneous velocity of the macromolecule along the pore (x-direction) is
| (3) |
where −ζdx/dt is the frictional force (with ζ = kBT/D being the friction coefficient), frandom is the random force arising from the collisions of solvent molecules with the protein, and f is the net force on the protein from the external electric field fE, counterion cloud fcloud, confinement inside the pore fconf, and pore-protein interaction fp,
| (4) |
We address below successively the three major components of the problem, viz., collective dynamics of the protein and its counterion cloud, confined hydrodynamic transport inside the pore, and the effect of potential interaction between the protein and the pore wall.
FIG. 1.
Counterion cloud drags the protein in the opposite direction and gives an apparent charge different from protein's bare charge. The pore-protein interaction, modeled as a periodic potential, dramatically changes the diffusion coefficient and the voltage dependence of velocity of the protein.
II. PROTEIN-COUNTERION DYNAMICS
Without any confinement effects, the velocity v(r) of the fluid at location r outside the protein is given by the linearized Navier-Stokes equation in the steady state limit
| (5) |
where ρ(r) is the local charge density from all small ions surrounding the protein and E(r) is the local electric field and p is the pressure. By combining Eqs. (3)–(5) for a spherical particle and taking E(r) as , and using the Debye-Hückel theory and no-slip boundary condition at the particle surface, Hückel derived the velocity U = ⟨dx/dt⟩ as15
| (6) |
where the two terms on the right-hand side are from fE and fcloud, respectively. κ is the inverse Debye length, and for an aqueous solution at 25°C of monovalent salt at concentration cs in molarity, nm−1.
Combining the two terms on the right-hand side of Eq. (6),
| (7) |
where the macromolecule and its counterion cloud are taken as a collective entity (quasiparticle) with an effective charge Qeff which is much less than the bare charge Q. Several attempts to improve Hückel's theory are found in the literature.19–22 The most notable is from Henry (by accounting for local electric field near the particle arising from the continuity of current at the particle surface) with the result19
| (8) |
where the Henry correction g(κRg) varies between 1 and 3/2 for nonconducting macroions, given by
| (9) |
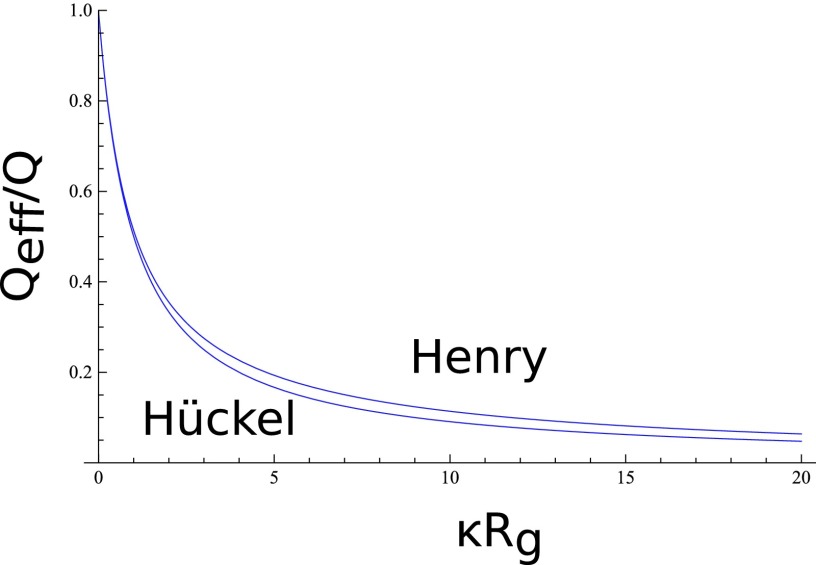
with E1(α) being the exponential integral . The ratio Qeff/Q is plotted in Figure 2 as a function of κRg for the Hückel (Eq. (7)) and Henry (Eq. (8)) expressions. The drag force from the counterion cloud significantly affects the inference of the protein charge in electrophoresis measurements. For example, for a protein of radius 1.5 nm in 1 M KCl, κRg = 5 and the effective charge is seen from Figure 2 to be reduced to about 20% of the bare charge.
FIG. 2.
The apparent charge Qeff is much smaller than Q depending on κRg.
