Abstract
Study Design
Retrospective database analysis.
Objective
To assess the effect glycemic control has on perioperative morbidity and mortality in patients undergoing elective degenerative lumbar spine surgery.
Summary of background data
Diabetes Mellitus (DM) is a prevalent disease of glucose dysregulation that has been demonstrated to increase morbidity and mortality following spine surgery. However, there is limited understanding of whether glycemic control influences surgical outcomes in DM patients undergoing lumbar spine procedures for degenerative conditions.
Methods
The Nationwide Inpatient Sample was analyzed from 2002 to 2011. Hospitalizations were isolated based on International Classification of Diseases Ninth Revision, Clinical Modification procedural codes for lumbar spine surgery and diagnoses codes for degenerative conditions of the lumbar spine. Patients were then classified into three cohorts: controlled diabetics, uncontrolled diabetics and non-diabetics. Patient demographic data, acute complications and hospitalization outcomes were determined for each cohort.
Results
A total of 403,629 (15.7%) controlled diabetics and 19,421(0.75%) uncontrolled diabetics underwent degenerative lumbar spine surgery from 2002-2011. Relative to non-diabetics, uncontrolled diabetics had significantly increased odds of cardiac complications, deep venous thrombosis and post-operative shock; additionally, uncontrolled diabetics also had an increased mean length of stay (approximately 2.5 days), greater costs (1.3-fold) and a greater risk of inpatient mortality (odds ratio=2.6, 95% confidence interval=1.5-4.8, p < .0009). Controlled diabetics also had increased risk of acute complications and inpatient mortality when compared to non-diabetics, but not nearly to the same magnitude as uncontrolled diabetics.
Conclusion
Suboptimal glycemic control in diabetic patients undergoing degenerative lumbar spine surgery leads to increased risk of acute complications and poor outcomes. Patients with uncontrolled DM, or poor glucose control, may benefit from improving glycemic control prior to surgery.
Keywords: diabetes mellitus, degenerative lumbar spine surgery, glycemic control, acute complications, controlled diabetes, uncontrolled diabetes, glucose control, mortality, length of stay, costs, nationwide inpatient sample
Introduction
Diabetes Mellitus (DM) is a disease of blood glucose dysregulation that is known to cause complications of the microvasculature, often leading to cardiovascular, ophthalmic, renal and peripheral vascular disease.1 In 2010, DM was estimated to affect 8.3% of the population in the United States (U.S.), or roughly 25.8 million people.2 The disease burden is several times greater in the elderly, affecting 26.9% of U.S. residents over the age of 65. A large fraction of patients undergoing degenerative lumbar spine surgery are elderly and, therefore, the impact of DM on surgical outcomes is of great interest.
Currently, only a few studies have described the outcomes of spine surgery in patients with DM. Furthermore, previous reports have relatively small sample sizes, which limit the conclusions that can be drawn from their findings.3-5 In patients with DM who underwent degenerative lumbar spine surgery, studies have shown reduced pain improvement, increased incidence of fusion complications and increased incidence of surgical site infections relative to those patients without DM.4-6 A recent study from 2011, comparing patient centered outcomes in diabetics versus non-diabetics undergoing surgical intervention for common degenerative spinal diseases, showed some pain improvement following treatment of spinal stenosis and degenerative spondylolisthesis but no improvement following treatment of intervertebral disc (IVD) herniation.7 However, this study did not stratify patients between varying levels of glycemic control, making it impossible to determine whether the severity of a patient’s diabetic condition also influenced surgical outcomes.
The purpose of this study was to assess the impact glycemic control has on in-hospital outcomes in patients undergoing lumbar surgery for degenerative conditions. We hypothesized that patients with uncontrolled DM will have more perioperative complications, extended hospital stay, greater hospital costs and an increased risk of inpatient mortality relative to patients with controlled DM or no DM.
Materials and Methods
The Nationwide Inpatient Sample (NIS) database, under the auspices of the Healthcare Cost and Utilization Project (HCUP) and administered by the Agency for Healthcare Research and Quality, was queried from 2002 to 2011. The NIS, which comprises a 20% stratified sample of all hospital discharges, is the largest all-payer hospital inpatient database in the U.S.8 Unweighted, this sample is approximately 8 million hospitalizations each year and an estimated 40 million hospitalizations when appropriately weighted. The NIS contains valuable information including patient characteristics (e.g. race, age and gender), hospital characteristics (e.g. teaching status, location and size) and hospitalization outcomes (e.g. mortality, costs and length of stay). The NIS allows identification of hospitalizations according to procedures, diagnoses and comorbidities of interest using codes in accordance with the International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM).
