The highly pubescent abaxial side of holm oak leaves is unwettable and water repellent, while the adaxial side is wettable and can take up water, which may be an adaptation to growing under Mediterranean conditions.
Abstract
Plant trichomes play important protective functions and may have a major influence on leaf surface wettability. With the aim of gaining insight into trichome structure, composition, and function in relation to water-plant surface interactions, we analyzed the adaxial and abaxial leaf surface of holm oak (Quercus ilex) as a model. By measuring the leaf water potential 24 h after the deposition of water drops onto abaxial and adaxial surfaces, evidence for water penetration through the upper leaf side was gained in young and mature leaves. The structure and chemical composition of the abaxial (always present) and adaxial (occurring only in young leaves) trichomes were analyzed by various microscopic and analytical procedures. The adaxial surfaces were wettable and had a high degree of water drop adhesion in contrast to the highly unwettable and water-repellent abaxial holm oak leaf sides. The surface free energy and solubility parameter decreased with leaf age, with higher values determined for the adaxial sides. All holm oak leaf trichomes were covered with a cuticle. The abaxial trichomes were composed of 8% soluble waxes, 49% cutin, and 43% polysaccharides. For the adaxial side, it is concluded that trichomes and the scars after trichome shedding contribute to water uptake, while the abaxial leaf side is highly hydrophobic due to its high degree of pubescence and different trichome structure, composition, and density. Results are interpreted in terms of water-plant surface interactions, plant surface physical chemistry, and plant ecophysiology.
Plant surfaces have an important protecting function against multiple biotic and abiotic stress factors (Riederer, 2006). They may, for example, limit the attack of insects (Eigenbrode and Jetter, 2002) or pathogenic fungi (Gniwotta et al., 2005; Łaźniewska et al., 2012), avoid damage caused by high intensities of UV and visible radiation (Reicosky and Hanover, 1978; Karabourniotis and Bormann, 1999), help to regulate leaf temperature (Ehleringer and Björkman, 1978; Ripley et al., 1999), and chiefly prevent plant organs from dehydration (Riederer and Schreiber, 2001).
The epidermis of plants has been found to have a major degree of physical and chemical variability and may often contain specialized cells such as trichomes or stomata (Roth-Nebelsick et al., 2009; Javelle et al., 2011). Most aerial organs are covered with an extracellular and generally lipid-rich layer named the cuticle, which is typically composed of waxes embedded in (intracuticular waxes) or deposited on (epicuticular waxes) a biopolymer matrix of cutin, forming a network of cross-esterified hydroxy C16 and/or C18 fatty acids, and/or cutan, with variable amounts of polysaccharides and phenolics (Domínguez et al., 2011; Yeats and Rose, 2013). Different nano- and/or microscale levels of plant surface sculpturing have been observed by scanning electron microscopy (SEM), generally in relation to the topography of epicuticular waxes, cuticular folds, and epidermal cells (Koch and Barthlott, 2009). Such surface features together with their chemical composition (Khayet and Fernández, 2012) may lead to a high degree of roughness and hydrophobicity (Koch and Barthlott, 2009; Konrad et al., 2012). The interactions of plant surfaces with water have been addressed in some investigations (Brewer et al., 1991; Brewer and Smith, 1997; Pandey and Nagar, 2003; Hanba et al., 2004; Dietz et al., 2007; Holder, 2007a, 2007b; Fernández et al., 2011, 2014; Roth-Nebelsick et al., 2012; Wen et al., 2012; Urrego-Pereira et al., 2013) and are a topic of growing interest for plant ecophysiology (Helliker and Griffiths, 2007; Aryal and Neuner, 2010; Limm and Dawson, 2010; Kim and Lee, 2011; Berry and Smith, 2012; Berry et al., 2013; Rosado and Holder, 2013; Helliker, 2014). On the other hand, the mechanisms of foliar uptake of water and solutes by plant surfaces are still not fully understood (Fernández and Eichert, 2009; Burkhardt and Hunsche, 2013), but they may play an important ecophysiological role (Limm et al., 2009; Johnstone and Dawson, 2010; Adamec, 2013; Berry et al., 2014).
The importance of trichomes and pubescent layers on water drop-plant surface interactions and on the subsequent potential water uptake into the organs has been analyzed in some investigations (Fahn, 1986; Brewer et al., 1991; Grammatikopoulos and Manetas, 1994; Brewer and Smith, 1997; Pierce et al., 2001; Kenzo et al., 2008; Fernández et al., 2011, 2014; Burrows et al., 2013). Trichomes are unicellular or multicellular and glandular or nonglandular appendages, which originate from epidermal cells only and develop outwards on the surface of plant organs (Werker, 2000). Nonglandular trichomes are categorized according to their morphology and exhibit a major variability in size, morphology, and function. On the other hand, glandular trichomes are classified by the secretory materials they excrete, accumulate, or absorb (Johnson, 1975; Werker, 2000; Wagner et al., 2004). Trichomes can be often found in xeromorphic leaves and in young organs (Fahn, 1986; Karabourniotis et al., 1995). The occurrence of protecting leaf trichomes has been also reported for Mediterranean species such as holm oak (Quercus ilex; Karabourniotis et al., 1995, 1998; Morales et al., 2002; Karioti et al., 2011; Camarero et al., 2012). There is limited information about the nature of the surface of trichomes, but they are also covered with a cuticle similarly to other epidermal cell types (Fernández et al., 2011, 2014).
In this study and using holm oak as a model, we assessed, for the first time, the leaf surface-water relations of the abaxial (always pubescent) versus the adaxial (only pubescent in developing leaves and for a few months) surface, including their capacity to absorb surface-deposited water drops. Based on membrane science methodologies (Fernández et al., 2011; Khayet and Fernández, 2012) and following a new integrative approach, the chemical, physical, and anatomical properties of holm oak leaf surfaces and trichomes were analyzed, with the aim of addressing the following questions. Are young and mature adaxial and abaxial leaf surfaces capable of absorbing water deposited as drops on to the surfaces? Are young and mature abaxial and adaxial leaf surfaces similar in relation to their wettability, hydrophobicity, polarity, work of adhesion (Wa) for water, solubility parameter (δ), and surface free energy (γ)? What is the physical and chemical nature of the adaxial versus the abaxial trichomes, chiefly in relation to young leaves?
RESULTS
Leaf Surface Water Absorption
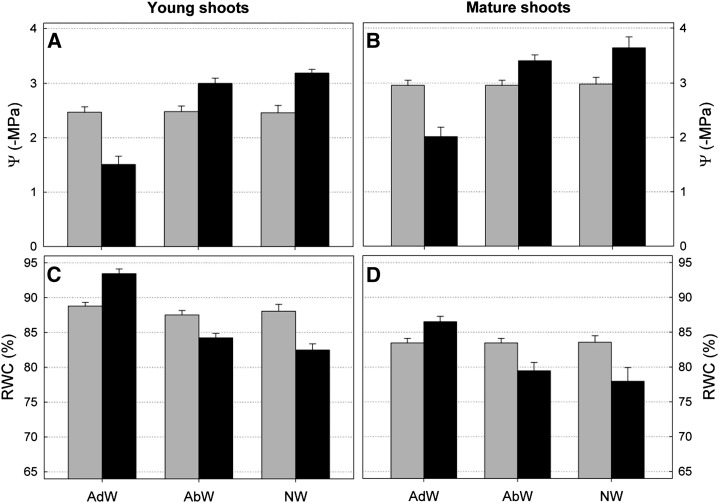
The water absorption results for both young and mature holm oak shoots are shown in Figure 1. The initial shoot water potential (Ψo) measured for young shoots was –2.47 ± 0.10 MPa. Twenty-four hours after wetting the adaxial side of the leaves, the final water potential (Ψf) became –1.51 ± 0.15 MPa. By contrast, after wetting the abaxial side, Ψf reached –3.00 ± 0.09 MPa. As expected, the untreated (nonwetted) shoots reached a lower Ψf of –3.19 ± 0.07 MPa (Fig. 1A). All Ψf values were statistically different from Ψo (α = 0.05). A similar trend was found for mature shoots (Fig. 1B). In addition, the relative water content (RWC) varied according to the water potential variations. When the adaxial holm oak leaf side was wetted, RWC increased from 88.79% ± 0.54% to 93.45% ± 0.67%. When drops were applied on to the abaxial side, RWC decreased from 87.52% ± 0.63% to 84.22% ± 0.65%. Finally, the RWC of untreated leaves (nonwetted) decreased from 88.05% ± 0.99% to 82.48% ± 0.89% (Fig. 1C). Similar variations were recorded for mature leaves (Fig. 1D).
Figure 1.
Leaf water potential (Ψ) and RWC for young (A and C) and mature (B and D) shoots of holm oak before (gray bars) and after (black bars) surface wetting. Significant differences before and after surface wetting were found in all cases. AdW, Leaf adaxial wetting; AbW, leaf abaxial wetting; NW, no wetting.
The adaxial and abaxial surface cuticular conductances of young holm oak leaves were 3.8 ± 0.2 and 3.4 ± 0.1 m s–1 10–5, respectively. The cuticular conductance values measured for mature leaves were 3.1 ± 0.2 (adaxial) and 3.3 ± 0.3 (abaxial) m s–1 10–5. Regardless of leaf age, no significant cuticular conductance differences were found between upper and lower leaf sides. Statistically significant conductance differences were, however, recorded for the adaxial cuticle of young versus mature leaves (P < 0.05).
