Arabidopsis hybrids show different growth patterns in outperforming parents for biomass and yield associated with differences in gene expression patterns, suggesting multiple routes for hybrid vigor.
Abstract
Heterosis is important for agriculture; however, little is known about the mechanisms driving hybrid vigor. Ultimately, heterosis depends on the interactions of specific alleles and epialleles provided by the parents, which is why hybrids can exhibit different levels of heterosis, even within the same species. We characterize the development of several intraspecific Arabidopsis (Arabidopsis thaliana) F1 hybrids that show different levels of heterosis at maturity. We identify several phases of heterosis beginning during embryogenesis and culminating in a final phase of vegetative maturity and seed production. During each phase, the hybrids show different levels and patterns of growth, despite the close relatedness of the parents. For instance, during the vegetative phases, the hybrids develop larger leaves than the parents to varied extents, and they do so by exploiting increases in cell size and cell numbers in different ratios. Consistent with this finding, we observed changes in the expression of genes known to regulate leaf size in developing rosettes of the hybrids, with the patterns of altered expression differing between combinations. The data show that heterosis is dependent on changes in development throughout the growth cycle of the hybrid, with the traits of mature vegetative biomass and reproductive yield as cumulative outcomes of heterosis at different levels, tissues, and times of development.
Heterosis or hybrid vigor occurs in both plants and animals. In plants, F1 hybrid offspring exhibit enhanced yield compared with the parents and are extensively used in agriculture across a variety of crop species (for review, see Schnable and Springer, 2013). Heterosis is generated through interactions between the two parental genomes and epigenomes in the nucleus of the hybrid. The advantage of hybrid vigor is confined to the F1, with the level of phenotypic uniformity and heterosis reduced in the F2 and subsequent generations.
Despite the obvious benefits of heterosis for crop production, the molecular mechanisms underlying F1 hybrid vigor and its subsequent decay remain elusive. Hybrid vigor is suggested to be dependent on heterozygosity between the parents, which is generally thought of as variation in gene sequences with increased heterozygosity correlated with a greater level of heterosis (for review, see Birchler et al., 2010). However, variation between the parent epigenome also seems to be a contributing factor. Changes to small interfering RNA populations and DNA methylation patterns have been reported in various hybrid systems and contribute to altering the transcriptome of the F1 hybrid (for review, see Chen, 2013; Groszmann et al., 2013). The remodeling processes occur predominantly at regions that differ in their epigenetic states between the parents (Groszmann et al., 2011b; for review, see Groszmann et al., 2011a; Greaves et al., 2012). The epigenome diverges at a much faster rate than the genetic sequence (Schmitz et al., 2011), which could be especially important for heterosis in intraspecific hybrids, where genetic variation may not be extensive.
Although instances of single-gene heterosis have been reported, in most cases, quantitative trait loci provide evidence that heterosis is a polygenic trait (for review, see Schnable and Springer, 2013). Dominance, overdominance, epistasis, gene dosage, and metabolic synthesis models are suggested modes of allelic interaction explaining the generation of heterosis in the F1 hybrid (for review, see Goff, 2011; Baranwal et al., 2012; Kaeppler, 2012; Schnable and Springer, 2013).
The levels of growth and heterosis attained by a given hybrid combination depend on the interactions of the specific alleles and epialleles contributed by the parents. This is consistent with hybrid vigor being the cumulative outcome of different levels of heterosis in different tissues at different times of development (for review, see Schnable and Springer, 2013). Adding to the complexity is that increases in some traits, such as seed yield, are likely to be the result of heterotic changes earlier in the developmental cycle. With the advent of large-scale analytical methods to document the genome, epigenome, proteome, and metabolome, new opportunities have arisen to uncover the mechanisms generating heterosis. It is now feasible to carry out these various omic studies on many different tissue and cell types at different stages of development across a variety of hybrids.