III. CONFINEMENT EFFECTS
The diffusion and convection of a macromolecule are hindered by the physical boundary of the pore. Considering an uncharged spherical particle confined inside a cylindrical pore, the hard excluded volume effect and hydrodynamic interaction among the particle along the pore axis and the pore wall were addressed by Ferry23 and Faxén,24 respectively. As a result, the diffusion coefficient Dp of the particle inside the pore is substantially reduced, given by (known as the Renkin equation25)
| (10) |
More recently, Dechadilok and Deen26 have provided a better formula for Dp given by
| (11) |
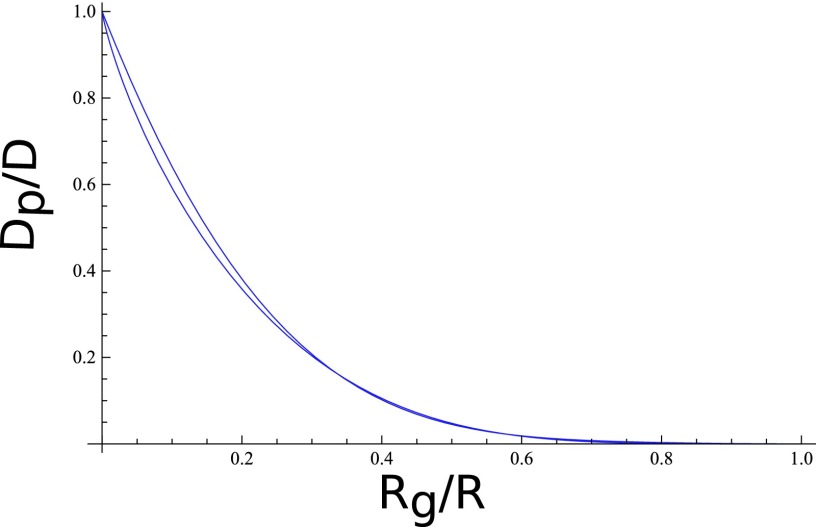
A plot of Dp/D versus Rg/R is given in Figure 3 for both Ferry-Faxén-Renkin and Dechadilok-Deen equations. Clearly, Dp is substantially smaller than D. A generalization of Faxén's result for restricted hydrodynamics involving macroions and counterion clouds in an electrolyte solution is nontrivial and entails numerical work. As we expect the dominant effect from the finite pore volume to arise from the excluded volume effect and as the hydrodynamic and electrostatic interactions are to be screened to some extent, we are here content with the above formulas for Dp and Qeff.
FIG. 3.
Effect of pore confinement on diffusion coefficient according to Ferry-Faxén-Renkin (top curve) and Dechadilok-Deen (bottom curve).
IV. PORE-PROTEIN INTERACTION
We now consider the effect of the periodic potential energy profile along the pore on the diffusion and drift of the protein molecule. The Langevin equation from Eqs. (3) and (4) is
| (12) |
where the confinement effect is subsumed into Dp and the fluctuation-dissipation theorem for the random noise Γ(t) is satisfied. The force fp due to pore-protein interaction is −∂Fp(x)/∂x, where Fp is taken as the saw-tooth periodic potential,
| (13) |
ε is the strength of the attractive energy in units of kBT as sketched in Figure 1. Since the net effect of fE and fcloud is to yield the apparent charge Qeff, we write fE + fcloud = Qeff E as −∂(−Qeff Ex)/∂x. Therefore, Eq. (12) becomes
| (14) |
with
| (15) |
where . The Fokker-Planck equation for the probability of finding the protein at x and time t, and the flux J, follows from Eq. (12) as10,28
| (16) |
A. Effective diffusion coefficient