Holm Oak Leaf Surface Structure
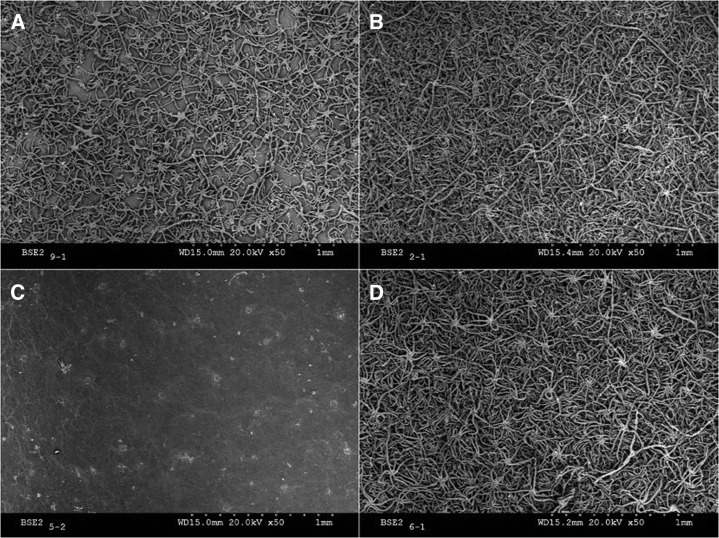
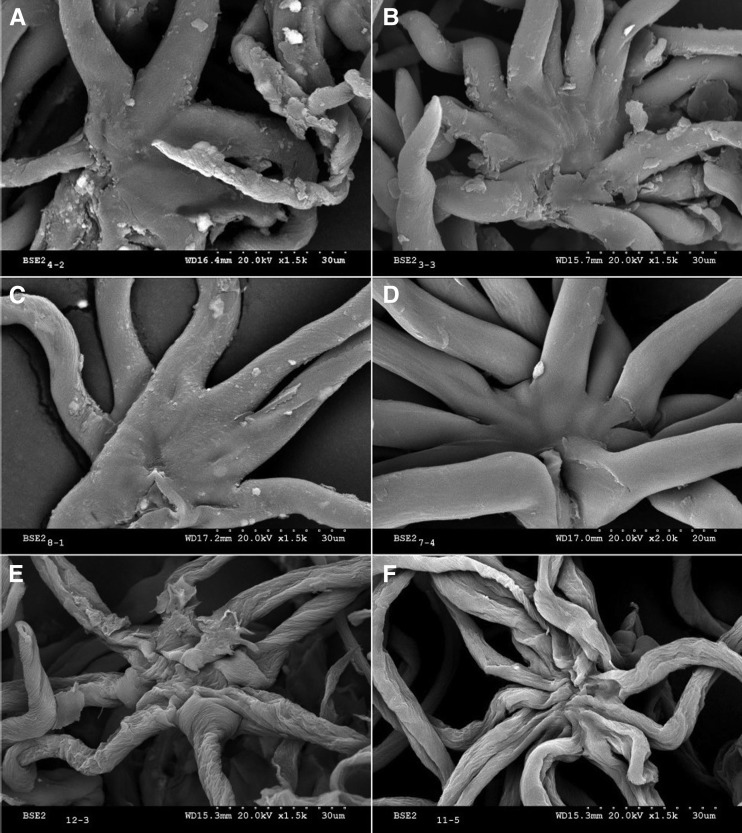
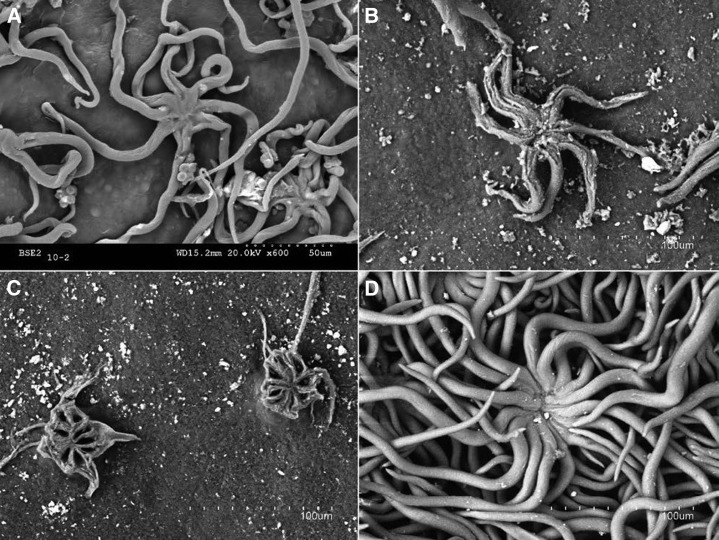
Fresh, young, and mature holm oak leaves were examined by SEM. The young leaves were covered with trichomes, the adaxial surfaces being much less pubescent than the abaxial surfaces, where dense, imbricated trichome layers were found (Fig. 2, A and B). An abaxial dense indumentum was also present in mature leaves, but their upper surfaces had almost no trichomes (Fig. 2, C and D).
Figure 2.
SEM micrographs of intact adaxial and abaxial holm oak leaf surfaces. A and B, Adaxial and abaxial sides of young leaves. C and D, Adaxial and abaxial sides of mature leaves.
Concerning the adaxial leaf epidermis, a rather smooth epicuticular wax layer could be observed in mature leaves, which was only partially visible as patches in the young leaves (Fig. 2, A and C). The adaxial side of young leaves had a trichome density of approximately 87 per mm2 leaf surface. The cuticle surface covered with trichomes was estimated to be about 43% (Fig. 2A). Some protruding trichome scars were especially visible in the mature leaf adaxial surface (Fig. 2C).
Trichomes were observed to be nonglandular, multicellular, and stellate, with the arms fusing into a short, erect stipe and then diverging horizontally (Hardin, 1976). Most of the adaxial trichomes of the young leaves had eight arms, while single long hairs and from four- to seven-arm trichomes were also eventually found. Their length and diameter were measured on eight-arm trichomes from the fusing point and at 10 µm above, respectively. The length of the arms was approximately 115 ± 24 µm, and the diameter was 6.2 ± 0.6 µm. The overlapping of trichome arms and the existence of various trichome layers on the lower leaf sides prevented the accurate estimation of the number of arms, arm length, and diameter.
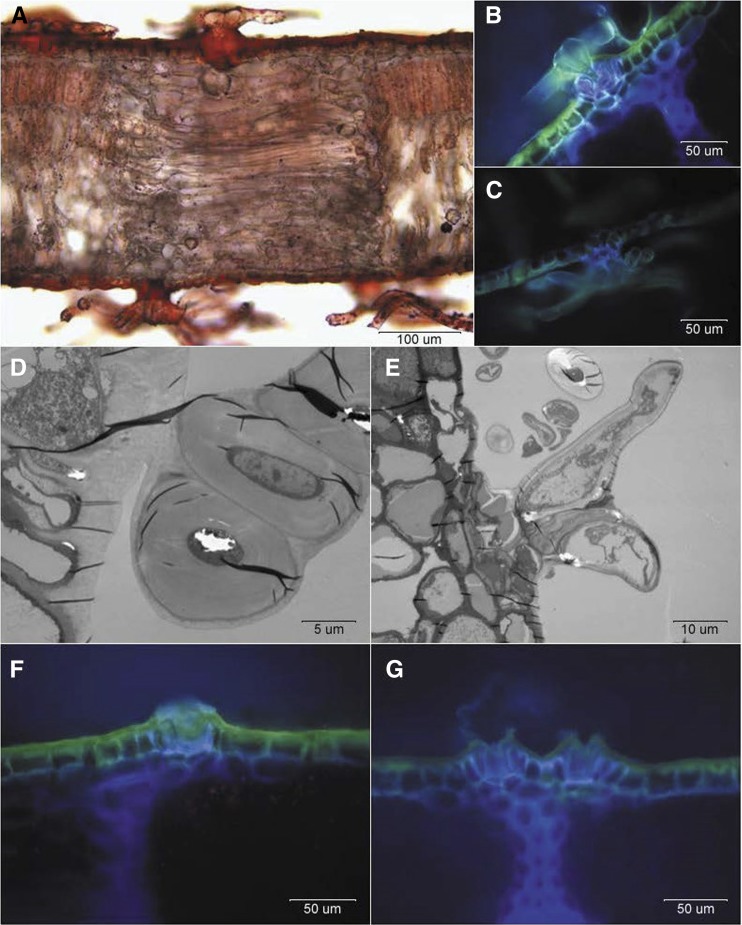
When analyzing the epidermal structure of holm oak leaves, we noticed that the base of abaxial and adaxial trichomes was cutinized, because it was stained with Sudan IV (Fig. 3A). The structure of the base of adaxial versus abaxial trichomes is different (Fig. 3, B–E). Abaxial trichomes are anchored deeper into the epidermis by a group of cells (Fig. 3, C and E), while those present in the upper side of young leaves are bound to the underlying tissue in a more superficial manner, the union largely formed by cuticle and cell wall material appearing more susceptible to shedding (Fig. 3, B and D). The scars remaining in the upper leaf sides after trichome abscission seemed to have a heterogeneous topography in both young (Fig. 3F) and mature leaves (Fig. 3G). Trichomes and trichome scars were often seen directly on or in the vicinity of bundle sheath extensions (Fig. 3, A and G).
