Abstract
Brown adipose tissue (BAT) is specialized for energy expenditure, a process called adaptive thermogenesis. PET-CT scans recently demonstrated the existence of metabolically active BAT in adult humans, which revitalized our interest in BAT. Increasing the amount and/or activity of BAT holds tremendous promise for the treatment of obesity and its associated diseases. PGC1α is the master regulator of UCP1-mediated thermogenesis in BAT. A number of proteins have been identified to influence thermogenesis either positively or negatively through regulating the expression or transcriptional activity of PGC1α. Therefore, BAT activation can be achieved by either inducing the expression of positive regulators of PGC1α or by inhibiting the repressors of the PGC1α/UCP1 pathway. Here, we review the most important negative regulators of PGC1α/UCP1 signaling and their mechanism of action in BAT-mediated thermogenesis.
Keywords: PGC1a, UCP1, Oxidative phosphorylation, Obesity, Thermogenesis
Introduction
The relatively easy availability of calorie-rich diets compounded with technology-driven sedentary life styles have caused epidemic levels of obesity in the developed world (Malik et al., 2013). Since obesity is identified as a significant risk factor for a vast number of diseases, such as cardiovascular disease, type 2 diabetes, hypertension, fatty liver disease, atherosclerosis, degenerative disorders, and numerous cancers, it has become a substantial health concern around the world (Hursting, 2014; Mirza, 2011; Poirier and Eckel, 2002; Vucenik and Stains, 2012). In the US, ~35% of adults and 17% of children are obese; overall, ~60% of US adults are either obese or overweight, which costs ~$150 billion per year for direct healthcare and other indirect costs.
Obesity results from a shift in the energy balance of the body. Adipose (fat) tissues play a central role in maintaining energy homeostasis (Klaus, 2004; Spiegelman and Flier, 1996). If the energy intake persistently exceeds energy expenditure, the excessive energy is stored as triglycerides in the form of unilocular lipid droplets in the white adipose tissue (WAT). WAT not only functions as a simple storehouse of energy but is also an active endocrine organ and secretes cytokines (also known as adipokines) such as leptin and adiponectin (Guerre-Millo, 2004). The adipokine levels fluctuate in response to body energy levels and communicate with other organs, such as brain, muscle and liver to regulate energy homeostasis (Trayhurn and Wood, 2004). WAT represents ~10% of the body weight of a healthy adult human. However, WAT has an incredible ability to expand by both hypertrophy and hyperplasia in response to excessive energy influx (Hausman et al., 2001). Although initially it was considered as an evolutionary ‘gift’ to cope with periods of food scarcity, this quickly turned out to be a ‘curse’ in the present times of easy availability of energy-rich food.
As the storehouse of energy, WAT has become the primary organ of interest to target and treat obesity. However, strategies aimed at directly targeting and inhibiting differentiation and/or expansion of WAT may not be very successful. This is mainly because in absence of a sufficient amount of WAT reserves or an inability of WAT to expand in response to an extra influx of energy could lead to ectopic accumulation of lipids in other organs, such as liver, kidney, and skeletal muscle, leading to the development of metabolic disorders such as insulin resistance and diabetes. For example, in response to adipocyte-specific deletion of PPARγ, a crucial regulator of adipocyte differentiation, there is severe loss of WAT (lipoatrophy) but the body weights of adult PPARγ-null mice are similar to wild-type litter mates. Moreover, the null mice suffered from severe insulin resistance, diabetes and fatty liver due to ectopic accumulation of lipid. These mice also displayed abnormalities of bone, skin, and mammary glands, all of which contain adipose tissue (Wang et al., 2013). Similar discouraging results were observed when WAT is completely abolished in a fatless (A-ZIP/F-1) mouse model.
These mice displayed increased levels of inflammation, developed diabetes, and are also susceptible to spontaneous and induced carcinogenesis (Nunez et al., 2006). Therefore, strategies aimed at directly targeting and reducing WAT will work only when it is coupled with strictly controlling energy intake. However, if we have the ability to control energy intake we would not have the problem of obesity in the first place. As a result, the attention has now quickly turned to exploring ways to increase body energy expenditure to combat excessive energy intake.
Brown adipose tissue
The existence of a second type of adipose tissue, called brown adipose tissue (BAT), is well described in rodents and in infant humans (Cannon and Nedergaard, 2004). For example, newborn humans have ~30 g of BAT, which represents ~1% of body weight (Cannon and Nedergaard, 2004; Hull, 1976). The function of BAT is very different from WAT’s lipid storage function. BAT is specialized for energy expenditure, a process called adaptive thermogenesis, a physiological mechanism during which energy is dissipated to generate heat in response to cold temperatures and possibly diet (Cannon and Nedergaard, 2004; Seale et al., 2009). The very densely packed mitochondria, which give the brown color to BAT, execute heat production through a unique protein called uncoupling protein-1 (UCP1), which is present in the inner mitochondrial membrane. UCP1 uncouples mitochondrial oxidative phosphorylation from ATP production and dissipates chemical energy as heat, thereby profoundly increasing energy expenditure (Klingenberg, 1999; Kozak and Anunciado-Koza, 2008). Since UCP1-mediated thermogenesis is driven by oxidative metabolism, BAT is metabolically highly active and utilizes predominantly lipid, which is stored in BAT in multiple small fat droplets (multilocular). BAT also actively takes up glucose. The prevailing role of UCP1 in BAT-mediated thermogenesis is further elevated when Ucp1 genetic knockout mice displayed an impaired ability to produce heat by nonshivering thermogenesis and exhibited cold intolerance (Enerback et al., 1997). Moreover, Ucp1 null mice gained more body weight when mice were housed at thermoneutral temperature (Feldmann et al., 2009), suggesting that UCP1-mediated thermogenesis can be activated not only in response to cold but also by diet. These findings suggest that activation of UCP1-mediated thermogenesis in BAT could have anti-obesity effects, which can be exploited for therapeutic benefits (Costford et al., 2007; Kozak and Anunciado-Koza, 2008).