Whole-genome transcriptome studies of hybrids in Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), maize (Zea mays), and wheat (Triticum aestivum) have been conducted (for review, see Baranwal et al., 2012; Chen, 2013; Schnable and Springer, 2013), and although interpretations of these data are complex, indications are that hybrids have some combination of changes to energy production, metabolism, stress response, senescence, and hormone signaling.
In this study, we characterize a number of intraspecific Arabidopsis hybrids throughout their lifecycle. Despite the close relatedness of these Arabidopsis accessions, the parental combinations produce hybrids with different patterns of heterosis for vegetative biomass and seed yield. In some combinations, hybrid vigor was first apparent during embryo development, indicating an early morphological genesis for heterosis. After germination, all hybrids exhibit different patterns of rosette growth as well as morphological differences of the rosette leaves and changes in the architecture of the reproductive structures that affect fruit and seed yields. Consistent with their differences in leaf development, the hybrids were found to have altered expression levels of genes regulating leaf size through modulating cell size and cell number, with the expression profiles differing between hybrids. The results show that, even between closely related hybrids, heterosis for biomass and yield can be achieved through multiple processes, some of which are common to all hybrids and others that are specific to a hybrid combination.
RESULTS
Hybrids with Different Levels of Heterosis Have Different Patterns of Vegetative Growth
Heterosis is assessed by comparing the trait value of the F1 hybrid with the performance of the parents. Agronomically important traits, such as vegetative biomass or seed yield, generally need to exceed the better parent value (BPV) for the hybrid to be considered commercially beneficial. These mature traits are comprised of smaller component traits that may only exceed the average performance of the two parents (midparent value [MPV]) but collectively result in BPV for important traits. Therefore, the performance of a hybrid is often best evaluated using comparisons with both BPV and MPV. Traits exceeding MPV and BPV are categorized as exhibiting midparent heterosis (MPH) and better parent heterosis (BPH), respectively.
The Arabidopsis accessions C24, Landsberg erecta (Ler), and Columbia (Col) show phenotypic differences in vegetative growth and reproductive yields. Crosses between these accessions produce F1 hybrid combinations that show heterosis at some point(s) during vegetative development and in traits associated with reproductive yield. The reciprocal combinations of each hybrid mostly show similar patterns of development and when appropriate, were considered jointly in the assessment of the hybrids. The C24/Ler, C24/Col, and Col/Ler hybrids (slash denotes both reciprocal combinations) show differences in growth vigor compared with the parents at various time points throughout vegetative development (Fig. 1, A and B; Supplemental Data Set S1). We recognized three phases of heterosis during vegetative development based on differences in the heterotic growth patterns among the hybrids (Fig. 1B). These vegetative phases are influenced by a preceding embryogenic phase of heterosis and affect the final phase of vegetative maturity and seed production.
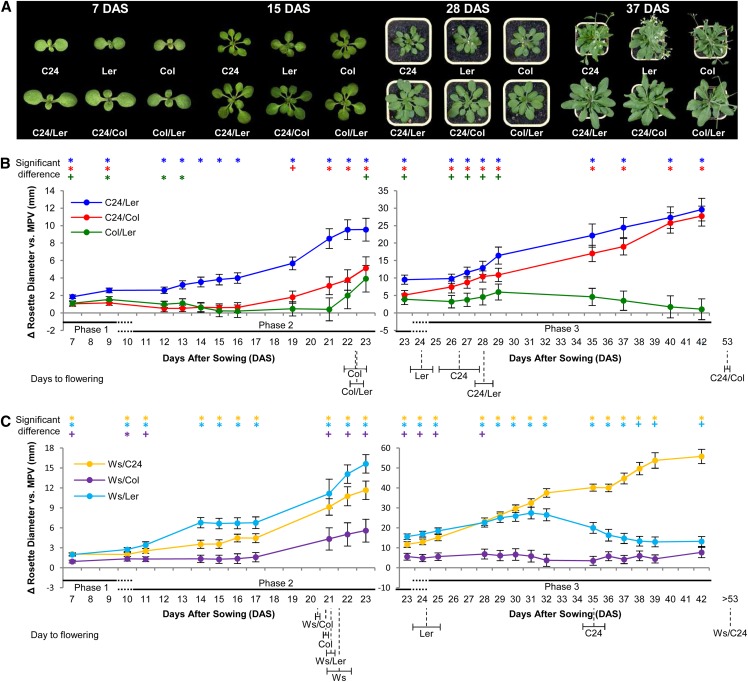
Figure 1.