Figure 3.
Optical and TEM micrographs of intact adaxial and abaxial surfaces of holm oak young (A–F) and mature (G) leaves. A, Transversal section of a young leaf stained with Sudan IV. B, Adaxial leaf trichome. C, Abaxial leaf trichome. D, Base of an adaxial leaf trichome observed by TEM. E, Base of an abaxial leaf trichome observed by TEM. F, Detail of scar on a young, adaxial leaf surface after trichome shedding. G, Detail of a scar on a mature, adaxial leaf surface after trichome shedding. Note that the bases are near the bundle sheath extension (dark blue).
Chemical Composition of Trichomes
The proportion of chemical constituents of isolated holm oak trichomes was assessed by the weight loss after successive chemical treatments, coupled with Fourier transform infrared spectroscopy (FTIR) measurements. Isolated trichome SEM observations, however, led us to discard the data concerning the adaxial side, because epidermal pieces of considerable size were removed together with the trichomes due to their scarcity. Hence, we are only showing the chemical composition of trichomes isolated from the lower leaf side to avoid misinterpretations.
The fractions corresponding to depolymerized material (chiefly cutin) represented the greatest percentage of the trichomes (49% [w/w]). After cutin depolymerization, a residue (mainly of polysaccharides) of 42.9% (w/w) was determined. A proportion of soluble waxes of 7.9% (w/w) of the trichome mass was recorded after chloroform extraction.
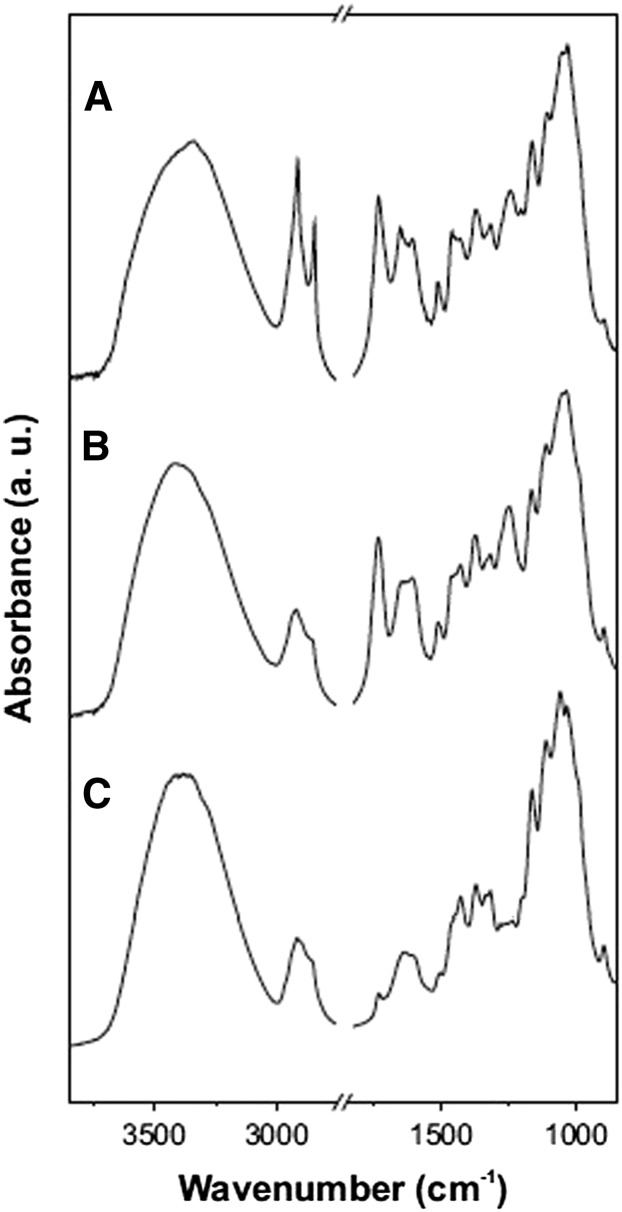
The FTIR spectra of isolated intact and chemically treated abaxial holm oak trichomes are shown in Figure 4. Cuticular material assignment was based on previous data (Ramírez et al., 1992; Villena et al., 2000). For intact hairs, bands associated with different chemical functional groups, and consequently to cuticle components, were identified (Fig. 4A): long-chain aliphatic waxes and cutin (intense and thin asymmetric and symmetric CH2-stretching absorptions at 2,919 and 2,849 cm–1 and the CH2-bending one at 1,463 cm–1), the polyester cutin (C-O-stretching vibration in ester environments band at 1,729 cm–1 and the asymmetrical and symmetrical C-O-C-stretching absorptions at 1,166 and 1,107 cm–1, respectively), polysaccharides (mainly for the band associated with the glycosidic bond at 1,054 cm–1), and some unsaturated/aromatic compounds (stretching of C=C double bonds, aromatic rings, and aromatic rings conjugated with double bonds at 1,653, 1,610, and 1,515 cm–1, respectively). The broad and very strong band at 3,350 c–1, assigned to the O-H-stretching vibration, could be related to tissue hydration. When waxes were extracted in chloroform, a remarkable reduction of the aliphatic bands was observed (CH2-stretching absorptions decreased and broadened; Fig. 4B). Finally, the saponification step led to the depolymerization of cutin and hence decreasing its characteristic absorption peaks. The remaining material can be considered a polysaccharide fraction with a small contribution of unsaturated/aromatic compounds (Fig. 4C).
Figure 4.
FTIR spectra of isolated (A), dewaxed (B), and cutin-depolymerized (C) abaxial holm oak leaf trichomes.
When examining the isolated trichomes of young holm oak leaves by SEM, a generally smoother surface with some shallow cracks was noticed after wax removal compared with the intact trichomes (Fig. 5, A–D). This phenomenon could be observed more clearly in the abaxial trichomes, because they initially had a rougher epicuticular wax layer compared with adaxial ones (Fig. 5, B and D). After cutin depolymerization, trichomes appeared to be flatter, with twisted arms, showing the cell wall structure (Fig. 5, E and F). Adaxial, intact (isolated and attached to the leaf surface), and chloroform-extracted trichomes seemed to be covered with only a partially developed cuticle (Figs. 5, A and C, and 6A).
Figure 5.
Isolated trichomes of adaxial and abaxial holm oak leaf surfaces after gradual extraction of chemical components. A and B, Intact trichomes. C and D, Trichomes after chloroform extraction. E and F, Trichomes after cutin depolymerization.
Figure 6.
Effect of cellulase hydrolysis on holm oak adaxial (A, B, and C) and abaxial (D) leaf surfaces. A, Intact, adaxial leaf surface. B, Adaxial leaf surface after 4 d in cellulase solution. C, Adaxial leaf surface after 7 d in cellulase solution. D, Abaxial leaf surface after 7 d in cellulase solution.
The effect of immersing holm oak leaves in cellulase and pectinase solutions for 4 or 7 d is shown in Figure 6. The adaxial trichomes of young leaves were hydrolyzed when immersed in 2% cellulase (Fig. 6C). The process of trichome degradation seemed to take place from the fusing point of each arm at the side which is exposed to the environment (Fig. 6B). After 7 d of cellulase hydrolysis, almost only the cuticle covering the base of the trichomes remained attached to the epidermis underneath (Fig. 6C), which finally led to trichome shedding. The opening of the cuticle fusing line (observed as a central furrow in the arms) located near the base in some arms of intact trichomes (Fig. 6A) may somehow facilitate cellulase accessibility and cell wall degradation. These cuticular fractures in the adaxial trichomes were more common in the fusing point and toward the tip of the arms (see Fig. 6A as an example). Leaf immersion in cellulase did not alter the structure of abaxial leaf trichomes, which were similar to those present in intact holm oak surfaces (Fig. 6D). Leaf pectinase digestion did not affect the structure of either the adaxial or the abaxial trichomes (data not shown).
Contact Angle Measurements
The mean contact angle (θ) values measured after depositing drops of three liquids with different polar and apolar surface tension components on to the adaxial and abaxial surfaces of young and mature holm oak leaves are summarized in Table I. The presence of trichomes in young leaf adaxial surfaces led to initially high θ values for water and especially glycerol, which sharply decreased over time before reaching a steady value when the drop became static. This phenomenon was, however, not observed for the fully apolar diiodomethane drops, which remained static when deposited on to the adaxial young leaf surfaces. This indicates that water and glycerol drops (having a significant polar [acid-base] surface tension component) interacted with the trichomes present in the adaxial surface of young leaves, which led to a sharp θ decrease shortly after drop deposition (Fig. 2A; Table I). On the contrary, the glabrous upper leaf surface of mature leaves and the highly pubescent abaxial young and mature leaf surfaces enabled the measurement of static θ values immediately after drop deposition, which implies a lower degree of chemical interactions between such liquids and the plant surfaces analyzed (Fig. 2, B–D).