Sample Selection
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for lumbar spine procedures and diagnosis codes for degenerative conditions of the lumbar spine. The following procedural codes were included: anterior dorsal lumbar fusion (81.04), posterior dorsal lumbar fusion (81.04), anterior lumbar fusion (81.06), lumbar fusion, lateral transverse technique (81.07), posterior lumbar fusion (81.08), posterior lumbar decompression without fusion (03.09), anterior dorsal lumbar fusion revision (81.41), posterior dorsal lumbar fusion revision (81.35), anterior lumbar fusion revision (81.36), lumbar lateral transverse process fusion revision (81.37) and posterior lumbar fusion revision (81.38).
Procedures were then stratified to include only those with concurrent diagnosis codes that best described a degenerative lumbar pathology or associated condition. The following degenerative lumbar conditions were included: lumbar spondylosis with and without myelopathy (721.42, 721.3), displacement of lumbar IVD without myelopathy (722.10), degeneration of lumbar IVD (722.52), lumbar IVD disorder with myelopathy (722.73), post-laminectomy syndrome in the lumbar region (722.83), other and unspecified disc disorders in lumbar region (722.93) and lumbar spinal stenosis (724.02). Procedures were then organized into three groups: lumbar fusion, lumbar fusion revision and lumbar decompression without fusion. Diabetes codes were chosen based on ICD-9-CM codes for uncontrolled diabetics or controlled diabetics regardless of secondary manifestation. For example, Type 1(250.53) and Type 2 (250.52) diabetics with ophthalmic manifestation are described as uncontrolled and thus were included in the uncontrolled diabetic cohort. Diabetes ICD-9-CM diagnoses codes not stated as uncontrolled were included in the controlled diabetic cohort. Patients fell into one of three cohorts: controlled diabetics, uncontrolled diabetics or non-diabetic. Diagnosis codes for diabetic cohorts and acute complications can be found in the Appendix.
Outcome Measures
We analyzed demographic data of uncontrolled DM, controlled DM and non-DM cohorts including age (mean and age group distributions), pay schedule, gender, race, Elixhauser Comorbidity Index, hospital characteristics (size, setting) and surgical procedure. We chose the Elixhauser Comorbidity Index because of its validated capacity to accurately predict mortality as well as patient burden of comorbidities in administrative database studies. A larger index indicates those patients at greater risk of death during hospitalization.9-11
Perioperative complications were also chosen based on ICD-9-CM diagnosis codes (Appendix). The following acute complications were investigated: cerebrovascular accident, respiratory, cardiac, deep venous thromboembolism (DVT), peripheral vascular, neurological, genitourinary, postoperative shock, pulmonary embolism, postoperative infection and acute postoperative hemorrhage. We further analyzed hospitalization outcomes such as mean and median length of stay (LOS), costs and mortality rates. All hospital charges were adjusted for inflation using the U.S. Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the last year included in our study.12 Charges were then converted into costs with the HCUP cost to charge ratio tool.13
Data Analysis
Statistical analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Chi-squared test was used for analysis of categorical variables and Student t test was used for continuous variables. Analysis took into account the complex survey design of the NIS and procedures such as surveyfreq, surveymeans and surveylogistic were used for data analysis. Discharge weights, NIS_stratum and cluster (hospital identification) variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Regression modeling was done to examine odds ratios for complication covariates referencing controlled, uncontrolled DM and all diabetic patients (controlled and uncontrolled DM combined) with those patients without DM. Multivariate logistic regression modeling was performed to assess the role uncontrolled or controlled DM had on inpatient mortality. Cochran-Armitage trend test was performed to assess diabetic cohorts’ trend of prevalence over time. Statistical significance was maintained at P < 0.05 for all comparisons, trends and regression modeling.