Hybrids show differences in their vegetative growth patterns and levels of heterosis. A, Representative hybrid plants showing increased vegetative growth compared with the parental lines at different time points in development. B and C, Levels of vegetative heterosis represented as the difference in rosette diameter between the hybrid and MPV. Error bars are sem. n = 15 to 28 (Supplemental Table S11). Significant deviations of hybrid values from MPV (+), or exceeding BPV (*; Student's t-test P < 0.05) located along the top of the graphs. The three vegetative phases of heterosis are marked below x axes. Flowering time is indicated below x axes labels; horizontal error bars are sem. C24 flowering time was variable between sowings but on average, flowered at the same time as the C24/Ler hybrid (Supplemental Table S1).
The first vegetative phase of heterosis occurs in the period from germination to approximately 7 to 9 d after sowing (DAS; phase 1), at which point the cotyledons are the largest aerial organ. The C24 and Col parents have an equivalent diameter across both cotyledons of the seedlings, with Ler being smaller at this early stage (Supplemental Data Set S1). All hybrids had 16% to 32% increase over MPV in seedling diameter (Fig. 1B; Supplemental Data Set S1). The C24/Ler and C24/Col hybrids produce seedlings that are 11% to 12% larger than the better parent, whereas Col/Ler hybrids are slightly smaller and similar to BPV (Supplemental Data Set S1). The early processes of germination, marked by primary root emergence through to splaying of the cotyledons, occur in some hybrid combinations faster than the MPV but not significantly faster than the more advanced parent (Supplemental Fig. S1). This indicates that the larger sizes of the hybrid seedlings in vegetative phase 1 result from increased postgermination growth rates and not from disparity in emergence times.
The disparity in size between the C24, Col, and Ler seedlings ends by 13 DAS, after which the parents show similar-sized rosettes for the remainder of the vegetative growth phase (Supplemental Data Set S1). The hybrids involving C24, Ler, and Col exhibit similar growth until approximately 9 DAS, after which their growth rates diverge during the second vegetative phase of heterosis, which ends at approximately 23 DAS (Fig. 1B). During this period, C24/Ler has the greatest levels of heterosis and largest rosette size of the three hybrids, being always larger than the parents (Fig. 1B; Supplemental Data Set S1). The growth rates of the C24/Col and Col/Ler hybrids were markedly less than C24/Ler until 16 DAS, at which point the C24/Col rosette size increased (Fig. 1B; Supplemental Data Set S1).
Phase three of vegetative heterosis extends from approximately 24 to approximately 42 DAS when rosette sizes of both parents and hybrids (except for C24/Col) plateau before a reduction in size as senescence of the rosette proceeds (Fig. 1B; Supplemental Data Set S1). During this phase, both the C24/Ler and C24/Col hybrids show an almost linear trend of increased rosette diameter over MPV at levels that exceed the better parent (Fig. 1B; Supplemental Data Set S1). The Col/Ler hybrid, which has the smaller maximum rosette size of the three hybrids, initially shows a steady rate of growth above MPV that then decreases to a level close to MPV (Fig. 1B; Supplemental Data Set S1).