Table I. θ of water (θw), glycerol (θg), and diiodomethane (θd) with the adaxial and abaxial surface of young and mature holm oak leaves.
| Leaf Age |
θw |
θg |
θd |
|||
|---|---|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | Adaxial | Abaxial | |
| ° | ||||||
| Young | 40.7 ± 3.8 | 130.1 ± 6.5 | 50.2 ± 5.1 | 141.4 ± 4.4 | 56.6 ± 4.9 | 128.4 ± 6.5 |
| 91.1 ± 6.0s | 143.5 ± 6.0s | |||||
| Mature | 55.5 ± 5.1 | 134.3 ± 10.1 | 68.6 ± 5.9 | 142.5 ± 3.9 | 59.1 ± 4.1 | 119.3 ± 10.2 |
With regard to leaf age, higher water and glycerol θ values were measured on the upper side of mature versus young leaves, but similar results were recorded for all abaxial leaf surfaces. In contrast, diiodomethane θ measurements were similar for the adaxial surface of young and mature leaves and slightly higher for the abaxial side of young leaves.
The total γ of the holm oak leaf surfaces evaluated was determined from θ measurements (Fernández et al., 2011, 2014) using the so-called van Oss, Good, and Chaudhury method (van Oss et al., 1987, 1988). The γ of young leaf adaxial surfaces was higher than that of mature surfaces (Table II). By contrast, the abaxial surface of mature leaves had a higher γ value compared with that of young leaves. On the other hand, the total γ results of young and mature adaxial leaf surfaces were higher than the values determined for the corresponding abaxial surfaces. The γ values of the upper surfaces can be mainly ascribed to their relatively higher dispersive component (Lifshitz-van der Waals component [γLW]). For the adaxial surface, the nondispersive component (acid-base component [γAB]) was higher for young leaves and decreased with maturity (Table II).
Table II. γ per unit of area.
γLW, γAB, γ, and surface polarity (γAB γ–1) of the adaxial and abaxial surface of young and mature holm oak leaves.
The highest degree of surface polarity was generally determined for the abaxial leaf side. For the upper side, the surface polarity values of young leaves were, however, higher than those determined for mature leaves (Table II).
The Wa (Kwok and Neumann, 1999) for the three liquids and the δ were calculated as described by Fernández et al. (2011, 2014) and Khayet and Fernández (2012; Table III). The adaxial surface of young and mature holm oak leaves exhibited a higher adhesion for water drops, followed by glycerol and diiodomethane, compared with the water drop repellence of the lower leaf side. Of the three liquids, the highest Wa values were recorded for water drops deposited on to the upper leaf side. Regardless of leaf age, the abaxial surfaces were found to have similar Wa values for water and diiodomethane, which were higher than those for glycerol. The δ values of the adaxial surface of young and mature leaves were considerably higher than those of the lower leaf side. Regarding the effect of leaf age, younger leaves had higher δ values for the upper side.
Table III. δ and Wa for water (Wa,w), glycerol (Wa,g), and diiodomethane (Wa,d) and δ of intact, adaxial, and abaxial surfaces of young and mature holm oak leaves.
| Leaf Age |
Wa,w |
Wa,g |
Wa,d |
δ |
||||
|---|---|---|---|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | Adaxial | Abaxial | Adaxial | Abaxial | |
| mJ m–2 | MJ1/2 m–3/2 | |||||||
| Young | 127.99 | 25.93 | 104.98 | 14.00 | 78.79 | 19.25 | 20.85 | 3.67 |
| Mature | 114.04 | 21.99 | 87.40 | 13.25 | 76.91 | 25.94 | 15.77 | 4.62 |
DISCUSSION
In this study, we analyzed the physicochemical properties of the adaxial and abaxial surface of holm oak leaves as a model for a typically Mediterranean species and also in relation to leaf maturity. We selected this species because it constitutes an interesting system for assessing the nature and functionality of adaxial and abaxial leaf surfaces and also for evaluating leaf water absorption and solid-liquid interactions. As a preliminary trial, we assessed the water absorption capacity of holm oak leaves by measuring the leaf water potential 24 h after depositing water drops either on to the adaxial or abaxial leaf side. After gaining evidence for leaf hydration via the upper leaf side (independently of leaf age), we analyzed in detail the physicochemical and structural properties of abaxial and adaxial holm oak leaf surfaces, focusing on trichomes. The structure and morphology of the adaxial and abaxial trichomes were similar to those described in previous holm oak leaf studies (Karabourniotis et al., 1998; Karioti et al., 2011; Camarero et al., 2012).
Trichome Chemical Composition and Structure
The presence of trichomes in developing organs has been reported in previous studies (Karabourniotis et al., 1995). The adaxial and abaxial holm oak leaf side performed differently in terms of wettability (Table I), as previously found for other plant species (Brewer and Smith, 1997; Fernández et al., 2014). We showed that the hydrophobic character of the abaxial leaf side is clearly associated with the roughness provided by the high density, chemical composition, and structure of trichomes. According to the classification suggested by Brewer et al. (1991) and Brewer and Smith (1997), the abaxial holm oak leaf trichomes may belong to the lifting strategy group, while further trials will require characterizing the performance of liquid water with the adaxial oak leaf side at a macroscopic level.
Leaf surface hydrophobicity has been often interpreted as either an adaptive trait to dry climates (Holder, 2007a) or to wet environments (Holder, 2007b). The occurrence of trichome layers on the abaxial leaf surface may be considered as a xeromorphic character, which provides adaptive advantages mainly under biotic and abiotic stress conditions (Karabourniotis et al., 1992; Karabourniotis and Bormann, 1999; Liakopoulos et al., 2006). In spite of this, the relationship between leaf surface hydrophobicity and the ecological conditions to which a plant species is native remains controversial.
By measuring the θ values of three liquids with different polarity, we gained evidence for the wettable character of holm oak adaxial leaf surfaces. The increased wettability and Wa for water of the adaxial young leaf surfaces is associated with the occurrence of hydrophilic trichomes, which interacted with polar liquids (i.e. water and glycerol) immediately after drop deposition. The degradation of adaxial trichomes after leaf immersion in cellulase showed that they were permeable to water, in contrast to the abaxial ones, which were not altered by the enzymatic treatment. The cuticular irregularities observed in the surface and base of the adaxial trichomes are likely to be the main uptake pathway for the cellulase solution, which finally led to trichome degradation.
The gradual extraction of cuticular components of the abaxial leaf surface trichomes coupled with SEM observations facilitated the quantification of cuticular constituents, which amounted to 8% soluble waxes, 49% depolymerized material (likely cutin), and 43% residue (chiefly polysaccharides). The abaxial holm oak leaf trichomes were largely found to have a higher proportion of cutin and a lower content of polysaccharides compared with the peach (Prunus persica) fruit trichomes analyzed by Fernández et al. (2011; having 15% waxes, 19% cutin, and 66% polysaccharides). SEM and transmission electron microscopy (TEM) observations also in relation to the chemical removal of cuticular components showed that both the adaxial and abaxial trichomes are covered with a cuticle, which seemed to be homogeneously distributed along the surface of the more long-lasting abaxial trichomes but appeared to develop heterogeneously over the cell wall of the adaxial trichomes of young holm oak leaves. Apart from being hydrophilic, such trichomes fractured more easily. Moreover, trichome shedding left a scar on the adaxial leaf surface, and the base of the abscised trichomes may represent a site for water entry because the cuticle in this area was observed to be thinner and more heterogeneous (Fig. 3, F and G). In addition, adaxial trichomes were often located in the vicinity of bundle sheath extensions (Fig. 3, F and G). Bundle sheath extensions may facilitate the transport of water in such heterobaric leaves (Wylie, 1943) and may be related to water economy (Nikolopoulos et al., 2002).
Leaf Surface Wettability, Polarity, δ, and Wa
Measurement of the water θ provides information about the wettability and retention of water drops by different leaf surfaces and may serve as a tool to classify species growing in contrasting environmental conditions (Brewer et al., 1991; Brewer and Nuñez, 2007; Holder, 2007a, b). Additional determinations of the θ of liquids with different polarity (Fernández et al., 2011; Khayet and Fernández, 2012) facilitate the quantitative characterization of plant surfaces and their solid-liquid interactions in relation to the combined effect of surface chemistry and roughness.
The most abundant epicuticular waxes found on holm oak young leaves are n-alkyl esters, representing up to 56% of the total chloroform soluble waxes, followed by n-primary alcohols (Martins et al., 1999). While an increased surface roughness due to pubescence (Fernández et al., 2011) seems again to have a major effect on leaf surface wettability, the contribution of surface chemistry should also be considered and is the major factor affecting water-solid interactions in the adaxial surface of mature leaves (rather flat and glabrous). The predominant role of surface chemistry in relation to the adaxial, mature leaf side was supported by the δ obtained experimentally (approximately 16 MJ1/2 m–3/2), which is within the range calculated by Khayet and Fernández (2012) for model epicuticular wax molecular structures (between 16 to 17 MJ1/2 m–3/2). By contrast, the dense indumentum of the lower leaf sides led to extremely low δ values, which were even below those determined for the almost superhydrophobic juvenile Eucalyptus globulus leaf surface (Khayet and Fernández, 2012). This may be due to the high level of surface roughness and to the occurrence of air pockets within the trichome layers covering the abaxial leaf side (Fernández et al., 2011), which may be even denser when leaves are young.