Results
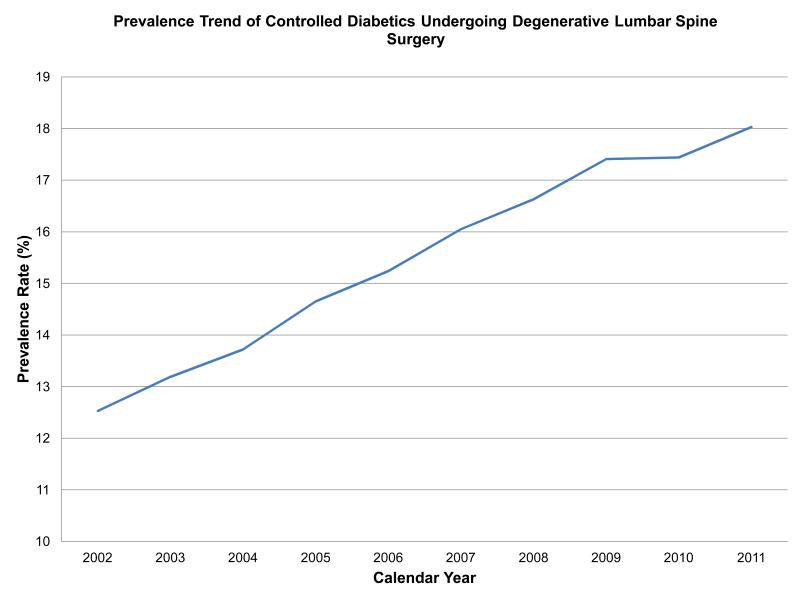
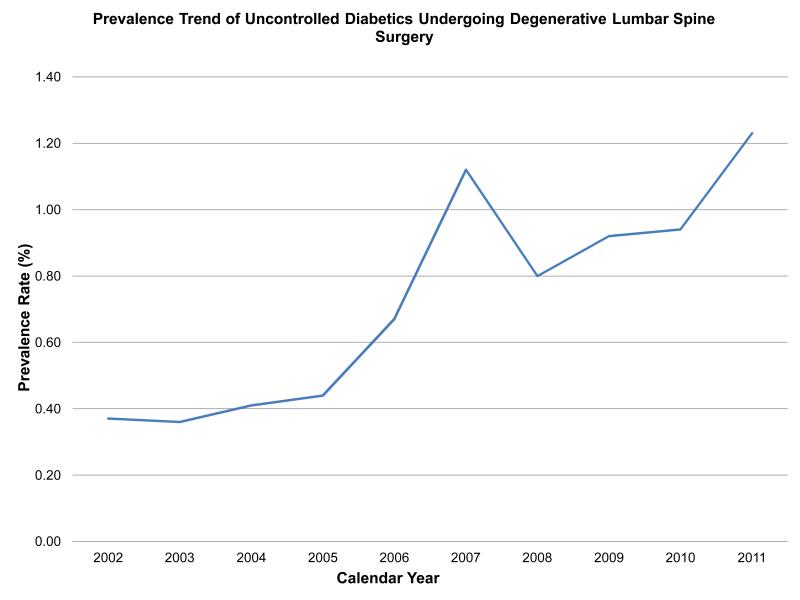
There was a total of 2,568,994 elective degenerative lumbar spine procedures performed from 2002-2011. Controlled diabetics made up 403,629 (15.7%) of these surgeries while uncontrolled DM was only seen in 19,421(0.75%) of all cases. Moreover, the prevalence of these two diabetic cohorts significantly increased over time (Figure 1a and 1b)(p < 0.0001, for both). Controlled diabetics and uncontrolled were significantly older than those patients without diabetes (Table 1). Both diabetic cohorts were significantly more likely to have Medicare when compared to non-diabetics; however, slightly more patients were uninsured in the uncontrolled DM cohort (1.3% versus 0.89% in those without DM, p < 0.0001) than in those with controlled DM (0.5% versus .89% in those without, p < 0.0001).
Figure 1a.
The prevalence of controlled diabetics undergoing degenerative lumbar spine surgery significantly increased from 2002-2011(p < 0.0001).
Figure 1b.
The prevalence of uncontrolled diabetics undergoing degenerative lumbar spine surgery significantly increased from 2002-2011(p < 0.0001).
Table 1.
Demographic information of diabetic patients undergoing degenerative lumbar spine surgery.
| Demographics | No DM | Cont DM | Uncont DM | P value No vs. Cont |
P value No vs. Uncont |
P value Cont vs. Uncont |
|---|---|---|---|---|---|---|
| Weighted N (%) | 2,145,944 | 403,629 | 19,421 | |||
| Average age (yr) | 57.88 | 64.52 | 63.01 | <0.0001 | <0.0001 | <0.0001 |
| Age group, yr (%) | <0.0001 | <0.0001 | <0.0001 | |||
| 0-44 | 20.89 | 5.65 | 7.26 | |||
| 45-64 | 42.23 | 40.23 | 43.94 | |||
| 65< | 36.78 | 54.10 | 48.77 | |||
| Sex (%) | ||||||
| Female | 53.20 | 51.00 | 51.43 | <0.0001 | 0.033 | 0.632 |
| Race (%) | <0.0001 | <0.0001 | 0.002 | |||
| White | 65.72 | 62.20 | 59.60 | |||
| Black | 4.35 | 7.21 | 9.64 | |||
| Hispanic | 3.92 | 5.35 | 7.21 | |||
| Asian or Pacific | 0.72 | 1.11 | 0.75 | |||
| Native American | 0.27 | 0.39 | 0.37 | |||
| Other | 1.75 | 1.78 | 1.52 | |||
| Missing Race | 23.28 | 21.94 | 20.92 | |||
|
Hospital teaching
status (%) |
0.791 | 0.368 | 0.323 | |||
| Nonteaching | 48.64 | 48.66 | 46.65 | |||
| Teaching | 51.36 | 51.34 | 53.35 | |||
| Hospital size (%) | 0.001 | 0.301 | 0.630 | |||
| Small | 13.37 | 12.35 | 10.93 | |||
| Medium | 21.92 | 22.18 | 22.25 | |||
| Large | 64.71 | 65.46 | 66.82 | |||
| Pay Schedule | <0.0001 | <0.0001 | 0.039 | |||
| Medicare | 38.66 | 55.43 | 52.55 | |||
| Medicaid | 6.17 | 7.14 | 9.51 | |||
| Private | 43.96 | 30.72 | 29.88 | |||
| Uninsured | 0.86 | 0.63 | 1.34 | |||
| Other | 10.15 | 5.93 | 6.60 | |||
| Missing | 0.19 | 0.16 | 0.12 | |||
|
Mean Elixhauser
Index |
0.46 | 0.73 | 1.72 | <0.0001 | <0.0001 | <0.0001 |
| Procedures (%) | ||||||
| Lumbar Decompression w/o fusion |
41.07 | 49.13 | 40.54 | <0.0001 | 0.679 | <0.0001 |
| Lumbar Fusion | 55.12 | 47.26 | 54.74 | <0.0001 | 0.771 | <0.0001 |
| Lumbar Fusion Revision |
3.81 | 3.62 | 4.72 | 0.013 | 0.008 | 0.001 |