The presence of the potential well (or a potential barrier) inside every period of the potential energy profile modifies the diffusion coefficient. For E = 0, the law of diffusion for each period λ obeys
| (17) |
where τ0 is the mean first passage time for one period (with reflecting boundary condition at x = 0 and absorbing boundary condition at x = λ, and E = 0),
| (18) |
Substituting Eq. (13) into Eq. (18), we get from Eq. (17)
| (19) |
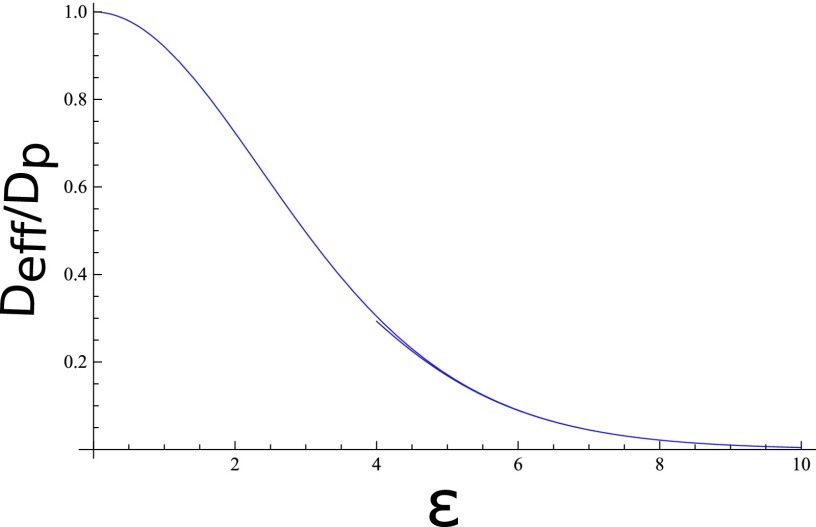
This exact result is plotted in Figure 4 as a function of ε (interaction energy in units of kBT). The diffusion coefficient is seen to decrease significantly as the barrier height increases. The asymptotic limits of Eq. (19) are
| (20) |
The large barrier result is reminiscent of the Kramer's formula for barrier crossing, but with a different prefactor ε2. This limit is also included in Figure 4. By measuring Deff and using Eq. (19), the pore-protein interaction energy can be determined.
FIG. 4.
Dependence of diffusion coefficient on pore-protein interaction energy. Bottom curve is from Eq. (20).
B. Electrophoretic mobility
The probability to find the macromolecule at x in the steady state (J = constant) follows from an integration of Eq. (16) as
| (21) |
For the periodic potential , the standard analysis gives (for m = integer)27–29
| (22) |
with
| (23) |
Averaging Eq. (14) over the normalized P(x) in each period, and with ⟨Γ(t)⟩ = 0, the average velocity of the protein is
| (24) |
where
| (25) |
Substituting Eq. (15) into Eqs. (23) and (25), we get
| (26) |
and
| (27) |
The exact analytical expression for the velocity is given by Eqs. (24), (26), and (27) as a function of the effective charge Qeff, pore diffusion coefficient Dp, pore-protein interaction energy ε, period of the potential λ, and the strength of the electric field E.
In the linear response regime (θ ≪ 1), Eq. (24)) reduces to
| (28) |
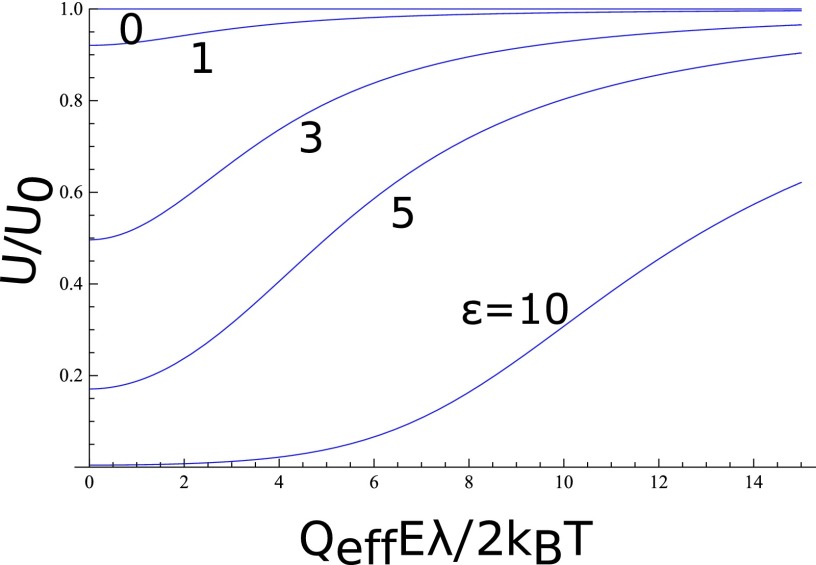
in view of Eq. (19). However, the linear response regime is valid only for very small values of θ for ε ≠ 0, as can be seen in Figure 5, where from Eq. (24) is plotted against for different values of ε.
FIG. 5.
Velocity reduction by pore-protein interaction energy ε and the onset of nonlinear dependence on the electric field. U0 is the velocity in the linear response regime .