Existence of BAT in adult humans
Although the existence of BAT has been well documented in rodents and newborn humans, for a long time it was believed that the steady loss of BAT during the growth process leaves too little BAT in adult humans, which is not enough to influence body weight. However, recent studies demonstrate that adult humans, in fact, have significant amounts of functionally active BAT. Using PET-CT (positron emission tomography) scans, the existence of metabolically active regions around the supraclavicular areas in adult humans was demonstrated. Biopsies from these regions revealed the presence of significant amounts of UCP1-expressing adipocytes, and the mass and activity of this tissue declined in obese and aged subjects (Virtanen et al., 2009). These findings revitalized the interest in BAT. Increasing the amount and/or activity of BAT thus holds tremendous promise for the treatment of obesity and its associated diseases. For example, in humans, as little as 50 g of BAT, which constitutes less than 0.1% of body weight, is estimated to consume up to 20% of the basal caloric needs if fully stimulated (Rothwell and Stock, 1983). Moreover, in a number of mouse models it was shown that increased activity of BAT protects from diet-induced and age-associated obesity (Seale et al., 2009). Together, these findings created exciting new prospects for the development of novel classes of drugs for the treatment of obesity and its associated metabolic diseases.
Regulation of BAT-mediated thermogenesis
Since UCP1 is the key factor in BAT-induced thermogenesis, understanding the regulation of UCP1 is essential to develop strategies to activate BAT thermogenesis. Earlier studies revealed peroxisome proliferator activated receptor γ coactivator 1α (PGC1α) regulates thermogenesis by directly inducing the expression of UCP1. Initially, PGC1α was identified as a cofactor that directly interacted with the nuclear receptor PPARγ in brown adipocytes (Puigserver et al., 1998). Later studies disclosed that, in addition to UCP1 and PPARγ, PGC1α also activates a number of other transcription factors and functions as the central regulator of numerous pathways involved in mitochondrial biogenesis and thermogenesis (Finck and Kelly, 2006; Lin et al., 2005a). For example, PGC1α coactivates nuclear respiratory factors 1 and 2 (NRF1 and NRF2), which regulate the expression of genes encoding respiratory chain subunits and other factors essential for mitochondrial function (Austin and St-Pierre, 2012; Wu et al., 1999). Moreover, PGC1α also activates a number of nuclear and non-nuclear receptor factors, such as PPARα, PPARβ, PPARδ (Austin and St-Pierre, 2012; Finck and Kelly, 2006; Handschin and Spiegelman, 2006; Lin et al., 2005a; Vega et al., 2000; Wang et al., 2003), retinoid receptors (Delerive et al., 2002), thyroid hormone receptor (Puigserver et al., 1998), glucocorticoid receptor (Knutti et al., 2000), estrogen receptor (Tcherepanova et al., 2000), hepatic nuclear factor-4 (HNF4) (Rhee et al., 2003), liver X receptor (LXR) (Lin et al., 2005a; Lin et al., 2005b), estrogen-related receptors (ERRs) (Huss et al., 2002), forkhead box O1 (FOXO1) (Puigserver et al., 2003) and SREBP1 (Lin et al., 2005b). By regulating these factors, PGC1α exerts tremendous influence on several aspects of mitochondrial energy metabolism, and thus has emerged as the most dominant regulator of mitochondrial biogenesis and oxidative metabolism.
Accordingly, deletion of PGC1α in mice resulted in significant impairment in cold-induced adaptive thermogenesis (Lin et al., 2004). Similarly, brown preadipocytes lacking PGC1α differentiated normally but showed impaired induction of thermogenesis genes, indicating that PGC1α is essential for thermogenesis but dispensable for brown fat differentiation (Uldry et al., 2006). Conversely, overexpression of PGC1α in white adipocytes is sufficient to induce various genes involved in mitochondrial biogenesis and thermogenesis including UCP1 (Puigserver et al., 1998; Tiraby et al., 2003), suggesting a central role of PGC1α in mitochondrial thermogenesis.
One of the well-known factors that strongly induce BAT-mediated thermogenesis is cold exposure. Following cold exposure, catecholamines, such as norepinephrine released by sympathetic nerve terminals, act on β-adrenergic receptors of the brown adipocytes (Cannon and Nedergaard, 2004). Activation of β-adrenergic receptor/cAMP signaling induces PGC1α via the PKA/Creb pathway (Herzig et al., 2001). In addition, the β-adrenergic/cAMP pathway can also induce PGC1α through p38 MAPK, which activates PGC1α by discharging p160-mediated repression and by increasing PGC1α protein stability (Cao et al., 2004). Overall, PGC1α expression, as well as its transcriptional activity is greatly induced in response to cold exposure. A number of factors such as FOXC2, SRC1, CREB, SIRT3 and p38 MAPK positively regulate PGC1α transcription (Seale et al., 2009). On the other hand, due to its critical role in thermogenesis, the expression and activity of PGC1α and/or UCP1 are tightly controlled by a variety of other factors that negatively regulate PGC1α and UCP1. These factors most likely serve as brakes and control thermogenesis to prevent adverse conditions such as hyperthermia, which has the potential to cause organ failure and death.
