Abstract
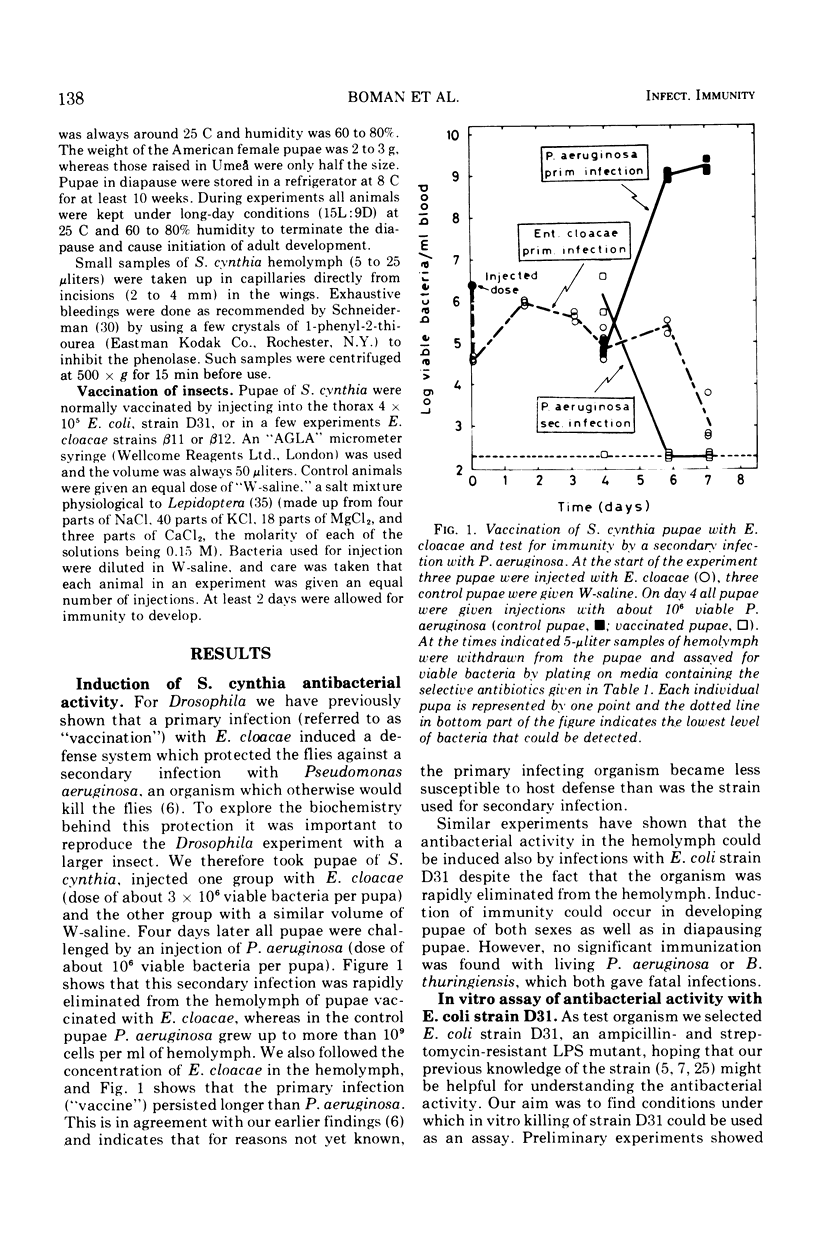
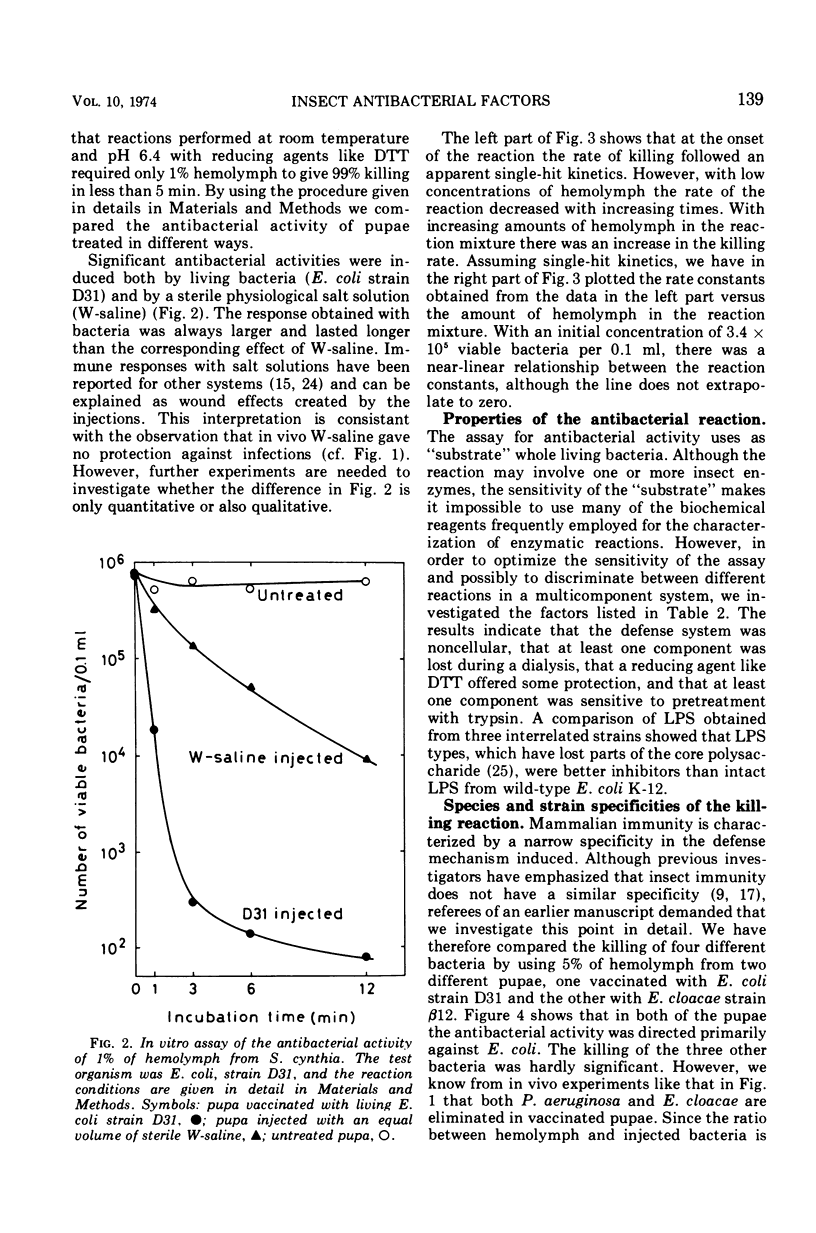
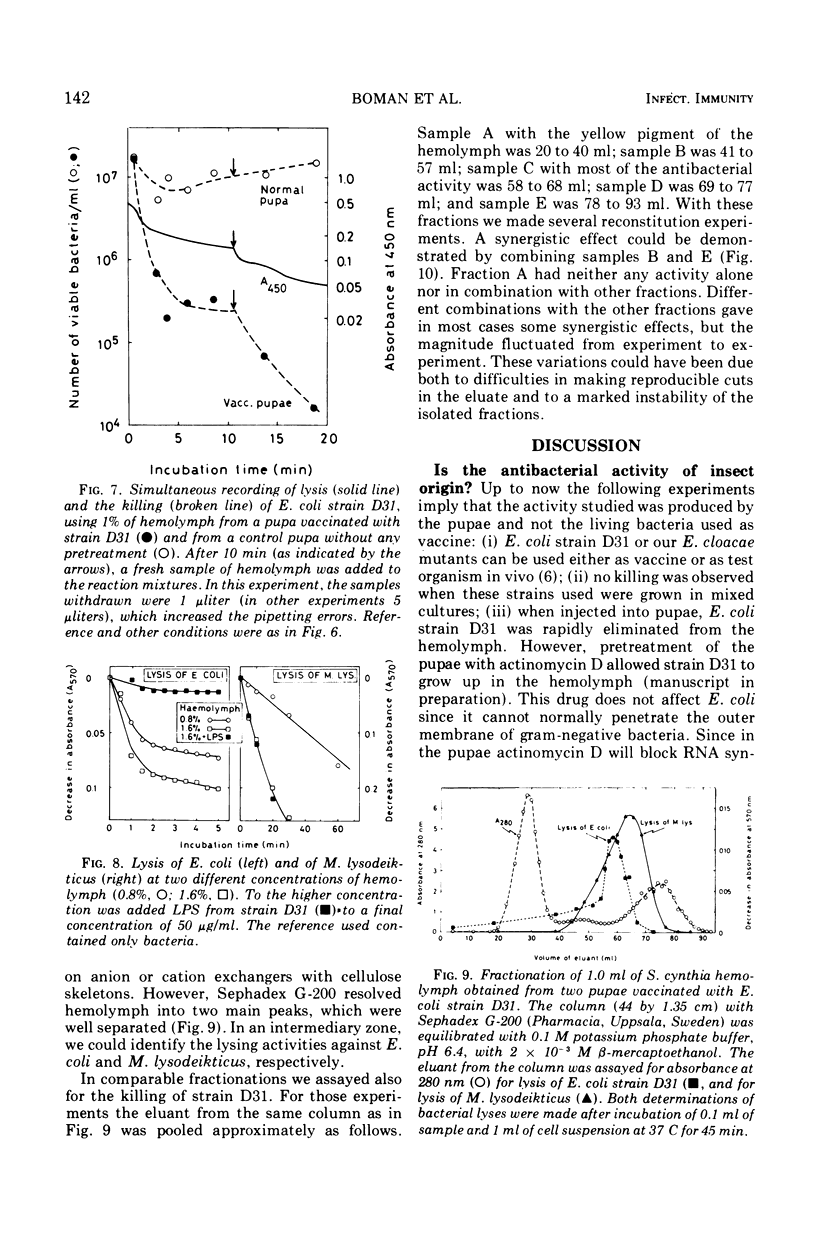
Pupae of the silk moth, Samia cynthia, were found to contain an inducible antibacterial activity in their hemolymph. This immunity response was provoked by primary infections with either Escherichia coli K-12 or Enterobacter cloacae. In both cases the antibacterial activity was directed chiefly towards E. coli. During standard conditions, 1% of hemolymph could kill 103 to 104 viable E. coli, strain D31, within 5 min. A lower level of antibacterial activity was induced by injections of a sterile salt solution. The killing of strain D31 followed single-hit kinetics, and increasing rate constants were obtained for increasing amounts of hemolymph. The reaction was sensitive to pretreatment with trypsin and it was protected by reducing agents. The activity was inhibited by microgram quantities of lipopolysaccharide (LPS) prepared from certain LPS mutants of E. coli K-12. A comparison of the susceptibility showed that “heptose-less” LPS mutants were more sensitive to killing than other strains. During standard conditions hemolymph will lyse both E. coli and Micrococcus lysodeikticus. Lysis of E. coli followed a multi-hit kinetics and it was inhibited by LPS, whereas lysis of M. lysodeikticus was unaffected by LPS. Hemolymph was fractionated on Sephadex G-200, and the lytic activities were recovered in partly overlapping peaks. Reconstitution with pooled fractions gave synergistic effects with the killing assay.