Abbreviations: Cont=Controlled, DM=Diabetes Mellitus, Uncont=Uncontrolled, Yr=Year, No=No DM
Major comorbidities were seen with greater prevalence in patients with controlled and uncontrolled DM when compared to non-diabetics (Table 2). In particular, congestive heart failure (CHF) was increased nearly 3-fold in patients with controlled DM (p < 0.0001) and almost 5-fold in patients with uncontrolled DM (p < 0.0001) compared to non-diabetics. Patients with controlled DM had a 4-fold increase in renal disease, while in uncontrolled diabetics this prevalence increased 8-fold relative to the non-diabetic cohort (Table 2). Patients with uncontrolled DM undergoing degenerative lumbar spine surgery were also significantly more obese compared to controlled diabetics (25.9% versus 18.6%, p < 0.0001).
Table 2.
Prevalence of comorbidities in patients undergoing degenerative lumbar spine surgery.
| Comorbidities | No DM | Cont DM | Uncont DM | P value No vs. Cont |
P value No vs. Uncont |
P value Cont vs. Uncont |
|---|---|---|---|---|---|---|
| Congestive heart failure | 1.46 | 4.31 | 7.17 | <0.0001 | <0.0001 | <0.0001 |
| Chronic pulmonary disease | 13.43 | 16.66 | 17.45 | <0.0001 | <0.0001 | 0.179 |
| Liver disease | 0.68 | 1.12 | 1.98 | <0.0001 | <0.0001 | <0.0001 |
| Neurological disorders | 3.15 | 3.39 | 4.08 | 0.001 | 0.001 | 0.019 |
| Renal disease | 1.11 | 4.40 | 8.99 | <0.0001 | <0.0001 | <0.0001 |
| Obesity | 8.42 | 18.59 | 25.92 | <0.0001 | <0.0001 | <0.0001 |
| Depression | 10.86 | 11.36 | 12.98 | 0.001 | 0.0001 | 0.004 |
Abbreviations: Cont=Controlled, DM=Diabetes Mellitus, Uncont=Uncontrolled, No=No DM
Controlled diabetics undergoing degenerative lumbar spine surgery had increased odds of acute complications including cerebrovascular, respiratory, cardiac, DVT, genitourinary, postoperative infection and postoperative hemorrhage (Table 3). These patients also had increased odds of inpatient mortality (Odds Ratio [OR]=1.4, 95 % Confidence Interval [CI]=1.13-1.68, p < 0.001). With the exception of cerebrovascular complications, patients with uncontrolled DM had increased odds of the same perioperative complications as those patients with controlled DM. Moreover, patients with uncontrolled DM had increased odds of postoperative shock (OR=3.6, 95% CI=1.9-7.1, p < 0.0001) when compared to non-diabetics, which represents a significant increase that was not seen in patients with controlled DM. Risk of inpatient mortality in uncontrolled diabetics undergoing degenerative spine surgery was significantly increased (OR=2.6, 95% CI=1.5-4.8, p < 0.001), which was greater than either controlled diabetics or all diabetics combined. Multivariate analyses also showed that uncontrolled DM was a significant independent predictor of inpatient mortality (OR=2.3, 95% CI=1.2-4.3, p = 0.009) despite other potential variables (Table 4). In contrast, controlled diabetics, in an identical multivariate regression model did not show the same effect on inpatient mortality (OR=1.1, 95% CI=0.92-1.4, p = 0.250).
Table 3.