Receptor Interacting Protein 140 (RIP140)
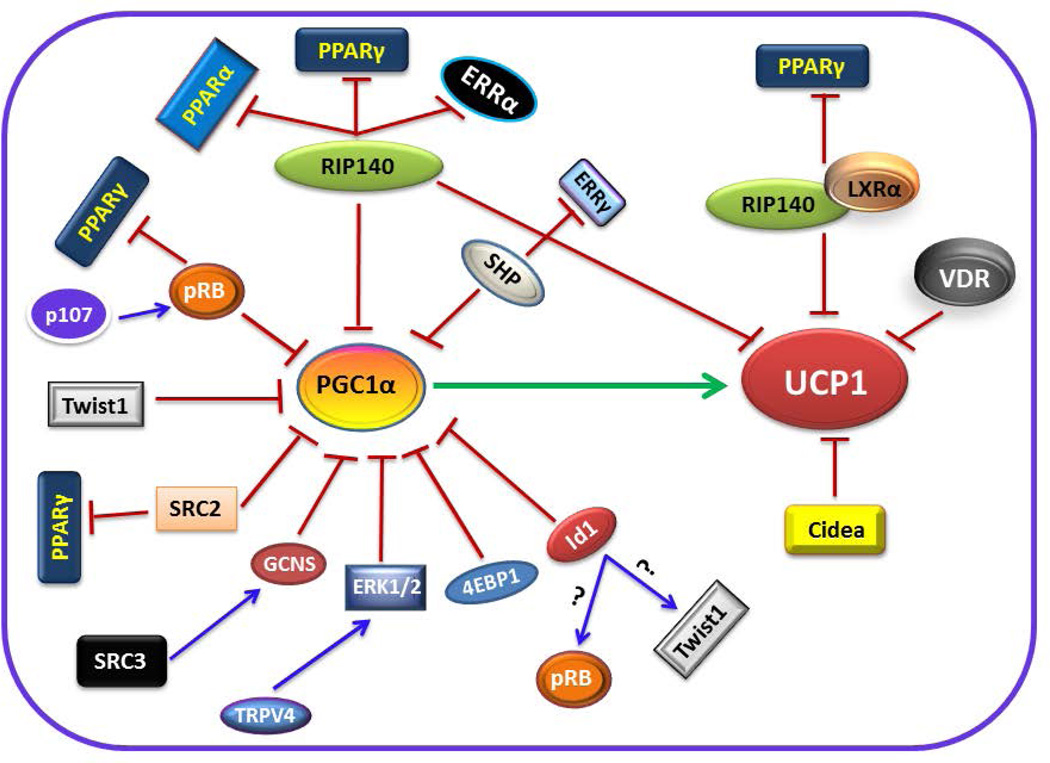
RIP140 is a transcriptional corepressor that is recruited to several nuclear receptors in a ligand-dependent manner (Christian et al., 2006). RIP140 and PGC1α share a number of downstream target gene promoters. RIP140 binds directly to PGC1α and inhibits its transcriptional activity on the target gene promoters shared by PGC1α and RIP140 (Hallberg et al., 2008). Presence or absence of RIP140 does not alter the expression of PGC1α but lack of RIP140 leads to increased PGC1α transcriptional activity. RIP140 also suppresses UCP1 expression by facilitating binding of repressive histone-modifying and DNA methylation enzymes to the UCP1 promoter to silence gene expression (Kiskinis et al., 2007). Lack of RIP140 leads to up-regulation of UCP1 gene expression due to derepression of UCP1 activating factors such as PPARα, PPARγ and ERRα (Fig. 1). Genetic ablation of RIP140 results in the formation of brown-like adipocytes within WAT with increased expression of PPARα and UCP1. Consequently, RIP140-deficient mice are lean and resistant to diet-induced obesity due to increased energy expenditure (Leonardsson et al., 2004). In contrast, constitutive overexpression of RIP140 in adipocytes and skeletal muscle suppresses expression of genes involved in mitochondrial biogenesis and oxidative metabolism (Christian et al., 2006; Leonardsson et al., 2004; Powelka et al., 2006).
Figure 1.
Cartoon showing an array of factors that negatively regulate PGC1α/UCP1-mediated thermogenesis. The detailed mechanism of action of the individual repressors is described in the text.
Liver X Receptor α (LXRα)
LXRα does not interfere with PGC1α expression or its transcriptional activity but rather functions as a direct transcriptional suppressor of UCP1 gene expression. The ligand-activated LXRα interferes with the transactivation of the Ucp1 promoter by competing with and dismissing PPARγ from the Ucp1 enhancer. LXRα achieves PPARγ discharge from the Ucp1 enhancer by recruiting RIP140 as a corepressor to the LXRα binding site. Since this region overlaps with the PPAR response element in the enhancer region of the Ucp1 promoter, binding of RIP140 leads to discharge of PPARγ (Fig. 1). In the absence of RIP140, LXRα fail to dismiss PPARγ from the Ucp1 promoter (Collins et al., 2010; Wang et al., 2008). Mice lacking both LXR isoforms (LXRα and LXRβ) (Kalaany et al., 2005), or LXRα alone have a lean phenotype with increased expression of UCP1 in BAT and WAT with no apparent effect on the expression of PGC1α (Wang et al., 2008). In addition, adipocytes from LXRα null mice have higher mitochondrial density and increased expression of Ucp1 and other genes that are involved in mitochondrial biogenesis and oxidative phosphorylation (Collins et al., 2010; Wang et al., 2008).