Irrespective of leaf age, the upper leaf side had a higher γ than the lower one, while polarity followed an inverse trend. The Wa for water provides a quantification of the degree of water drop adhesion or repellence to a certain plant surface (Fernández et al., 2014). The Wa for water was extremely high for the adaxial holm oak leaf surface compared with the values previously obtained for other pubescent or glabrous plant materials (Khayet and Fernández, 2012; Fernández et al., 2014). The presence of trichomes in the adaxial side of the young holm oak leaves additionally increased the adhesion of water drops to such surfaces. However, the lower leaf sides had a degree of water drop repulsion similar to the one of the peach fruit (Fernández et al., 2011), but their Wa for water was above the values estimated for the even more water-repellent and unwettable, hairy, adaxial wheat (Triticum aestivum) leaf surface (Fernández et al., 2014).
Leaf Water Uptake and Ecophysiological Implications
The ability to capture water via the leaf has been indirectly reported in species occurring in deserts (Martin and von Willert, 2000), tropical climates (Yates and Hutley, 1995), cloud-immersed mountain habitats (Berry and Smith, 2012; Berry et al., 2013, 2014), and coastal mountain regions where fog is a significant climatic contributor (Burgess and Dawson, 2004). However, this is the first time that foliar water uptake has been analyzed in strict physicochemical terms, while recording significant water potential changes in response to leaf hydration.
Regardless of leaf age, we observed a markedly different performance of the adaxial (wettable and retaining water drops) versus the abaxial (unwettable and water-repellent) holm oak leaf surfaces when in contact with water drops. We have demonstrated that the major wettability of the adaxial side of holm oak leaves allows for leaf rehydration both in terms of RWC and leaf water potential (Fig. 1). Drop adherence to the plant surface is a prerequisite for foliar uptake to occur (Fernández and Brown, 2013; Fernández et al., 2014). Thereby, in the case of holm oak leaves, foliar absorption of pure water (i.e. in the absence of surfactants) may only take place through the upper leaf side to which water drops strongly adhere (having a high Wa for water; Table III). By contrast, the repulsion of water drops by the abaxial leaf side will impede the penetration of liquid water. The hydrophobic nature of the abaxial leaf trichomes will ensure the occurrence of an air layer above stomatal pores and hence help to preserve an adequate gas exchange rate even under wet conditions.
The permeability of plant surfaces to water and solutes has been a matter of scientific interest since the last century, but the mechanisms involved are still not fully characterized (Fernández and Eichert, 2009). Water deposited onto a leaf surface may penetrate via stomata, the cuticle, cuticular cracks, and irregularities or through specialized epidermal cells such as trichomes (Fernández and Brown, 2013). In the case of holm oak adaxial leaf surfaces, trichomes and the remaining scars after trichome shedding may play a key role in water absorption. The high surface tension, polarity, and H-bonding capacity of water theoretically pose restrictions for the penetration of this liquid through the cuticle (Guzmán et al., 2014 a, 2014b) and also via stomata (Schönherr and Bukovac, 1972; Burkhardt et al., 2012). The actual contribution of leaf trichomes to the absorption of water and solutes remains unclear (Fernández et al., 2014). While some studies suggest that trichomes may actively participate in the uptake of foliar-applied nutrient solutions (e.g. Benzing et al., 1976; Schlegel and Schönherr, 2002) and water in Bromeliads (e.g. Pierce et al., 2001; Reyes-García et al., 2012), the contribution of Phlomis fruticosa leaf trichomes to the absorption of water could not be clarified (Grammatikopoulos and Manetas, 1994). A traditional problem when investigating the mechanisms of foliar penetration is the occurrence of technical constraints associated with optical and fluorescence microscopy and attempting to observe the uptake of specific dyes and solutes by leaf surface micro- and nanostructures (Fernández and Eichert, 2009). Fahn (1986) and, more recently, Burrows et al. (2013) reported that leaf trichomes having a cutinized base would fail to take up the surface-applied dye solutions and transport them into the leaf interior. While the base of the adaxial and abaxial holm oak leaf trichomes that we analyzed was cutinized, when applying water drops onto the adaxial surface of young holm oak leaves, we gained evidence for hydration, as derived from the resulting leaf water potential increase. Our results suggest that there may be a major degree of variability among trichome structures and functions that may sometimes impede or facilitate the uptake of water.
Foliar water uptake due to natural phenomena such as fog or dew may be an important mechanism of hydration in some areas of the world subjected to temporary drought (e.g. Burgess and Dawson, 2004; Oliveira et al., 2005; Breshears et al., 2008; Limm et al., 2009; Simonin et al., 2009; Limm and Dawson, 2010; Berry et al., 2013, 2014; Gotsch et al., 2014).
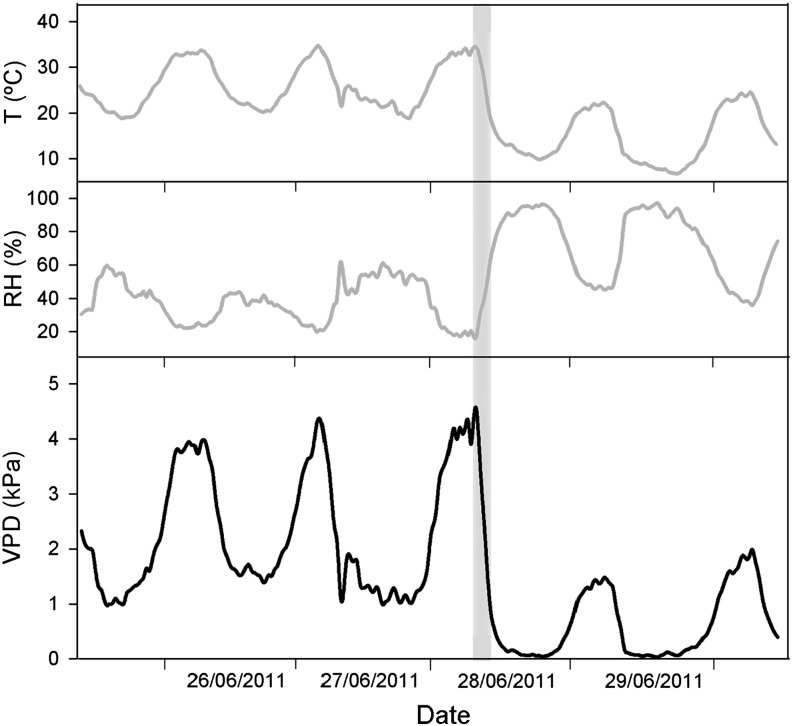
Holm oak is a species native to Mediterranean-type climates where summer drought is imposed by a combination of high temperatures and low precipitation. In spite of the lower precipitation level during this season, summer rainfall constitutes 24% of the whole-year precipitation (referring to the Iberian Peninsula; http://www.aemet.es). In this season, short-term storms are the most common precipitation form, being up to 100% of the fallen rain during July and August (Mosmann et al., 2004). While most of the water falling after high-precipitation summer storms is not available for plant roots due to storm water runoff, low precipitation storms (below 1 mm) are considered negligible in terms of soil water balance (Allen et al., 2000), the latter accounting for 54% of the total summer rainfall (http://www.aemet.es). Therefore, direct water uptake by the foliage may positively contribute to water economy of holm oak during the summer. From the beginning of a storm (high or low precipitation), the environmental conditions (air temperature and humidity) and vapor pressure deficit (VPD) sharply change, as shown in Figure 7 (data gathered with a Hobo Pro temp/RH [Onset Computer] and a Rain Collector II [Davis Instruments]). The extreme VPD reduction (close to 0 kPa, as simulated in our rehydration trials) provides optimal conditions for foliar water absorption, because the low VPD retards the evaporation of water drops, hence increasing the chance for water uptake by the foliage.
Figure 7.
Hourly evolution of temperature (T), relative humidity (RH), and VPD during five consecutive summer days in a typically Mediterranean holm oak area. The gray area indicates a precipitation event less than 1 mm.
An additional important source for leaf water absorption for evergreen holm oak is the formation of dew due to condensation, which may occur all year round but may be especially relevant during the summer. This mechanism occurs on approximately 20% of summer days in the holm oak growing areas of the Iberian Peninsula (http://www.aemet.es). Of the surface condensation mechanisms described (Nourbakhsh, 1989), two can be expected to occur under field conditions: (1) film condensation on the wettable adaxial leaf side and (2) dropwise condensation on the unwettable abaxial surface. While film condensation may lead to direct foliar water uptake, dropwise condensation will favor drop dripping either to the soil or to the foliage below, which may again turn available for foliar absorption. Finally, a further water deposition phenomenon of limited relevance under Mediterranean conditions is the occurrence of fog, in which the expected leaf wetting and water absorption mechanisms will be similar to those described above for dew deposition.
It could be reckoned that adaxial leaf trichomes and scars may also contribute to water loss and leaf dehydration. However, the mechanisms of uptake of liquid water and loss probably as water vapor (Rockwell et al., 2014) and processes of water adsorption and desorption in the cuticle (Reina et al., 2001) may be different and will require further investigation. The functional advantage of water absorption by the upper leaf side is not linked to a higher cuticular conductance, because no statistical differences between adaxial and abaxial cuticular conductances were found.