Acute complications in patients undergoing degenerative lumbar spine surgery.
| Complications | Prevalence | No DM vs Cont | No DM vs Uncont | No DM vs All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No DM | Cont DM |
Uncont DM |
All DM | OR (95% CI) |
P value | OR (95% CI) |
P valve | OR (95% CI) |
P valve | |
| Cerebrovascular | 0.06 | 0.09 | 0.07 | 0.09 | 1.58 (1.19-2.09) |
0.002 | 1.3 (0.42-4.05) |
0.65 | 1.56 (1.19-2.06) |
0.001 |
| Respiratory | 0.84 | 0.99 | 1.95 | 1.03 | 1.18 (1.09-1.28) |
<0.0001 | 2.35 (1.86-2.98) |
<0.0001 | 1.23 (1.14-1.33) |
<0.0001 |
| Cardiac | 0.71 | 0.96 | 1.74 | 0.99 | 1.36 (1.25-1.48) |
<0.0001 | 2.48 (1.98-3.11) |
<0.0001 | 1.41 (1.3-1.53) |
<0.0001 |
| DVT | 0.21 | 0.30 | 0.60 | 0.31 | 1.44 (1.23-1.69) |
<0.0001 | 2.85 (1.89-4.3) |
<0.0001 | 1.5 (1.29-1.75) |
<0.0001 |
| Peripheral Vascular | 0.07 | 0.07 | 0.12 | 0.07 | 1.02 (0.76-1.35) |
0.915 | 1.87 (0.77-4.58) |
0.161 | 1.05 (0.8-1.39) |
0.704 |
| Nervous System | 0.10 | 0.08 | 0.16 | 0.08 | 0.81 (0.61-1.06) |
0.125 | 1.58 (0.68-3.68) |
0.282 | 0.84 (0.64-1.11) |
0.219 |
| Genitourinary | 1.01 | 1.34 | 1.79 | 1.36 | 1.33 (1.25-1.43) |
<0.0001 | 1.78 (1.4-2.27) |
<0.0001 | 1.35 (1.27-1.44) |
<0.0001 |
| Postoperative Shock | 0.06 | 0.06 | 0.22 | 0.07 | 1.01 (0.75-1.37) |
0.943 | 3.65 (1.87-7.10) |
<0.0001 | 1.13 (0.85-1.51) |
0.396 |
| Pulmonary Embolism | 0.21 | 0.22 | 0.48 | 0.23 | 1.07 (0.9-1.28) |
0.416 | 2.35 (1.52-3.62) |
<0.0001 | 1.13 (0.96-1.33) |
0.133 |
| Postoperative Infection | 0.34 | 0.46 | 0.88 | 0.48 | 1.36 (1.21-1.52) |
<0.0001 | 2.61 (1.85-3.68) |
<0.0001 | 1.41 (1.27-1.58) |
<0.0001 |
| Acute Post-operative hemorrhage | 8.50 | 10.36 | 20.63 | 10.83 | 1.24 (1.2-1.29) |
<0.0001 | 2.8 (2.47-3.17) |
<0.0001 | 1.31 (1.26-1.36) |
<0.0001 |
| Inpatient Mortality | 0.11 | 0.15 | 0.28 | 0.15 | 1.38 (1.13-1.68) |
0.001 | 2.63 (1.45-4.78) |
0.001 | 1.44 (1.19-1.74) |
0.0001 |
Abbreviations: Cont=Controlled, DM=Diabetes Mellitus, Uncont=Uncontrolled, DVT= Deep Venous Thromboembolism. All cohorts were referenced to patients without DM.
Table 4.
Multivariate analysis for inpatient mortality in patients undergoing degenerative lumbar spine surgery.