Vitamin D receptor (VDR)
VDR is a member of the nuclear receptor superfamily. It mediates the actions of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D. The ligand-activated VDR appears to inhibit UCP1 expression since mice deficient for VDR or Cyp27b1 (1α-hydroxylase enzyme that generates 1,25(OH)2D3) display a lean phenotype and are resistant to diet-induced obesity, due to increased expression of UCP1, 2 and 3 and fatty acid oxidation genes in BAT and WAT (Narvaez et al., 2009; Wong et al., 2009). In contrast, adipocyte-specific overexpression of human VDR reduces energy expenditure and suppresses expression of genes involved in thermogenesis and fatty acid oxidation in WAT and BAT, leading to obesity (Wong et al., 2011). In vitro experiments in primary brown adipocyte cultures revealed that 1,25(OH)2D3 can directly suppress the expression of the UCPs (Wong et al., 2009). However, the mechanisms by which 1,25(OH)2D3, VDR or 1,25(OH)2D3/VDR complex repress UCP1 expression have not been clear until recently. By utilizing VDR mutant cells isolated from patients with HVDRR, it was shown that the unliganded VDR can directly regulate the expression of UCP1 by binding to a VDR-element (VDRE) in the proximal region of the UCP1 promoter and suppresses its expression (Fig. 1). However, mutant VDRs that cannot occupy a VDRE on the UCP1 promoter fail to suppress UCP1 expression (Malloy and Feldman, 2013).
Cell death-inducing DFFA-like Effector A (Cidea)
Cidea is a member of the CIDE apoptotic family. Cidea is highly expressed in BAT and directly interacts with UCP1 and suppresses its uncoupling function (Fig. 1), thereby reducing energy expenditure (Zhou et al., 2003). Cidea-deficient mice are resistant to obesity and showed an higher metabolic rate in BAT. Cidea-null mice also have higher body temperature when exposed to cold compared to wild type (Zhou et al., 2003), suggesting that thermogenesis is increased in the absence of Cidea. Moreover, Cidea is identified to regulate the metabolic sensor AMPK, which plays a critical role in energy homeostasis. Cidea forms a complex with the β subunit of AMPK, which leads to ubiquitin-mediated degradation of the AMPK-β subunit. In the absence of Cidea, the protein levels and enzymatic activity of AMPK is increased in BAT (Qi et al., 2008). Therefore, Cidea not only regulates UCP1 activity but also controls AMPK-associated energy homeostasis.
Retinoblastoma protein (pRB)
The retinoblastoma protein, pRB, functions as a molecular switch in white versus brown adipocyte differentiation programming (Hansen et al., 2004). For example, in response to PPARγ ligand rosiglitazone stimulation, pRB-deficient MEFs differentiate into brown-like adipocytes with high mitochondrial content, and increased expression of the thermogenic genes PGC1α and UCP1, and numerous other mitochondrial genes (Hansen et al., 2004). A number of studies addressed the in vivo requirement for pRB in BAT development and function. Targeted deletion of pRB along with p53, specifically in mesenchymal stem cells, resulted in the preferential formation of brown adipocyte-containing tumors known as hibernomas (Calo et al., 2010), suggesting that pRB could modulate a cell fate choice between muscle and BAT. Moreover, adipocyte-specific deletion of pRB in mice leads to browning of WAT and activation of BAT with concomitant increased energy expenditure. As a result, the mice are protected from diet-induced obesity (Dali-Youcef et al., 2007). Similarly, RB haploinsufficient (RB) mice displayed enhanced activation of BAT and higher expression of genes involved in mitochondrial oxidative metabolism in adipose tissues, and consequently, they are also protected from diet-induced obesity, insulin resistance and hepatosteatosis (Mercader et al., 2009). Interestingly, mice deficient in p107, another member of the pocket protein family, also contain abundant brown-like adipocytes within WAT. The WAT of p107-null mice is enriched in mitochondria with elevated levels of PGC1α and UCP1. Remarkably, pRB levels significantly declined in p107-deficient adipocyte precursors, indicating that p107 function could be facilitated through regulation of pRB (Scime et al., 2005). At the molecular level, it was described that pRB modulates energy metabolism by directly binding to the PGC1α promoter and suppresses its transcription (Scime et al., 2005) (Fig. 1). In addition, pRB functions as a PPARγ corepressor, and lack of pRB leads to increased PPARγ activity (Fajas et al., 2002). Simultaneously, pRB could also regulate oxidative metabolism through E2F1, as E2F1 was shown to suppress key genes in energy metabolism in a pRB-dependent manner in BAT (Blanchet et al., 2011). Browning of WAT, mediated by pRB, could be regulated through FOXC2, an adipocyte-specific forkhead transcription factor. FOXC2 expression is induced in pRB-deficient MEFs and its activity favors a PKA holoenzyme composition (RIα subunit) with lower threshold for activation by cAMP (Hansen et al., 2004). This ultimately results in induced expression of mitochondrial biogenesis and oxidative metabolism genes leading to browning of WAT.