Additional hybrids generated by crossing the Wassilewskija (Ws) accession with the other three accessions showed different patterns of heterosis during their lifecycle (Fig. 1C; Supplemental Data Set S1). The Ws/Ler hybrid was unique with rapid growth exceeding both parents until midway into vegetative phase 3 (approximately 31 DAS), when it drops off. The Ws/Col hybrid does not show much hybrid vigor and has a growth pattern comparable with that of Col/Ler (Fig. 1C; Supplemental Data Set S1). The Ws/C24 rosette produces more vegetative growth than the other hybrids, with a strong linear growth pattern resembling that of the C24/Ler hybrid (Fig. 1C; Supplemental Data Set S1).
Because rosette size is positively correlated with flowering time in Arabidopsis, changes to flowering time in the hybrids could contribute to the increased growth of the rosettes, especially during phase 3. The late flowering phenotypes of the C24/Col and Ws/C24 hybrids are a consequence of their genotypes at the FLOWERING LOCUS C (FLC) and FRIGIDA loci (Sheldon et al., 2000). Consistent with a delayed transition to flowering, C24/Col shows elevated mRNA levels of the flowering time repressor FLC (Supplemental Fig. S2A). FLC levels are undetectable in the earlier flowering Col/Ler hybrid and at MPV for C24/Ler (Supplemental Fig. S2A). Differences in flowering times probably contribute to the increased rosette sizes of C24/Col and Ws/C24; however, other factors must be operating, because at 42 DAS, the Ws/C24 hybrid is larger than the C24/Col hybrid, but neither initiates flowering before 53 DAS (Fig. 1, B and C; Supplemental Data Set S1).
The timing of the differentiation of successive leaves from the apical meristem is similar in parents and hybrids (Supplemental Table S2), indicating that the larger rosettes of the hybrid result from differences in growth processes of the lamina and petiole of each leaf and are not a result of accelerated leaf initiation timing. In addition, the larger leaves of the hybrids have a more rapid rate of growth as opposed to a prolonged duration of growth compared with the parents (Supplemental Fig. S2B).
Hybrids Have Increased Photosynthetic Cell Size and/or Cell Number