| Risk factor | Odds Ratio | Lower 95% CI | Upper 95% CI | P Value |
|---|---|---|---|---|
|
Uncontrolled DM (Ref = No Diabetes) |
2.31 | 1.24 | 4.33 | 0.009 |
| Age | 1.07 | 1.05 | 1.08 | <0.0001 |
|
Race (Ref = White) |
||||
| Black | 1.88 | 1.27 | 2.80 | 0.059 |
| Hispanic | 1.03 | 0.63 | 1.68 | 0.720 |
| Asian or Pacific | 1.00 | 0.31 | 3.23 | 0.812 |
| Native American | 1.14 | 0.17 | 7.71 | 0.990 |
| Other | 0.95 | 0.38 | 2.39 | 0.690 |
| Female vs. Male | 0.46 | 0.37 | 0.57 | <0.0001 |
|
Hospital Bed Size (Ref = Large) |
||||
| Small | 0.53 | 0.36 | 0.77 | 0.012 |
| Medium | 0.75 | 0.57 | 0.98 | 0.847 |
|
Hospital Region (Ref = Northeast) |
||||
| Midwest | 0.76 | 0.53 | 1.09 | 0.329 |
| South | 0.89 | 0.69 | 1.16 | 0.674 |
| West | 0.81 | 0.58 | 1.14 | 0.607 |
|
Hospital Location (Ref = Rural) |
||||
| Urban | 1.83 | 0.95 | 3.54 | 0.072 |
|
Hospital Teaching Status (Ref = Non-teaching) |
||||
| Academic | 1.22 | 0.97 | 1.52 | 0.085 |
|
Pay Schedule (Ref = Private Insurance) |
||||
| Medicare | 1.25 | 0.87 | 1.78 | <0.0001 |
| Medicaid | 2.00 | 1.23 | 3.26 | <0.0001 |
| Uninsured | 1.83 | 0.59 | 5.67 | <0.0001 |
| Other | 1.14 | 0.69 | 1.90 | <0.0001 |
| Missing | <0.001 | <0.001 | <0.001 | <0.0001 |
|
Surgical Approach (Ref = Lumbar |
||||
| Decompression without Fusion) | ||||
| Lumbar Fusion | 1.72 | 1.38 | 2.15 | <0.0001 |
| Lumbar Fusion Revision | 1.05 | 0.55 | 1.98 | 0.889 |
Abbreviations: CI=Confidence Interval, Ref=Reference
Hospital outcomes were affected by the diagnosis of DM (Table 5). The controlled DM cohort had lower mean costs than those without diabetes ($20,806 versus $21,249.8, p < 0.0001). This was not the case in patients with uncontrolled DM, in which costs were significantly greater ($26,476 versus $21,249, p < 0.0001). Diabetics undergoing degenerative lumbar spine surgery also had extended LOS. Relative to non-diabetics, controlled DM patients had an increased LOS of approximately half a day (1.1 fold, p < 0.0001) while patients with uncontrolled DM had an increase of approximately 2.4 days (1.7 fold, p < 0.0001).
Table 5.
Hospitalization outcomes of patients undergoing degenerative lumbar spine surgery.
| Outcomes | Normal | Controlled DM |
Uncontrolled DM |
P Value Normal vs Cont |
P Value Normal vs Uncont |
|---|---|---|---|---|---|
| Mean Costs($) | 21,250 | 20,806 | 26,476 | <0.0001 | <0.0001 |
| Median Cost ($) (IQR) |
18,140 (8,748-28,065) |
17,989 (8,636-28,034) |
18,181 (8,783-28,119) |
… | … |
| Mean LOS(days) | 3.65 | 4.07 | 6.02 | <0.0001 | <0.0001 |
| Median LOS(days) (IQR) |
2.58 (1.45-3.88) |
2.79 (1.60-4.36) |
3.88 (2.49-6.31) |
… | … |
Abbreviations: DM=Diabetes Mellitus, LOS= Length of Stay, IQR= Interquartile Range.
Costs reported in ($USD)
Discussion
Diabetes is a common systemic disease that poses a significant health burden in the general population.2 Furthermore, due to its growing incidence and prevalence, diabetic patients are likely to be encountered by spine surgeons and other health care providers throughout their careers. Although Type 1 and Type 2 diabetics may initially acquire diabetes through different mechanisms, treatment is focused on glycemic control for both.14 Several studies have shown that hyperglycemia, or poor glycemic control, independently leads to increased rates of perioperative complications and poorer outcomes relative to those with better glycemic control.15-18 A secondary analysis of the Spine Outcomes Research Trial (SPORT) showed that some diabetic patients with degenerative spinal disease might benefit from surgery; however, this analysis did not stratify patients by their glycemic control, which is of great importance when treating diabetic patients.7 To our knowledge, this is the first comprehensive study analyzing outcomes and complications in diabetic patients with varying glycemic control undergoing degenerative lumbar surgery.
Glycemic control in diabetics can be diagnosed and monitored in several ways including the use of glycated hemoglobin (HbA1c), which gives an accurate 3-month representation of glucose control in patients.19 After studies evaluating increased risk of diabetic complications after a HbA1c threshold, both the American Diabetes Association and American Association of Clinical Endocrinologists advocate the use of HbA1c > 6.5% to diagnose DM.20,21 Stryker et al22 demonstrated that a perioperative HbA1c of > 6.7% increased the OR of postoperative wound complications to 9.0 after total joint arthroplasty. Similarly, Koutsoumbelis and colleagues’23 investigation of posterior lumbar instrumented arthrodesis found that a clinical diagnosis of diabetes prior to surgery significantly increased the risk of postoperative infection. Our own analysis confirmed these findings showing that controlled, uncontrolled and all diabetic patients undergoing degenerative lumbar spine surgery had increased likelihood of postoperative infection with these odds further amplified in patients with poor glycemic control (OR= 1.36, 2.61 and 1.41 for controlled, uncontrolled and all diabetics, respectively, p < 0.0001). Importantly, the significantly increased odds in patients with uncontrolled DM was seen in nearly all complications, exhibiting the profound effect poor glucose control has on perioperative morbidity in patients undergoing degenerative lumbar spine surgery.