Twist basic helix-loop-helix transcription factor 1 (Twist1)
Twist1 is a basic helix-loop-helix (bHLH) transcription factor that regulates diverse cellular processes such as embryogenesis, cellular differentiation and apoptosis (Puisieux et al., 2006). Recent studies discovered Twist1 as a negative regulator of BAT-mediated thermogenesis. Twist1 transgenic mice with adipocyte-specific overexpression results in reduced mitochondrial density and suppression of UCP1 and fatty acid oxidation genes in BAT, leading to diet-induced obesity (Pan et al., 2009). In contrast, Twist1 heterozygous knockout mice are protected from diet-induced obesity due to higher mitochondrial density and increased expression of UCP1 and fatty acid oxidation genes. The lipid sensor PPARδ regulates Twist1 expression by directly binding to the Twist1 promoter and inducing its expression in BAT. Once induced, Twist1 does not influence the expression or localization pattern of PGC1α but rather directly binds to PGC1α and suppresses its transcriptional activity (Pan et al., 2009) (Fig. 1). PGC1α recruits Twist1 to the promoters of specific PGC1α downstream target genes. Once recruited Twist1 causes a reduction in histone H3 acetylation by recruiting histone deacetylase 5 (HDAC5) to the promoters of PGC1α target genes, leading to suppression of their induction, thereby directly regulates BAT-mediated thermogenesis (Pan et al., 2009).
Steroid receptor coactivators (SRCs)
The members of the SRC family, SRC1 (NcoA1), SRC2 (TIF2), and SRC3 (p/CIP), have divergent functions in the regulation of thermogenesis (Louet and O’Malley, 2007). In contrast to SRC1, which positively regulates PGC1α/UCP1-mediated thermogenesis, both SRC2 and SRC3 function as negative regulators of the PGC1α/UCP1 thermogenic pathway. SRC1 mediates its effects through PGC1α as it augments coactivation of PPARγ by PGC1α. As a result, genetic ablation of SRC1 leads to impaired thermogenesis due to reduced expression of UCP1, the downstream target of PGC1α, in BAT (Picard et al., 2002). In contrast to SRC1, SRC2 inhibits the interaction between PPARγ and PGC1α, leading to reduced activity of PGC1α. Consequently, deletion of SRC2 results in increased thermogenesis and improved energy expenditure (Picard et al., 2002). Similar to SRC2, SRC3 regulates BAT-mediated thermogenesis through PGC1α but by a distinct mechanism. SRC3 represses the transcriptional activity of PGC1α by inducing GCN5, the primary acetyltransferase of PGC1α (Lerin et al., 2006), which acetylates and suppresses PGC1α transcriptional activity (Fig. 1). Deletion of SRC3 decreases acetylation of PGC1α, resulting in increased mitochondrial biogenesis and thermogenesis (Coste et al., 2008).
Transient receptor potential cation channel 4 (TRPV4)
TRPV4 receptor belongs to a family of ion channels. In a recent study it was demonstrated that TRPV4 negatively regulates the expression of PGC1α, UCP1, and cellular respiration (Ye et al., 2012). Knockdown of TRPV4 results in induced expression of PGC1α and UCP1, and TRPV4-null mice showed increased energy expenditure and elevated UCP1 expression in fat tissues. Moreover, TRPV4 also controls the proinflammatory cytokine gene program that contributes to the development of insulin resistance. Consequently, mice treated with a TRPV4 inhibitor displayed increased expression of thermogenic genes and are protected from diet-induced obesity, inflammation, and insulin resistance. At the molecular level, the repression of PGC1α and the induction of proinflammatory cytokines by TRPV4 are mediated through ERK1/2 protein kinases (Ye et al., 2012) (Fig. 1).
Orphan nuclear receptor SHP (NR0B2)
SHP inhibits the transactivation of other nuclear receptors by either functioning directly as a transcriptional repressor or by competing with other coactivators for binding to activated nuclear receptors (Borgius et al., 2002; Johansson et al., 2000). SHP is strongly expressed in BAT, and it was demonstrated that SHP inhibits the ERRγ-mediated promoter transactivation of PGC1α, therefore functioning as a negative regulator of PGC1α expression in BAT (Fig. 1). SHP-deficient mice displayed increased energy expenditure and are resistant to diet-induced obesity due to elevated expression of PGC1α and UCP1 in BAT (Wang et al., 2005). However, in contrast to the expectation that SHP overexpression reduces energy expenditure, adipose-specific overexpression of SHP resulted in increased whole-body energy metabolism and enhanced BAT function by activating β1AR expression and mitochondrial biogenesis (Tabbi-Anneni et al., 2010). These unexpected results could be explained by the fact that in SHP-null mice whole-body deletion of SHP could have affected the metabolic functions of other organs such as liver and muscle, leading to a phenotype that is different and difficult to compare with studies in SHP transgenic mice where SHP is specifically overexpressed in adipose tissues.
Eukaryotic translation initiation factor 4E–binding protein-1 (4E–BP1)
Eukaryotic translation initiation complex, eIF4F, recognizes the 5′ cap structure of mRNAs and recruits them to the 40S ribosomal subunit. 4E–BPs (eIF4E–binding proteins) inhibit cap-dependent translation by sequestering eIF4E, thus interfering with eIF4F complex formation (Gingras et al., 1999). The translational inhibitor 4E–BP1 specifically represses the translation of PGC1α mRNA, leading to reduced levels of PGC1α protein in adipose tissues (Fig. 1). Mice deficient for 4E–BP1 (Eif4ebp1−/−) showed increased translation of PGC1α and induced expression of UCP1 in the adipose tissues. Consequently, Eif4ebp1-deficient mice exhibited an increased metabolic rate and reduced WAT mass (Tsukiyama-Kohara et al., 2001).