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Bakula M. Antibacterial compounds in the cell-free haemolymph of Drosophila melanogaster. J Insect Physiol. 1970 Jan;16(1):185–197. doi: 10.1016/0022-1910(70)90125-3. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Jonsson S., Monner D., Normark S., Bloom G. D. Cell-surface alterations in Escherichia coli K-12 with chromosmal mutations changing ampicillin resistance. Ann N Y Acad Sci. 1971 Jun 11;182:342–357. doi: 10.1111/j.1749-6632.1971.tb30670.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nilsson I., Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972 May 26;237(5352):232–235. doi: 10.1038/237232a0. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Chadwick J. S. Serological responses of insects. Fed Proc. 1967 Nov-Dec;26(6):1675–1679. [PubMed] [Google Scholar]
- Chadwick J. S., Vilk E. Endotoxins from several bacterial species as immunizing agents against Pseudomonas aeruginosa in Galleria mellonella. J Invertebr Pathol. 1969 May;13(3):410–415. doi: 10.1016/0022-2011(69)90193-1. [DOI] [PubMed] [Google Scholar]
- Day N. K., Gewurz H., Johannsen R., Finstad J., Good R. A. Complement and complement-like activity in lower vertebrates and invertebrates. J Exp Med. 1970 Nov;132(5):941–950. doi: 10.1084/jem.132.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Harshbarger J. C., Faust R. M. Environmental factors internal to the host that affect the sucess of microbial insecticides. Ann N Y Acad Sci. 1973 Jun 22;217:131–140. doi: 10.1111/j.1749-6632.1973.tb32755.x. [DOI] [PubMed] [Google Scholar]
- Heimpel A. M., Harshbarger J. C. Symposium on microbial insecticides. V. Immunity in insects. Bacteriol Rev. 1965 Sep;29(3):397–405. doi: 10.1128/br.29.3.397-405.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen R., Anderson R. S., Good R. A., Day N. K. A comparative study of the bactericidal activity of horseshoe crab (Limulus polyphemus) hemolymph and vertebrate serum. J Invertebr Pathol. 1973 Nov;22(3):372–376. doi: 10.1016/0022-2011(73)90167-5. [DOI] [PubMed] [Google Scholar]
- Landureau J. C., Jollès P. Lytic enzyme produced in vitro by insect cells: lysozyme or chitinase? Nature. 1970 Mar 7;225(5236):968–969. doi: 10.1038/225968a0. [DOI] [PubMed] [Google Scholar]
- Marchialonis J. J., Edelman G. M. Isolation and characterization of a hemagglutinin from Limulus polyphemus. J Mol Biol. 1968 Mar 14;32(2):453–465. doi: 10.1016/0022-2836(68)90022-3. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powning R. F., Davidson W. J. Studies on insect bacteriolytic enzymes. I. Lysozyme in haemolymph of Galleria mellonella and Bombyx mori. Comp Biochem Physiol B. 1973 Jul 15;45(3):669–686. [PubMed] [Google Scholar]