The definite association between uncontrolled DM and complications in patients undergoing surgery, although represented in our analysis, is not entirely clear in the literature. Marchant et al24 distinctly showed the relationship between uncontrolled DM and increased risk of perioperative complications in patients undergoing total joint arthroplasty. However, Adams et al25, in a cohort investigation of preoperative HbA1c in total knee arthroplasty, was not able to find any association between poor glycemic control and increased perioperative complications. There are a few reasons for the differences in Marchant and Adams’s findings. Adams et al25 utilized preoperative laboratory reports of HbA1c > 7% to categorize patients as uncontrolled diabetics, while Marchant et al24 and our study classified patients based on a ICD-9-CM diagnosis code algorithm. Unfortunately, there are currently no clear guidelines indicating what is considered uncontrolled diabetes in the perioperative setting. Therefore, it is difficult to corroborate either method. A HbA1c of > 7% may have been acceptable as a threshold for the classification of uncontrolled diabetes. However, this method has already yielded contrasting results in different studies; therefore, it is difficult for it be accepted universally. For example, in contrast to the lack of association found by Adams et al25, a study investigating the risk of surgical site infection after thoracic and lumbar spinal instrumentation, also using the Adam’s threshold schema to classify uncontrolled diabetics, showed increased risk of surgical site infection in patients with poor glycemic control.26 Although there is the potential that diabetic patients undergoing lumbar spine surgery are at a higher risk of complications relative to those undergoing joint arthroplasty, a proper classification schema, especially in the perioperative period, for controlled and uncontrolled diabetics is warranted. This will make future studies, not only in spine but also other surgical fields, more unified and easier to validate.
By eliminating cases of trauma, infection and severe or rare pathology, our analysis was performed on a population of patients mostly undergoing elective surgical cases for degenerative lumbar spine pathology. Hospital outcomes, therefore, are likely to be representative of the common patient seen by a spine surgeon undergoing lumbar surgery. Compared to a non-diabetic, uncontrolled diabetics had significantly increased costs and LOS. Increased costs may be due to the fact that more lumbar fusion revisions are performed in this population (p < 0.0001). Uncontrolled DM patients also have extended LOS, possibly due to a difficult post-operative course arising from complications secondary to their diabetic condition. Additionally, because patients with uncontrolled DM are less likely to have private insurance and more likely to have Medicaid or no insurance, these patients may furthermore represent a large subset of patients with a greater severity of the diagnoses chosen possibly due to suboptimal healthcare access.
There are a few limitations that must be addressed in our study. One limitation of our study is that the NIS does not have information regarding preoperative blood glucose levels or HbA1c. Without these data, we are restricted to the use of ICD-9-CM diagnosis codes limiting our interpretations to less precisely defined dichotomous groups (controlled and uncontrolled diabetes), which does not allow us to further stratify uncontrolled diabetics based on the severity of their condition. Furthermore, the NIS does not provide information after discharge or readmission, which can possibly underestimate or overestimate, respectively, the incidence of complications in the three cohorts investigated.
Conclusion
Diagnosis of DM confers significant perioperative morbidity and increased risk of mortality in patients undergoing degenerative lumbar spine surgery, and this is particularly notable in patients with uncontrolled DM. Uncontrolled DM patients scheduled for elective lumbar spine surgery will benefit from improved glycemic control. Overall, patients with superior glucose control as represented by patients with controlled or no DM, are likely to achieve better outcomes when undergoing degenerative lumbar surgery as compared to patients with uncontrolled DM.
Supplementary Material
Key Points.
Controlled diabetics made up 15.7% and uncontrolled diabetics 0.75% of all degenerative lumbar spine procedures with steady increase in their prevalence from 2002-2011.
Medical comorbidities such as obesity, renal disease and congestive heart failure were significantly more likely to be seen in uncontrolled diabetics compared to controlled diabetics or non-diabetics.
The proportion of fusion revisions in the lumbar spine was significantly greater in the uncontrolled diabetic cohort than in controlled diabetics or non-diabetics.
Controlled and non-diabetics cohorts had similar hospitalization outcomes; in contrast, the uncontrolled diabetic cohort had significantly increased costs and extended length of stay.
Acknowledgements
Authors gratefully acknowledge helpful discussions on interpretations of findings with Dr. Tim Yoon.
The manuscript submitted does not contain information about medical device(s)/drug(s). No funds were received in financial activities outside the submitted work: Relevant support of this work. consultancy, grants, royalties.