Inhibitor of DNA binding 1 (Id1)
Id1 belongs to the Id family (Id1-Id4) of helix-loop-helix (HLH) transcription factors. Id1 lacks a basic DNA binding domain; therefore, it functions by dimerization with other transcriptional regulators such as basic helix-loop-helix (bHLH) factors. Id1 expression is strongest in BAT compared to other metabolic organs. The expression levels of PGC1α and UCP1 are up-regulated in the BAT of Id1−/− mice, suggesting that Id1 deficiency results in increased thermogenesis (Satyanarayana et al., 2012). However, the molecular mechanism behind induced expression of thermogenic genes in the absence of Id1 still needs to be established. We are currently working to identify the molecular link between Id1 and the PGC1α/UCP1 thermogenic pathway. Since Id1 functions as a dominant negative regulator of other transcription factors, we are investigating whether Id1 co-operates with other negative regulators of PGC1α such as Rb and Twist1, thereby controlling the expression and activity of the PGC1α network of proteins involved in thermogenesis (Fig. 1).
Other negative regulators
In addition to the above described factors, a number of other factors have been identified that negatively regulate BAT-mediated thermogenesis. Deletion of these genes in mice resulted in increased expression of PGC1α and/or UCP1, leading to increased thermogenesis (Table 1).
Table I.
Genes that negatively control thermogenesis and their deletion lead to increased BAT-mediated thermogenesis
| Gene deleted | Effect on thermogenesis | Ref |
|---|---|---|
| Atf4 | Increased expression of PGC1α, PPARγ, Ucp1, Ucp2 and Ucp3 in BAT | (Wang et al., 2010) |
| Bace1 | Increased expression of UCP1 in BAT | (Meakin et al., 2012) |
| Foxo1 | Adipose tissue-specific overexpression of dominant negative Foxo1 leads to increased BAT-mediated thermogenesis due to induced expression of PGC1α, Ucp1, Ucp2 and Adrb3. |
(Nakae et al., 2008) |
| Oprd1 | Enhanced BAT-mediated thermogenesis due to induced expression of PGC1α and UCP1 |
(Czyzyk et al., 2012) |
| Pctp | Enlarged mitochondria and enhanced expression of thermogenic genes in BAT | (Kang et al., 2009) |
| Prkar2b | Increased PKA activity, induced expression of UCP1 leading to enhanced thermogenesis |
(Cummings et al., 1996) |
| Pref1 | Increased expression of PGC1α and UCP1 in BAT | (Armengol et al., 2012) |
| Smad3 | Increased mitochondrial biogenesis and induced expression of genes involved in thermogenesis |
(Yadav et al., 2011) |
| Tnfr1 | Increased expression of UCP1 in BAT | (Romanatto et al., 2009) |
| Them1 | Increased mitochondrial content and induced expression of thermogenic genes PGC1α, PPARγ and UCP1 in BAT |
(Zhang et al., 2012) |
Conclusions
Obesity results from a shift in the energy balance of the body. Therefore, strategies aimed at treating obesity should restore energy balance by increasing energy expenditure. Since the predominant function of BAT is to waste energy as heat, body energy expenditure could be raised by either increasing the amount of BAT or activating the existing BAT (Fig. 2). This concept is supported by numerous genetic studies in animal models where it has been demonstrated that an increase in the amount and/or activation of BAT causes a lean and healthy phenotype. Understanding the in-depth molecular mechanisms controlling BAT-mediated thermogenesis will facilitate the development of effective therapeutics to combat obesity and metabolic disorders. So far tremendous progress has been made in identifying genes that play critical roles not only in BAT development and differentiation but also activation and negative regulation. This knowledge can be effectively utilized to develop strategies to increase or activate BAT. One such therapeutic strategy could be identifying drugs that specifically target the repressors of BAT-mediated thermogenesis. Since the repressors or the negative regulators not only suppress thermogenesis but also differentiation and expansion of BAT, selective inhibition of these repressors could lead to BAT expansion as well as activation. However, it will be very challenging to specifically target these factors without causing unintended side-effects, because at least part of the molecular machinery that is involved in BAT-mediated thermogenesis is also active in other metabolic organs such as liver and muscle. Therefore, these organs will have to face unintended consequences from drugs that are directed towards BAT activation.
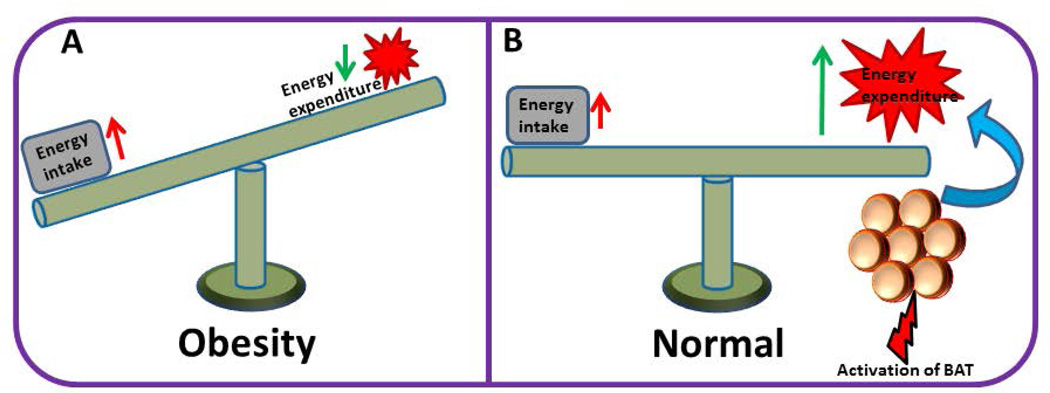
Figure 2.