In summary, in this study, we analyzed the water-leaf surface interactions of the adaxial and abaxial leaf side of holm oak, a typical evergreen Mediterranean species. While the adaxial trichomes present in young leaves were hydrophilic, the dense indumentum of the lower leaf side led to a high degree of hydrophobicity. The upper holm oak leaf side of young and mature leaves was wettable and absorbed water, and the uptake mechanisms have been related to the presence of trichomes and trichomes scars. It is concluded that trichome structure, chemical composition, and function may vary, even for the same organ, but in relation to their occurrence in the abaxial versus the adaxial leaf side. The water absorption capacity of holm oak leaves may be ecologically advantageous for competition under Mediterranean conditions and in relation to different means of water precipitation (e.g. condensation, rain, or fog) all year round, especially during summer and when growing in soils with low water storage capacity.
MATERIALS AND METHODS
Plant Material
Holm oak (Quercus ilex) seeds were planted in 500-mL containers filled with a mixture of 80% substratum and 20% perlite and were kept in a greenhouse. Seedlings were transplanted to 25-L containers after the first growing season, and they were subsequently transplanted to an experimental field plot (Centro de Investigación y Tecnología Agroalimentaria). Plants always grew under typically Mediterranean environmental conditions and were irrigated when necessary, avoiding also the incidence of pests and diseases. Ten-year-old trees were finally selected for the development of this study. Shoots with undamaged, fully developed, 2-month-old leaves with pubescent adaxial and abaxial sides (referred to as young leaves) were collected in early summer, whereas shoots having 8-month-old leaves (referred to as mature leaves) with a glabrous adaxial and a pubescent abaxial surface were collected in late autumn.
Leaf Surface Water Absorption
By mid-June, two holm oak twigs per tree from five different trees of holm oak were cut and enclosed in plastic bags until measurement. The Ψo and initial fresh weight were measured in three young shoots of each of the 10 twigs selected. Then, 2 mL of distilled water were carefully deposited onto (1) the adaxial side of the leaves of one of the shoots and (2) the abaxial side of the leaves of another shoot. For comparison, the third shoot was used as an untreated control shoot. Thereafter, young shoots were enclosed in single plastic bags and stored in the dark at room temperature (at approximately 20°C). After 24 h, the weight and water potential were measured again in all shoots to obtain the Ψf and final fresh weight. Then, shoots were dried in an oven (65°C for 24 h), and they were weighed to obtain the dry weight (DW). With the remaining young shoots of each twig not used in this process, we obtained a relationship between shoot full saturation weight (turgid weight) and DW. Afterward, weight measurements were used to calculate the RWC for each shoot as RWC = (fresh weight – DW)/(turgid weight – DW). A Student’s t test paired sample comparison was used to compare leaf water potential and RWC before (Ψo) and after (Ψf) surface wetting. This leaf surface water absorption process was repeated again in late autumn on mature leaves.
Cuticular Conductance
Cuticular water losses were measured gravimetrically on fully rehydrated young and mature leaves (Anfodillo et al., 2002). To differentially assess the transpiration loss of adaxial and abaxial surfaces, silicon grease was applied onto the opposite surface to seal it against water loss. After initially weighing the leaves, they were placed for 72 h in a dark chamber with an electric fan ensuring air circulation under constant humidity and temperature conditions (22.4°C ± 0.1°C and 76.4% ± 0.1%). Cuticular conductance (ms–1) was calculated from the transpiration data and according to Anfodillo et al. (2002).
Quantitative and Qualitative Estimation of Chemical Components of Holm Oak Trichomes
Adaxial and abaxial trichomes of young holm oak leaves were mechanically isolated by gently scraping the leaf surfaces with a scalpel. Trichomes were subjected to the successive removal of soluble cuticular lipids and cutin, while performing simultaneous FTIR analyses using a Nexus 670-870 NICOLET FTIR spectrometer (Thermo Fisher Scientific; transmission mode, 3,850 to 850 cm–1 with 4 cm–1 resolution, and accumulating 64 scans). For FTIR, samples were ground with 1% potassium bromide, and thin tablets were subsequently formed and set into the apparatus. A blank corresponding to the spectrum of potassium bromide was also recorded and substracted from sample spectra. For chemical removal, hairs were first immersed in chloroform for 4 h, and then cutin was depolymerized by saponification in 1 m sodium hydroxide for 24 h under reflux conditions. Percentages of each chemical fraction (soluble waxes, cutin, and remaining residue) were calculated according to the corresponding weight loss.
Additionally, young holm oak leaves were enzymatically digested for 4 and 7 d in either 2% cellulase or 2% pectinase (both from Novozymes) plus 2 mm sodium azide, adjusting the pH to 5.0 by adding sodium citrate.
Microscopy
Gold-sputtered intact and enzymatically digested adaxial and abaxial holm oak leaf surfaces were examined with a variable-pressure SEM (Hitachi S-3400 N; acceleration potential, 20 kV; working distance, 15–17 mm). Changes in adaxial and abaxial isolated trichome structure were analyzed in intact hairs and after soluble lipid extraction and alkaline hydrolysis.
For TEM, samples were fixed in 2.5% glutaraldehyde-4% paraformaldehyde (both from Electron Microscopy Sciences) for 6 h at 4°C, rinsed in ice-cold phosphate buffer, pH 7.2, four times within a period of 6 h, and left overnight. Tissues were then postfixed in a 1:1 2% aqueous osmium tetroxide (TAAB Laboratories) and 3% aqueous potassium ferrocyanide (Sigma-Aldrich) solution for 1.5 h. Samples were then washed with distilled water (three times), dehydrated in a graded series of 30%, 50%, 70%, 80%, 90%, 95%, and 100% acetone (two times, 15 min each concentration), and embedded in acetone-Spurr’s resin (TAAB Laboratories) solutions (3:1, 2 h; 1:1, 2 h; 1:3, 3 h [v/v]) and in pure resin overnight at room temperature. Final embedding was done in blocks that were incubated at 70°C for 3 d for complete polymerization. Prior to TEM observation, sections were poststained with Reynolds lead citrate (Electron Microscopy Sciences) for 5 min.
Thin, cryosectioned leaf tissues (30 μm thick) were observed with a Olympus BX40 fluorescence microscope. Transversal sections were examined by either visible light transmission or under UV excitation (emission of blue fluorescence by simple phenols and lignin) after immersion in 10% (w/v) sodium hydroxide for 2 min, followed by a thorough distilled water rinse. An ultraviolet mirror unit's wavelength filter combination (exciter filter, 330–385 nm; barrier filter, 420 nm) was used. Leaf sections were also stained with Sudan IV. Microphotographs were taken using an Olympus DP71 digital camera.
The average number of trichome arms, arm length, arm diameter, trichome densities, and leaf area covered with hairs were assessed by image analysis of adaxial and abaxial SEM micrographs (ImageJ 1.45s; Rasband, 2014).
Determinations and Leaf Surface Properties
The advancing θ of drops of double-distilled water, glycerol, and diiodomethane (both 99% purity; Sigma-Aldrich) were measured at room temperature (25°C) using a CAM 200 Contact Angle Meter (KSV Instruments) equipped with a CCD camera, frame grabber, and image analysis software. θ values were determined on adaxial and abaxial surfaces of intact, young, and mature leaves. After removing the midrib and margins, leaf sections of approximately 2 × 0.5 cm2 were cut with a scalpel and mounted on a microscope slide with double-sided adhesive tape. Two-microliter drops of each liquid were deposited onto the adaxial or abaxial holm leaf surfaces with a manual dosing system holding a 1-mL syringe with a 0.5-mm-diameter needle (30 repetitions). Side-view images of the drops were captured at a rate of 6 frames s–1. θ values were automatically calculated by fitting the captured drop shape to the one calculated from the Young-Laplace equation. For mature leaves and all abaxial leaf surfaces, θ values for all the liquids were measured shortly after drop deposition. On young adaxial surfaces, however, θ values of water and, chiefly, glycerol were initially high and decreased over time. Hence, θ values were recorded immediately after drop deposition and also when they were static (taking approximately 15 s for water and 10 min for glycerol).
For all the surfaces evaluated, the γ or surface tension and its components, i.e. γLW and γAB (with the contributions of electron acceptor [γ+] and electron donor [γ–] interactions), as well as the Wa for the three liquids and the δ were calculated, considering the surface tension components of water (γLW = 21.8 mJ m–2 and γ+ and γ– = 25.5 mJ m–2), glycerol (γLW = 34.0 mJ m–2, γ+ = 3.92 mJ m–2, and γ– = 57.4 mJ m–2), and diiodomethane (γLW = 50.8 mJ m–2 and γ+ and γ– = 0 mJ m–2; Fernández et al., 2011; Khayet and Fernández, 2012).
Glossary
- SEM
scanning electron microscopy
- Ψo
initial water potential
- Ψf
final water potential
- RWC
relative water content
- FTIR
Fourier transform infrared spectroscopy
- γ
total surface free energy
- γLW
Lifshitz-van der Waals component
- γAB
acid-base component
- θ
contact angle
- Wa
work of adhesion
- δ
solubility parameter
- VPD
vapor pressure deficit
- DW
dry weight
- TEM
transmission electron microscopy
Footnotes
This work was supported by the Spanish Ministry of Economy and Competitiveness (projects AGL2010–21153–C02–02 and AGL2012–35580), a Ramon y Cajal contract to V.F. [cofinanced by the European Social Fund], and a Juan de la Cierva post-doctorial contract to J.J.P.-P.); the Technical University of Madrid (pre-doctoral grant to P.G.), and European Union's Seventh Framework Program for Research (Marie Curie Intra-European Fellowship to J.A.H.-G.).