- Riddiford L. M. Artificial diet for cecropia and other saturniid silkworms. Science. 1968 Jun 28;160(3835):1461–1462. doi: 10.1126/science.160.3835.1461. [DOI] [PubMed] [Google Scholar]
- Schwalbe C. P., Boush G. M. Clearance of 51Cr-labeled endotoxin from hemolymph of actively immunized Galleria mellonella. J Invertebr Pathol. 1971 Jul;18(1):85–88. doi: 10.1016/0022-2011(91)90012-f. [DOI] [PubMed] [Google Scholar]
- Watson J., Trenkner E., Cohn M. The use of bacterial lipopolysaccharides to show that two signals are required for the induction of antibody synthesis. J Exp Med. 1973 Sep 1;138(3):699–714. doi: 10.1084/jem.138.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weevers R. D. A lepidopteran saline: effects of inorganic cation concentrations on sensory, reflex and motor responses in a herbivorous insect. J Exp Biol. 1966 Feb;44(1):163–175. doi: 10.1242/jeb.44.1.163. [DOI] [PubMed] [Google Scholar]
- Werner R. A., Jones J. C. Phagocytic haemocytes in unfixed Galleria mellonella larvae. J Insect Physiol. 1969 Mar;15(3):425–437. doi: 10.1016/0022-1910(69)90291-1. [DOI] [PubMed] [Google Scholar]
- Zacharuk R. Y. Ultrastructural changes in the midgut epithelium of an elaterid larva (Coleoptera) infected enterically with Pseudomonas aeruginosa. Can J Microbiol. 1973 Jul;19(7):811–821. doi: 10.1139/m73-131. [DOI] [PubMed] [Google Scholar]