C. Average translocation time
The average translocation time for the protein through the nanopore of length L with N periods (L = Nλ) is given by the mean first passage time
| (29) |
where the reflecting boundary condition at x = 0 and absorbing boundary condition at x = L are used with the initial position of the protein being at x = 0. Using Eqs. (13), (15), and (22),
| (30) |
where τ1 is the mean first passage time over one period (with replacing in Eq. (18), as a generalization to E ≠ 0). Combination of Eqs. (13), (15), (25), and (30) gives
| (31) |
and
| (32) |
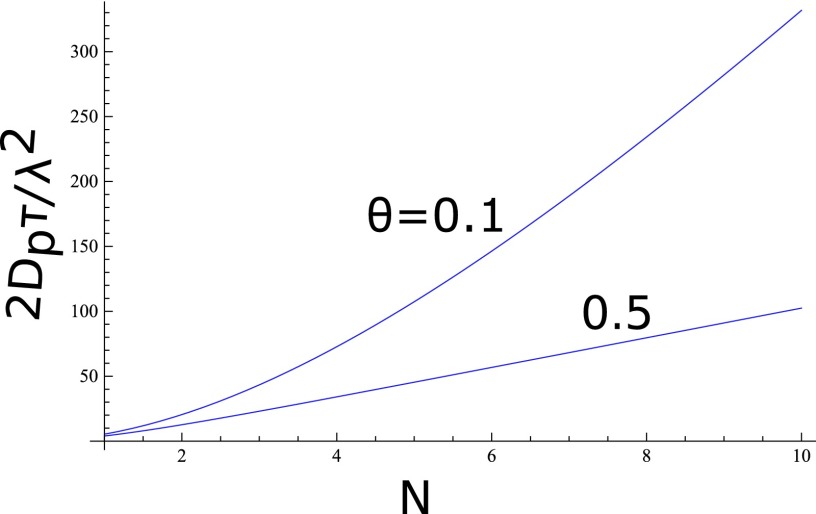
with I± given in Eq. (26). The above equation provides the complete dependence of the average translocation time on the pore length (L = Nλ) in terms of ε and E. As an illustration, a plot of Eq. (32) as 2Dpτ/λ2 vs. N is given in Figure 6. The above formula reduces to the expected limits. For E → 0, it becomes the law of diffusion,
| (33) |
and for ε = 0, it becomes the familiar drift-diffusion formula,10,30
| (34) |
In its own right, Eq. (34) is a crossover between the diffusive law τ = L2/(2Dp) for and the drift law for .
FIG. 6.
Dependence of mean translocation time on pore length for ε = 5 and (top) and 0.5 (bottom).
Combination of Eqs. (8), (11), (19), (24)–(27), and (32) gives the full set of equations for the effective charge, effective diffusion coefficient, velocity, and mean translocation time in terms of Q, D, pore radius R, inverse Debye length κ, protein radius Rg, period λ and strength ε of the pore-protein interaction potential, and the electric field. The effective values of diffusion coefficient and velocity obtained from fitting experimental translocation time histograms are shrouded by the various contributing factors in these equations. The derived formulas constitute a good starting point in sorting out Q, D, and μ from nanopore electrophoresis experiments.
ACKNOWLEDGMENTS
Author acknowledges the National Institutes of Health (Grant No. R01HG002776-11), National Science Foundation (Grant No. DMR 1104362), and AFOSR (Grant No. FA9550-14-1-0164).