Articles can be viewed online without a subscription.
References
- Adamec L. (2013) Foliar mineral nutrient uptake in carnivorous plants: what do we know and what should we know? Front Plant Sci 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RG, Walter IA, Elliott B, Mechan B, Jensen ME, Itentisu D, Howell TA, Snyder R, Brown P, Eching S, et al (2000) Issues, requirements and challenges in selecting and specifying and standardized ET equation. In RG Evans, BL Benham, TP Trooien, eds, National Irrigation Symposium: Proceedings of the 4th Decennial Symposium: November 14-16, 2000, Phoenix, Arizona. American Society of Agricultural Engineers, Saint Joseph, pp 201–204 [Google Scholar]
- Anfodillo T, Pasqua di Bisceglie D, Urso T. (2002) Minimum cuticular conductance and cuticle features of Picea abies and Pinus cembra needles along an altitudinal gradient in the Dolomites (NE Italian Alps). Tree Physiol 22: 479–487 [DOI] [PubMed] [Google Scholar]
- Aryal B, Neuner G. (2010) Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 162: 1–9 [DOI] [PubMed] [Google Scholar]
- Benzing DH, Henderson K, Kessel B, Sulak J. (1976) The absorptive capacities of bromeliad trichomes. Am J Bot 63: 1009–1014 [Google Scholar]
- Berry ZC, Hughes NM, Smith WK. (2013) Cloud immersion: an important water source for spruce and fir saplings in the southern Appalachian Mountains. Oecologia 173: 637–648 [DOI] [PubMed] [Google Scholar]
- Berry ZC, Hughes NM, Smith WK. (2014) Cloud immersion: an important water source for spruce and fir saplings in the southern Appalachian Mountains. Oecologia 174: 319–326 [DOI] [PubMed] [Google Scholar]
- Berry ZC, Smith WK. (2012) Cloud pattern and water relations in Picea rubens and Abies fraseri, southern Appalachian Mountains, USA. Agric For Meteorol 162: 27–34 [Google Scholar]
- Breshears DD, McDowell NG, Goddard KL, Dayem KE, Martens SN, Meyer CW, Brown KM. (2008) Foliar absorption of intercepted rainfall improves woody plant water status most during drought. Ecology 89: 41–47 [DOI] [PubMed] [Google Scholar]
- Brewer CA, Nuñez CI. (2007) Patterns of leaf wettability along an extreme moisture gradient in western Patagonia, Argentina. Int J Plant Sci 168: 555–562 [Google Scholar]
- Brewer CA, Smith WK. (1997) Patterns of leaf surface wetness for montane and subalpine plants. Plant Cell Environ 20: 1–11 [Google Scholar]
- Brewer CA, Smith WK, Vogelmann TC. (1991) Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant Cell Environ 14: 955–962 [Google Scholar]
- Burgess SSO, Dawson TE. (2004) The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant Cell Environ 27: 1023–1034 [Google Scholar]
- Burkhardt J, Basi S, Pariyar S, Hunsche M. (2012) Stomatal penetration by aqueous solutions: an update involving leaf surface particles. New Phytol 196: 774–787 [DOI] [PubMed] [Google Scholar]
- Burkhardt J, Hunsche M. (2013) “Breath figures” on leaf surfaces-formation and effects of microscopic leaf wetness. Front Plant Sci 4: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows GE, White RG, Harper JD, Heady RD, Stanton RA, Zhu X, Wu H, Lemerle D. (2013) Intrusive trichome bases in the leaves of silverleaf nightshade (Solanum elaeagnifolium; Solanaceae) do not facilitate fluorescent tracer uptake. Am J Bot 100: 2307–2317 [DOI] [PubMed] [Google Scholar]
- Camarero JJ, Olano JM, Arroyo Alfaro SJ, Fernández-Marín B, Becerril JM, Garcia-Plazaola JI. (2012) Photoprotection mechanisms in Quercus ilex under contrasting climatic conditions. Flora 207: 557–564 [Google Scholar]
- Dietz J, Leuschner C, Hölscher D, Kreilein H. (2007) Vertical patterns and duration of surface wetness in an old-growth tropical montane forest, Indonesia. Flora 202: 111–117 [Google Scholar]
- Domínguez E, Heredia-Guerrero JA, Heredia A. (2011) The biophysical design of plant cuticles: an overview. New Phytol 189: 938–949 [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Björkman O. (1978) A comparison of photosynthetic characteristics of Encelia species possessing glabrous and pubescent leaves. Plant Physiol 62: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrode SD, Jetter R. (2002) Attachment to plant surface waxes by an insect predator. Integr Comp Biol 42: 1091–1099 [DOI] [PubMed] [Google Scholar]
- Fahn A. (1986) Structural and functional properties of trichomes of xeromorphic leaves. Ann Bot (Lond) 57: 631–637 [Google Scholar]
- Fernández V, Brown PH. (2013) From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front Plant Sci 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V, Eichert T. (2009) Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit Rev Plant Sci 28: 36–68 [Google Scholar]
- Fernández V, Guzmán P, Peirce CAE, McBeath TM, Khayet M, McLaughlin MJ. (March 14, 2014) Effect of wheat phosphorus status on leaf surface properties and permeability to foliar applied phosphorus. Plant Soil http://dx.doi.org/10.1007/s11104-014-2052-6 [Google Scholar]
- Fernández V, Khayet M, Montero-Prado P, Heredia-Guerrero JA, Liakopoulos G, Karabourniotis G, Del Río V, Domínguez E, Tacchini I, Nerín C, et al. (2011) New insights into the properties of pubescent surfaces: peach fruit as a model. Plant Physiol 156: 2098–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gniwotta F, Vogg G, Gartmann V, Carver TL, Riederer M, Jetter R. (2005) What do microbes encounter at the plant surface? Chemical composition of pea leaf cuticular waxes. Plant Physiol 139: 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsch SG, Asbjornsen H, Holwerda F, Goldsmith GR, Weintraub AE, Dawson TE. (2014) Foggy days and dry nights determine crown-level water balance in a seasonal tropical montane cloud forest. Plant Cell Environ 37: 261–272 [DOI] [PubMed] [Google Scholar]
- Grammatikopoulos G, Manetas Y. (1994) Direct absorption of water by hairy leaves of Phlomis fruticosa and its contribution to drought avoidance. Can J Bot 72: 1805–1811 [Google Scholar]
- Guzmán P, Fernández V, García ML, Khayet M, Fernández A, Gil L. (2014b) Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol Biochem 76: 1–6 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Fernández V, Khayet M, Gil ML, Fernández A, Gil L (2014a) Ultrastructure of plant leaf cuticles in relation to sample preparation as observed by transmission electron microscopy. Sci World J 2014: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba YT, Moriya A, Kimura K. (2004) Effect of leaf surface wetness and wettability on photosynthesis in bean and pea. Plant Cell Environ 27: 413–421 [Google Scholar]
- Hardin JW. (1976) Terminology and classification of Quercus trichomes. J Elisha Mitchell Sci Soc 92: 151–161 [Google Scholar]
- Helliker BR. (2014) Reconstructing the δ18O of atmospheric water vapour via the CAM epiphyte Tillandsia usneoides: seasonal controls on δ18O in the field and large-scale reconstruction of δ18Oa. Plant Cell Environ 37: 541–556 [DOI] [PubMed] [Google Scholar]
- Helliker BR, Griffiths H. (2007) Towards a plant-based proxy for the isotope ratio of atmospheric water vapor. Glob Change Biol 13: 723–733 [Google Scholar]
- Holder CD. (2007a) Leaf water repellency of species in Guatemala and Colorado (USA) and its significance to forest hydrology studies. J Hydrol (Amst) 336: 147–154 [Google Scholar]
- Holder CD. (2007b) Leaf water repellency as an adaptation to tropical montane cloud forest environments. Biotropica 39: 767–770 [Google Scholar]
- Javelle M, Vernoud V, Rogowsky PM, Ingram GC. (2011) Epidermis: the formation and functions of a fundamental plant tissue. New Phytol 189: 17–39 [DOI] [PubMed] [Google Scholar]
- Johnson HB. (1975) Plant pubescence: an ecological perspective. Bot Rev 41: 233–258 [Google Scholar]
- Johnstone JA, Dawson TE. (2010) Climatic context and ecological implications of summer fog decline in the coast redwood region. Proc Natl Acad Sci USA 107: 4533–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G, Bormann JF. (1999) Penetration of UV-A UV-B and blue light through the leaf trichome of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiol Plant 105: 655–661 [Google Scholar]
- Karabourniotis G, Kofidis G, Fasseas C, Liakoura V, Drossopoulos I. (1998) Polyphenol deposition in leaf hairs of Olea europaea (Oleaceae) and Quercus ilex (Fagaceae). Am J Bot 85: 1007–1012 [PubMed] [Google Scholar]
- Karabourniotis G, Kotsabassidis D, Manetas Y. (1995) Trichome density and its protective potential against ultraviolet-B radiation damage during leaf development. Can J Bot 73: 376–383 [Google Scholar]