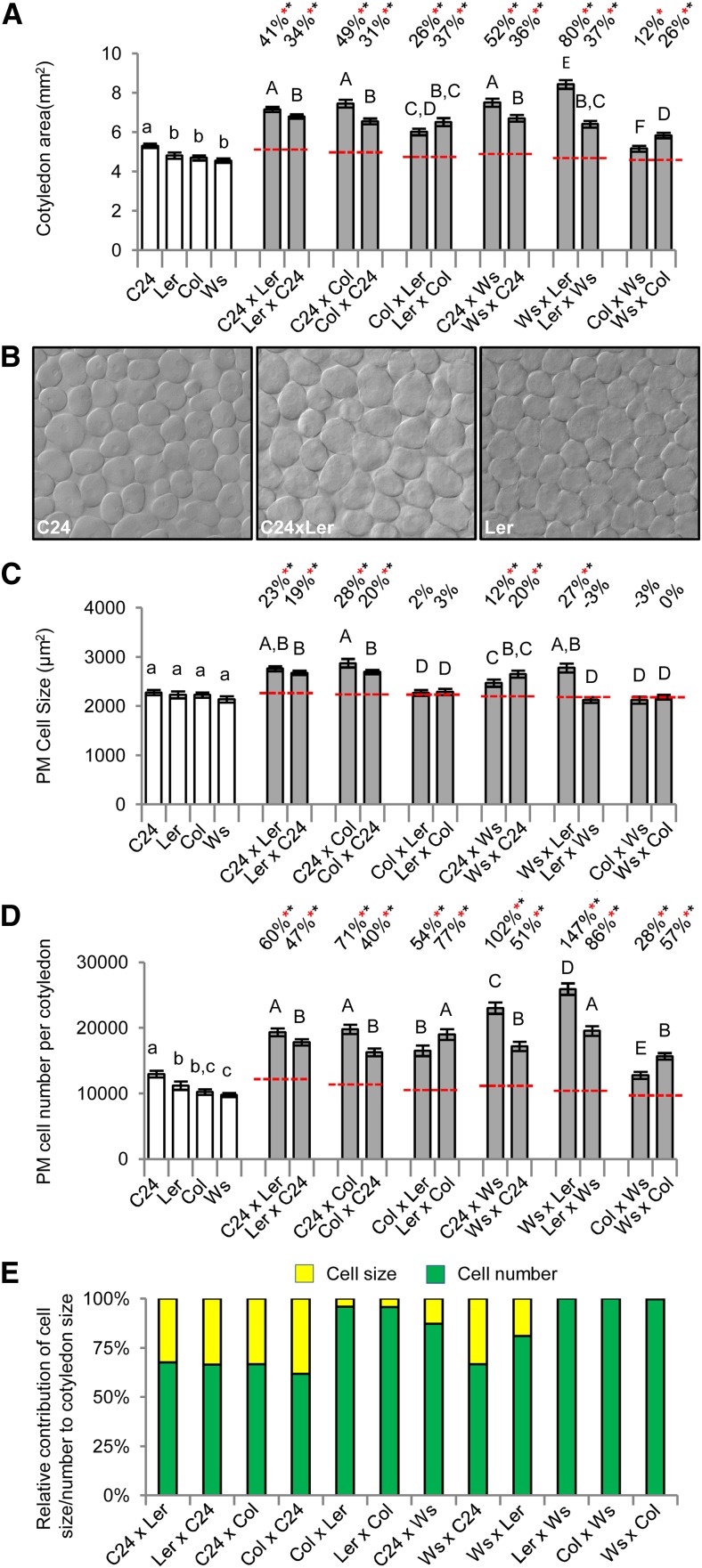
The larger rosette sizes of the hybrids indicate that leaf growth is greater in the hybrids than in the parents. This increased leaf size must result from changes in the size of the lamina and/or length of the petiole caused by increases in cell size, cell number, or both. The first phase of vegetative heterosis begins with an increased diameter across the cotyledons. At 7 DAS, the hybrid and parent seedlings are developmentally similar, with well-formed cotyledons and two emerging initial leaves that are already larger in the hybrids than in the parents. All hybrids have an increased whole-seedling biomass at 7 DAS, with BPH levels ranging from 23% (ColxWs) to 77% (WsxLer; Supplemental Table S3). The cotyledons are larger in area (Fig. 2A) and generally thicker (Supplemental Table S3) in the hybrids compared with the parents. The anatomical organization of the cotyledon, consisting of epidermal layers bounding the single palisade mesophyll (PM) layer and cells of the spongy mesophyll, remains unchanged in the hybrids. Cotyledon size differs among the hybrids, with WsxLer producing the largest cotyledons (BPH of 75%), and the C24 maternally derived hybrids (C24xLer, C24xCol, and C24xWs) also have large cotyledon areas (BPH of 35%–41%; Fig. 2A). The smallest cotyledons of the hybrids were produced by ColxWs, and they were the only combination not to show BPH with a 12% increase over MPV (Fig. 2A). The contributions of cell number and cell size to the increased area of the hybrid cotyledons were assessed by scoring paradermal optical sections of cleared cotyledons (see “Materials and Methods”; Fig. 2B). The photosynthetic cells of the PM are increased in area by 8% to 26% over BPV in the hybrids having C24 as a parent and the WsxLer hybrid (Fig. 2C), whereas the estimated number of PM cells per cotyledon was greater than in the parents in all hybrids, ranging from 25% to 132% BPH (Fig. 2D). These data show that the larger cotyledons of the hybrids are caused by an increase in cell number and in some cases, cell size, with the extent and ratios of these two properties differing among the hybrids (Fig. 2, C–E).
Figure 2.