REFERENCES
- 1.Movileanu L., Protein Pept. Lett. 21, 235 (2004). 10.2174/09298665113209990078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen P., Gu J., Brandin E., Kim Y.-R., Wang Q., and Branton D., Nano Lett. 4, 2293 (2004). 10.1021/nl048654j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han A., Schürmann G., Mondin G., Bitterli R. A., Hagelbach N. G., de Rooij N. F., and Staufer U., Appl. Phys. Lett. 88, 093901 (2006). 10.1063/1.2180868 [DOI] [Google Scholar]
- 4.Fologea D., Ledden B., McNabb D. S., and Li J., Appl. Phys. Lett. 91, 053901 (2007). 10.1063/1.2767206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han A., Creus M., Schürmann G., Linder V., Ward T. R., de Rooij N. F., and Staufer U., Anal. Chem. 80, 4651 (2008). 10.1021/ac7025207 [DOI] [PubMed] [Google Scholar]
- 6.Talaga D. S. and Li J., J. Am. Chem. Soc. 131, 9287 (2009). 10.1021/ja901088b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frinkes M., Pedone D., Knezevic J., Döblinger M., and Rant U., Nano Lett. 10, 2162 (2010). 10.1021/nl100861c [DOI] [PubMed] [Google Scholar]
- 8.Merchant C. A., Healy K., Wanunu M., Ray V., Peterman N., Bartel J., Fischbein M. D., Venta K., Luo Z., Johnson A. T. C., and Drndic M., Nano Lett. 10, 2915 (2010). 10.1021/nl101046t [DOI] [PubMed] [Google Scholar]
- 9.Kowalczyk S. W., Kapinos L., Blosser T. R., Megalhaes T., van Nies P., Bartel J., Lim R. Y. H., and Dekker C., Nat. Nanotechnol. 6, 433 (2011). 10.1038/nnano.2011.88 [DOI] [PubMed] [Google Scholar]
- 10.Muthukumar M., Polymer Translocation (Taylor & Francis, 2011). [Google Scholar]
- 11.Oukhaled A., Cressiot B., Bacri L., Pastoriza-Gallego M., Betton J.-M., Bourhis E., Jede R., Gierak J., Auvray L., and Pelta J., ACS Nano 5, 3628 (2011). 10.1021/nn1034795 [DOI] [PubMed] [Google Scholar]
- 12.Cressiot B., Oukhaled A., Patriarche G., Pastoriza-Gallego M., Betton J.-M., Auvray L., Muthukumar M., Bacri L., and Pelta J., ACS Nano 6, 6236 (2012). 10.1021/nn301672g [DOI] [PubMed] [Google Scholar]
- 13.Plesa C., Kowalczyk S. W., Zinsmeester R., Grosberg A. Y., Rabin Y., and Dekker C., Nano Lett. 13, 658 (2013). 10.1021/nl3042678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J., Henley R. Y., Muthukumar M., Rosenstein J. K., and Wanunu M., Biophys. J. 106, 696 (2014). 10.1016/j.bpj.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hückel E., Phys. Z. 25, 204 (1924). [Google Scholar]
- 16.Muthukumar M., Electrophoresis 17, 1167 (1996). 10.1002/elps.1150170629 [DOI] [PubMed] [Google Scholar]
- 17.Long D., Viovy J.-L., and Ajdari A., Phys. Rev. Lett. 76, 3858 (1996). 10.1103/PhysRevLett.76.3858 [DOI] [PubMed] [Google Scholar]
- 18.Muthukumar M., J. Chem. Phys. 107, 2619 (1997). 10.1063/1.474573 [DOI] [Google Scholar]
- 19.Henry D. C., Proc. R. Soc. London, Ser. A 133, 106 (1931). 10.1098/rspa.1931.0133 [DOI] [Google Scholar]
- 20.Booth F., J. Chem. Phys. 18, 1361 (1950). 10.1063/1.1747478 [DOI] [Google Scholar]
- 21.Saville D., Annu. Rev. Fluid Mech. 9, 321 (1977). 10.1146/annurev.fl.09.010177.001541 [DOI] [Google Scholar]
- 22.Lizana L. and Grosberg A. Y., EPL 104, 68004 (2013). 10.1209/0295-5075/104/68004 [DOI] [Google Scholar]
- 23.Ferry J. D., J. Gen. Physiol. 20, 95 (1936). 10.1085/jgp.20.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faxén H., Ann. Phys. 68, 89 (1922). [Google Scholar]
- 25.Renkin E. M., J. Gen. Physiol. 38, 225 (1954). [PMC free article] [PubMed] [Google Scholar]
- 26.Dechadilok P. and Deen W. M., Ind. Eng. Chem. Res. 45, 6953 (2006). 10.1021/ie051387n [DOI] [Google Scholar]
- 27.Lifson S. and Jackson J. L., J. Chem. Phys. 36, 2410 (1962). 10.1063/1.1732899 [DOI] [Google Scholar]
- 28.Risken H., The Fokker-Planck Equation (Springer, Berlin, Germany, 1989). [Google Scholar]
- 29.Hänggi P., Talkner P., and Borkovec M., Rev. Mod. Phys. 62, 251 (1990). 10.1103/RevModPhys.62.251 [DOI] [Google Scholar]
- 30.Muthukumar M., J. Chem. Phys. 111, 10371 (1999). 10.1063/1.480386 [DOI] [Google Scholar]