- Karabourniotis G, Papadopoulos K, Papamarkou M, Manetas Y. (1992) Ultraviolet-B radiation absorbing capacity of leaf hairs. Physiol Plant 86: 414–418 [Google Scholar]
- Karioti A, Sokovic M, Ciric A, Koukoulitsa C, Bilia AR, Skaltsa H. (2011) Antimicrobial properties of Quercus ilex L. proanthocyanidin dimers and simple phenolics: evaluation of their synergistic activity with conventional antimicrobials and prediction of their pharmacokinetic profile. J Agric Food Chem 59: 6412–6422 [DOI] [PubMed] [Google Scholar]
- Kenzo T, Yoneda R, Azani MA, Majid NM. (2008) Changes in leaf water use after removal of leaf lower surface hairs on Mallotus macrostachyus (Euphorbiaceae) in a tropical secondary forest in Malaysia. J For Res 13: 137–142 [Google Scholar]
- Khayet M, Fernández V. (2012) Estimation of the solubility parameters of model plant surfaces and agrochemicals: a valuable tool for understanding plant surface interactions. Theor Biol Med Model 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee X. (2011) Transition of stable isotope ratios of leaf water under simulated dew formation. Plant Cell Environ 34: 1790–1801 [DOI] [PubMed] [Google Scholar]
- Koch K, Barthlott W. (2009) Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Philos Trans A Math Phys Eng Sci 367: 1487–1509 [DOI] [PubMed] [Google Scholar]
- Konrad W, Ebner M, Traiser C, Roth-Nebelsick A. (2012) Leaf surface wettability and implications for drop shedding and evaporation from forest canopies. Pure Appl Geophys 169: 835–845 [Google Scholar]
- Kwok DY, Neumann AW. (1999) Contact angle measurement and contact angle interpretation. Adv Colloid Interfac 81: 167–249 [Google Scholar]
- Łaźniewska J, Macioszek VK, Kononowicz AK. (2012) Plant-fungus interface: the role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol Mol Plant Pathol 78: 24–30 [Google Scholar]
- Liakopoulos G, Nikolopoulos D, Klouvatou A, Vekkos KA, Manetas Y, Karabourniotis G. (2006) The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann Bot (Lond) 98: 257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limm EB, Dawson TE. (2010) Polystichum munitum (Dryopteridaceae) varies geographically in its capacity to absorb fog water by foliar uptake within the redwood forest ecosystem. Am J Bot 97: 1121–1128 [DOI] [PubMed] [Google Scholar]
- Limm EB, Simonin KA, Bothman AG, Dawson TE. (2009) Foliar water uptake: a common water acquisition strategy for plants of the redwood forest. Oecologia 161: 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, von Willert DJ. (2000) Leaf epidermal hydathodes and the ecophysiological consequences of foliar water uptake in species of Crassula from the Namib desert in Southern Africa. Plant Biol 2: 229–242 [Google Scholar]
- Martins CMC, Mesquita SMM, Vaz WLC. (1999) Cuticular waxes of the holm (Quercus ilex L. subsp. ballota (Desf.) Samp.) and cork (Q. suber L.) oaks. Phytochem Anal 10: 1–5 [Google Scholar]
- Morales F, Abadía A, Abadía J, Montserrat G, Gil-Pelegrín E. (2002) Trichomes and photosynthetic pigment composition changes: responses of Quercus ilex subsp. ballota (Desf.) Samp. and Quercus coccifera L. to Mediterranean stress conditions. Trees (Berl) 16: 504–510 [Google Scholar]
- Mosmann V, Castro A, Fraile R, Dessens J, Sanchez JL. (2004) Detection of statistically significant trends in the summer precipitation of mainland Spain. Atmos Res 70: 43–53 [Google Scholar]
- Nikolopoulos D, Liakopoulos G, Drossopoulos I, Karabourniotis G. (2002) The relationship between anatomy and photosynthetic performance of heterobaric leaves. Plant Physiol 129: 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourbakhsh HP (1989) Two-phase heat transfer. In EC Guyer, ed, Handbook of Applied Thermal Design. McGraw-Hill, New York, pp 75–84 [Google Scholar]
- Oliveira RS, Dawson TE, Burgess SO. (2005) Evidence for direct water absorption by the shoot of the dessication-tolerant plant Vellozia flavicans in the savannas of central Brazil. J Trop Ecol 21: 585–588 [Google Scholar]
- Pandey S, Nagar PK. (2003) Pattern of leaf surface wetness in some important medicinal and aromatic plants of western Himalaya. Flora 198: 349–357 [Google Scholar]
- Pierce S, Maxwell K, Griffiths H, Winter K. (2001) Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae. Am J Bot 88: 1371–1389 [PubMed] [Google Scholar]
- Ramírez FJ, Luque P, Heredia A, Bukovac MJ. (1992) Fourier transform IR study of enzymatically isolated tomato fruit cuticular membrane. Biopolymers 32: 1425–1429 [Google Scholar]
- Rasband WS. (2014) ImageJ. U.S. National Institutes of Health, Bethesda, MD http://imagej.nih.gov/ij (February 15, 2014) [Google Scholar]
- Reicosky DA, Hanover JW. (1978) Physiological effects of surface waxes. Plant Physiol 62: 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina JJ, Domínguez E, Heredia A. (2001) Water sorption-desorption in conifer cuticles: the role of lignin. Physiol Plant 112: 372–378 [DOI] [PubMed] [Google Scholar]
- Reyes-García C, Mejia-Chang M, Griffiths H. (2012) High but not dry: diverse epiphytic bromeliad adaptations to exposure within a seasonally dry tropical forest community. New Phytol 193: 745–754 [DOI] [PubMed] [Google Scholar]
- Riederer M (2006). Introduction: biology of the plant cuticle. In M Riederer, C Müller, eds, Biology of the Plant Cuticle, Vol 23. Blackwell Publishing, Oxford, pp 1–10 [Google Scholar]
- Riederer M, Schreiber L. (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52: 2023–2032 [DOI] [PubMed] [Google Scholar]
- Ripley BS, Pammenter NW, Smith VR. (1999) Function of leaf hairs revisited: the hair layer on leaves Arctotheca populifolia reduces photoinhibition, but leads to higher leaf temperatures caused by lower transpiration rates. J Plant Physiol 155: 78–85 [Google Scholar]
- Rockwell FE, Holbrook NM, Stroock AD. (2014) The competition between liquid and vapor transport in transpiring leaves. Plant Physiol 164: 1741–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado BHP, Holder CD. (2013) The significance of leaf water repellency in ecohydrological research: a review. Ecohydrol 6: 150–161 [Google Scholar]
- Roth-Nebelsick A, Ebner M, Miranda T, Gottschalk V, Voigt D, Gorb S, Stegmaier T, Sarsour J, Linke M, Konrad W. (2012) Leaf surface structures enable the endemic Namib desert grass Stipagrostis sabulicola to irrigate itself with fog water. J R Soc Interface 9: 1965–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Hassiotou F, Veneklaas EJ. (2009) Stomatal crypts have small effects on transpiration: a numerical model analysis. Plant Physiol 151: 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel TK, Schönherr J. (2002) Stage of development affects penetration of calcium chloride into apple fruits. J Plant Nutr Soil Sci 165: 738–745 [Google Scholar]
- Schönherr J, Bukovac MJ. (1972) Penetration of stomata by liquids: dependence on surface tension, wettability, and stomatal morphology. Plant Physiol 49: 813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin KA, Santiago LS, Dawson TE. (2009) Fog interception by Sequoia sempervirens (D. Don) crowns decouples physiology from soil water deficit. Plant Cell Environ 32: 882–892 [DOI] [PubMed] [Google Scholar]
- Urrego-Pereira YF, Martínez-Cob A, Fernández V, Cavero J. (2013) Daytime sprinkler irrigation effects on net photosynthesis of maize and alfalfa. Agron J 105: 1515–1528 [Google Scholar]
- van Oss CJ, Chaudhury MK, Good RJ. (1987) Monopolar surfaces. Adv Colloid Interface Sci 28: 35–64 [DOI] [PubMed] [Google Scholar]
- van Oss CJ, Chaudhury MK, Good RJ. (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev 88: 927–941 [Google Scholar]
- Villena JF, Domínguez E, Heredia A. (2000) Monitoring biopolymers present in plant cuticles by FT-IR spectroscopy. J Plant Physiol 156: 419–422 [Google Scholar]
- Yates DJ, Hutley LB. (1995) Foliar uptake of water by wet leaves of Sloanea woollsii, an Australian subtropical rainforest tree. Aust J Bot 43: 157–167 [Google Scholar]
- Yeats TH, Rose JK. (2013) The formation and function of plant cuticles. Plant Physiol 163: 5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot (Lond) 93: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XF, Lee X, Sun XM, Wang JL, Hu ZM, Li SG, Yu GR. (2012) Dew water isotopic ratios and their relationships to ecosystem water pools and fluxes in a cropland and a grassland in China. Oecologia 168: 549–561 [DOI] [PubMed] [Google Scholar]
- Werker E. (2000) Trichome diversity and development. Adv Bot Res 31: 1–35 [Google Scholar]
- Wylie RB. (1943) The role of the epidermis in foliar organization and its relations to the minor venation. Am J Bot 30: 273–280 [Google Scholar]