(A) Obesity results from a shift in energy balance. If the energy intake is persistently higher than energy expenditure, it ultimately leads to obesity. (B) Activation of BAT leads to increased energy expenditure that counter-balances increased energy intake, leading to normalization of the body energy balance.
Acknowledgments
The authors thank Dr. Rhea-Beth Markowitz for critically reviewing the manuscript. This research is supported by the National Cancer Institute (NCI) of the National Institutes of Health (K22CA168828).
Footnotes
Conflict of interest
The authors declare no competing financial conflicts of interest.
References
- Armengol J, Villena JA, Hondares E, Carmona MC, Sul HS, Iglesias R, Giralt M, Villarroya F. Pref-1 in brown adipose tissue: specific involvement in brown adipocyte differentiation and regulatory role of C/EBPdelta. The Biochemical journal. 2012;443:799–810. doi: 10.1042/BJ20111714. [DOI] [PubMed] [Google Scholar]
- Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. Journal of cell science. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- Blanchet E, Annicotte JS, Lagarrigue S, Aguilar V, Clape C, Chavey C, Fritz V, Casas F, Apparailly F, Auwerx J, et al. E2F transcription factor-1 regulates oxidative metabolism. Nature cell biology. 2011;13:1146–1152. doi: 10.1038/ncb2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgius LJ, Steffensen KR, Gustafsson JA, Treuter E. Glucocorticoid signaling is perturbed by the atypical orphan receptor and corepressor SHP. The Journal of biological chemistry. 2002;277:49761–49766. doi: 10.1074/jbc.M205641200. [DOI] [PubMed] [Google Scholar]
- Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Molecular and cellular biology. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, White R, Parker MG. Metabolic regulation by the nuclear receptor corepressor RIP140. Trends in endocrinology and metabolism: TEM. 2006;17:243–250. doi: 10.1016/j.tem.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Collins S, Yehuda-Shnaidman E, Wang H. Positive and negative control of Ucp1 gene transcription and the role of beta-adrenergic signaling networks. International journal of obesity. 2010;(34 Suppl 1):S28–33. doi: 10.1038/ijo.2010.180. [DOI] [PubMed] [Google Scholar]
- Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O’Malley BW, Auwerx J. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1{alpha} Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17187–17192. doi: 10.1073/pnas.0808207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costford S, Gowing A, Harper ME. Mitochondrial uncoupling as a target in the treatment of obesity. Current opinion in clinical nutrition and metabolic care. 2007;10:671–678. doi: 10.1097/MCO.0b013e3282f0dbe4. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature. 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Romero-Pico A, Pintar J, McKinzie JH, Tschop MH, Statnick MA, Nogueiras R. Mice lacking delta-opioid receptors resist the development of diet-induced obesity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:3483–3492. doi: 10.1096/fj.12-208041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dali-Youcef N, Mataki C, Coste A, Messaddeq N, Giroud S, Blanc S, Koehl C, Champy MF, Chambon P, Fajas L, et al. Adipose tissue-specific inactivation of the retinoblastoma protein protects against diabesity because of increased energy expenditure. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10703–10708. doi: 10.1073/pnas.0611568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, Wu Y, Burris TP, Chin WW, Suen CS. PGC-1 functions as a transcriptional coactivator for the retinoid X receptors. The Journal of biological chemistry. 2002;277:3913–3917. doi: 10.1074/jbc.M109409200. [DOI] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, Miard S, Auwerx J. The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Developmental cell. 2002;3:903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. The Journal of clinical investigation. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annual review of biochemistry. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes & metabolism. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- Hallberg M, Morganstein DL, Kiskinis E, Shah K, Kralli A, Dilworth SM, White R, Parker MG, Christian M. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Molecular and cellular biology. 2008;28:6785–6795. doi: 10.1128/MCB.00504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocrine reviews. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA, Petrovic N, Enerback S, Nedergaard J, Cinti S, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hull D. The function of brown adipose tissue in the newborn. Biochemical Society transactions. 1976;4:226–228. doi: 10.1042/bst0040226. [DOI] [PubMed] [Google Scholar]
- Hursting SD. Obesity, energy balance, and cancer: a mechanistic perspective. Cancer treatment and research. 2014;159:21–33. doi: 10.1007/978-3-642-38007-5_2. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. The Journal of biological chemistry. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Johansson L, Bavner A, Thomsen JS, Farnegardh M, Gustafsson JA, Treuter E. The orphan nuclear receptor SHP utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Molecular and cellular biology. 2000;20:1124–1133. doi: 10.1128/mcb.20.4.1124-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. LXRs regulate the balance between fat storage and oxidation. Cell metabolism. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kang HW, Ribich S, Kim BW, Hagen SJ, Bianco AC, Cohen DE. Mice lacking Pctp /StarD2 exhibit increased adaptive thermogenesis and enlarged mitochondria in brown adipose tissue. Journal of lipid research. 2009;50:2212–2221. doi: 10.1194/jlr.M900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, Parker MG. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. The EMBO journal. 2007;26:4831–4840. doi: 10.1038/sj.emboj.7601908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S. Adipose tissue as a regulator of energy balance. Current drug targets. 2004;5:241–250. doi: 10.2174/1389450043490523. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Uncoupling protein--a useful energy dissipator. Journal of bioenergetics and biomembranes. 1999;31:419–430. doi: 10.1023/a:1005440221914. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Molecular and cellular biology. 2000;20:2411–2422. doi: 10.1128/mcb.20.7.2411-2422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. International journal of obesity. 2008;(32 Suppl 7):S32–38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell metabolism. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell metabolism. 2005a;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005b;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Louet JF, O’Malley BW. Coregulators in adipogenesis: what could we learn from the SRC (p160) coactivator family? Cell cycle. 2007;6:2448–2452. doi: 10.4161/cc.6.20.4777. [DOI] [PubMed] [Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nature reviews Endocrinology. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Feldman BJ. Cell-autonomous regulation of brown fat identity gene UCP1 by unliganded vitamin D receptor. Molecular endocrinology. 2013;27:1632–1642. doi: 10.1210/me.2013-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin PJ, Harper AJ, Hamilton DL, Gallagher J, McNeilly AD, Burgess LA, Vaanholt LM, Bannon KA, Latcham J, Hussain I, et al. Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice. The Biochemical journal. 2012;441:285–296. doi: 10.1042/BJ20110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader J, Ribot J, Murano I, Feddersen S, Cinti S, Madsen L, Kristiansen K, Bonet ML, Palou A. Haploinsufficiency of the retinoblastoma protein gene reduces diet-induced obesity, insulin resistance, and hepatosteatosis in mice. American journal of physiology Endocrinology and metabolism. 2009;297:E184–193. doi: 10.1152/ajpendo.00163.2009. [DOI] [PubMed] [Google Scholar]
- Mirza MS. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN gastroenterology. 2011;2011:592404. doi: 10.5402/2011/592404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, Iskandar K, Suga K, Lombes M, Hayashi Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150:651–661. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez NP, Oh WJ, Rozenberg J, Perella C, Anver M, Barrett JC, Perkins SN, Berrigan D, Moitra J, Varticovski L, et al. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer research. 2006;66:5469–5476. doi: 10.1158/0008-5472.CAN-05-4102. [DOI] [PubMed] [Google Scholar]
- Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O’Malley BW, Chambon P, Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Poirier P, Eckel RH. Obesity and cardiovascular disease. Current atherosclerosis reports. 2002;4:448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. The Journal of clinical investigation. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. British journal of cancer. 2006;94:13–17. doi: 10.1038/sj.bjc.6602876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J, Li JZ, Wu J, Zhou HM, Li P. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. The EMBO journal. 2008;27:1537–1548. doi: 10.1038/emboj.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. The Journal of biological chemistry. 2009;284:36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rothwell NJ, Stock MJ. Effects of age on diet-induced thermogenesis and brown adipose tissue metabolism in the rat. Int J Obes. 1983;7:583–589. [PubMed] [Google Scholar]
- Satyanarayana A, Klarmann KD, Gavrilova O, Keller JR. Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:309–323. doi: 10.1096/fj.11-190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell metabolism. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes & development. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- Tabbi-Anneni I, Cooksey R, Gunda V, Liu S, Mueller A, Song G, McClain DA, Wang L. Overexpression of nuclear receptor SHP in adipose tissues affects diet-induced obesity and adaptive thermogenesis. American journal of physiology Endocrinology and metabolism. 2010;298:E961–970. doi: 10.1152/ajpendo.00655.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP. Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. The Journal of biological chemistry. 2000;275:16302–16308. doi: 10.1074/jbc.M001364200. [DOI] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. The Journal of biological chemistry. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. The British journal of nutrition. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E–BP1. Nature medicine. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell metabolism. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Molecular and cellular biology. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Annals of the New York Academy of Sciences. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Huang Z, Du Y, Cheng Y, Chen S, Guo F. ATF4 regulates lipid metabolism and thermogenesis. Cell research. 2010;20:174–184. doi: 10.1038/cr.2010.4. [DOI] [PubMed] [Google Scholar]
- Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Yehuda-Shnaidman E, Medvedev AV, Kumar N, Daniel KW, Robidoux J, Czech MP, Mangelsdorf DJ, Collins S. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Molecular and cellular biology. 2008;28:2187–2200. doi: 10.1128/MCB.01479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, Moore DD. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell metabolism. 2005;2:227–238. doi: 10.1016/j.cmet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Wong KE, Kong J, Zhang W, Szeto FL, Ye H, Deb DK, Brady MJ, Li YC. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. The Journal of biological chemistry. 2011;286:33804–33810. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KE, Szeto FL, Zhang W, Ye H, Kong J, Zhang Z, Sun XJ, Li YC. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. American journal of physiology Endocrinology and metabolism. 2009;296:E820–828. doi: 10.1152/ajpendo.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell metabolism. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Niepel MW, Kawano Y, Han S, Liu S, Marsili A, Larsen PR, Lee CH, Cohen DE. Targeted deletion of thioesterase superfamily member 1 promotes energy expenditure and protects against obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5417–5422. doi: 10.1073/pnas.1116011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nature genetics. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]