Appendix
ICD-9-CM diagnosis codes for diabetic cohorts and complications.
| Cohorts | ICD-9-CM diagnosis codes |
|---|---|
| Controlled Diabetes | 250.00, 250.01, 250.10, 250.11, 250.20, 250.21, 250.30, 250.31, 250.40, 250.41, 250.50, 250.51, 250.60, 250.61, 250.70, 250.71, 250.80, 250.81, 250.90, 250.91 |
| Uncontrolled Diabetes | 250.02, 250.03, 250.12, 250.13, 250.22, 250.23 250.32, 250.33, 250.42, 250.43, 250.52, 250.53, 250.62, 250.63, 250.72, 250.73, 250.82, 250.83, 250.92, 250.93 |
| Acute Complications | |
| Cerebrovascular | 997.02 |
| Respiratory | 997.3 |
| Cardiac | 997.1 |
| Deep Venous Thrombosis |
451.11, 453.4, 453.9, 451.19, 451.2, 451.81, 453.40, 453.41 |
| Peripheral Vascular | 997.2 |
| Nervous System | 997.00, 997.01, 997.0 |
| Genitourinary | 997.5 |
| Postoperative Shock | 998.0, 998.00, 998.01, 998.02, 998.09 |
| Pulmonary Embolism | 415.1, 415.11, 415.19 |
| Postoperative Infection | 998.59 |
| Acute post-operative hemorrhage |
285.1 |
References
- 1.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Annals of internal medicine. 2004;140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 3.Kawaguchi Y, Matsui H, Ishihara H, et al. Surgical outcome of cervical expansive laminoplasty in patients with diabetes mellitus. Spine. 2000;25:551–5. doi: 10.1097/00007632-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Satake K, Kanemura T, Matsumoto A, et al. Predisposing factors for surgical site infection of spinal instrumentation surgery for diabetes patients. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013;22:1854–8. doi: 10.1007/s00586-013-2783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi S, Suzuki A, Toyoda H, et al. Characteristics of diabetes associated with poor improvements in clinical outcomes after lumbar spine surgery. Spine. 2013;38:516–22. doi: 10.1097/BRS.0b013e318273583a. [DOI] [PubMed] [Google Scholar]
- 6.Pull ter Gunne AF, Hosman AJ, Cohen DB, et al. A methodological systematic review on surgical site infections following spinal surgery: part 1: risk factors. Spine. 2012;37:2017–33. doi: 10.1097/BRS.0b013e31825bfca8. [DOI] [PubMed] [Google Scholar]
- 7.Freedman MK, Hilibrand AS, Blood EA, et al. The impact of diabetes on the outcomes of surgical and nonsurgical treatment of patients in the spine patient outcomes research trial. Spine. 2011;36:290–307. doi: 10.1097/BRS.0b013e3181ef9d8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: 2002-2011. http://www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 9.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Medical care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 10.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Medical care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Medical care. 2012;50:1109–18. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 12.Bureau of Labor Statistics [Accessed November 26, 2013];CPI inflation calculator. Available at: http://data.bls.gov/cgi-bin/cpicalc.pl.
- 13.HCUP Cost-to-Charge Ratio Files (CCR) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: [Accessed November 2, 2013]. 2002-2011. http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. [Google Scholar]
- 14.Take Charge of Your Diabetes. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention 2014 Apr 03; 21 May 2011. Web. < http://www.cdc.gov/diabetes/pubs/tcyd/ktrack.htm>.
- 15.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke; a journal of cerebral circulation. 2001;32:2426–32. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 16.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 17.McAlister FA, Majumdar SR, Blitz S, et al. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes care. 2005;28:810–5. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 18.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. The Journal of trauma. 2003;55:33–8. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- 19.Committee IE International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diagnosis and classification of diabetes mellitus. Diabetes care. 2011;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 22.Stryker LS, Abdel MP, Morrey ME, et al. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. The Journal of bone and joint surgery. American volume. 2013;95:808–14. S1–2. doi: 10.2106/JBJS.L.00494. [DOI] [PubMed] [Google Scholar]
- 23.Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. The Journal of bone and joint surgery. American volume. 2011;93:1627–33. doi: 10.2106/JBJS.J.00039. [DOI] [PubMed] [Google Scholar]
- 24.Marchant MH, Jr., Viens NA, Cook C, et al. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. The Journal of bone and joint surgery. American volume. 2009;91:1621–9. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 25.Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. The Journal of bone and joint surgery. American volume. 2013;95:481–7. doi: 10.2106/JBJS.L.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hikata T, Iwanami A, Hosogane N, et al. High preoperative hemoglobin A1c is a risk factor for surgical site infection after posterior thoracic and lumbar spinal instrumentation surgery. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association. 2014;19:223–8. doi: 10.1007/s00776-013-0518-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.