Measurements of heterosis in cotyledons of 7-DAS hybrid seedlings. A, Cotyledon area in most hybrids exceeds BPV. B, Paradermal optical sections of the PM layer showing the larger cells of the C24xLer hybrid. C and D, Measurements of PM cell size and estimated cell number in the cotyledons. E, Relative contribution of the increases in cell size and cell number to the larger cotyledons of the hybrid. Percentages above bars are levels from MPV (red dashed line). Red asterisks indicate significant differences from MPV, and black asterisks indicate above BPV (Student's t test P < 0.01). Statistical comparisons among the hybrids (uppercase letters) and among the parents (lowercase letters) are shown, and categories with different letters are statistically different from the others (Student's t test P < 0.01). All error bars are sem. n = 40 to 136 (Supplemental Table S11).
During phase 2 of vegetative heterosis, rosette growth of the hybrids diverged, indicating differences in leaf growth. These differences were prominent at 15 DAS, when the two largest leaves on both parents and hybrids are the initial two leaves. Previous studies of the Col parental accession show that growth of the Arabidopsis leaf occurs initially through cell division followed by a phase of cell expansion (Donnelly et al., 1999; Beemster et al., 2005). Cell division rates of the first two leaves become minimal and localized to the most proximal region of the leaf blade at 12 DAS, at which point leaf growth occurs through cell expansion, with growth plateauing at approximately 21 DAS. Plant growth under our conditions was similar to the above studies, with leaves 1 and 2 first apparent at 6 DAS and leaf length plateauing at approximately 23 DAS for both parents and hybrids (Supplemental Fig. S2); at 15 DAS, the first two leaves, which are at >70% of their eventual length, are well into the cell expansion phase, with little to no cell division occurring.
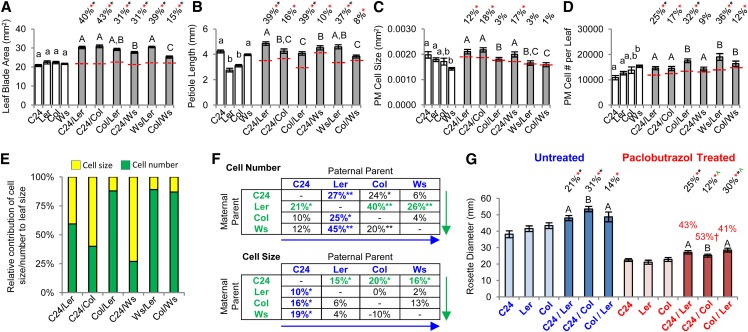
A comparison of the larger of the two leaves shows that all hybrids exhibit a greater leaf blade area than the parents through increases in both length and width (Fig. 3A; Supplemental Table S4), indicating that the hybrids have a significantly greater photosynthetic area than their parents. Most hybrids retain a leaf shape similar to that of the parents, except for Ws/Ler and Ws/Col, in which the ratio of leaf length and width deviates from the MPV (Supplemental Table S4). The younger leaves of the 15-DAS rosette are also larger and result in an aerial fresh weight for the hybrids greater than BPV for all except Col/Ws (Supplemental Table S5). Among the hybrids, the leaf blades were most separated in C24/Ler and Ws/Ler because of their longer petioles (Fig. 3B), probably increasing the exposed photosynthetic area of the rosette and contributing to these hybrids having the greatest rosette diameters at this stage. The Col/Ler and Ws/Col have the shortest petioles and subsequently, a more compact rosette (Fig. 3B). The PM cells in the leaf blade of the 15-DAS hybrids show increases in size and/or number compared with the parents, with the relative ratio differing between hybrids (Fig. 3, C–E). Marked increases in cell size occur only in hybrids where C24 is a parent (Fig. 3, C–F), whereas significant cell number increases occur in all hybrids except C24/Ws (Fig. 3D), with levels the greatest and exceeding BPV when Ler is a parent (Fig. 3F). These results suggest that the C24 parent provides alleles responsible for increasing cell size, whereas Ler alleles promote the greater increases in cell proliferation in the leaf lamina.
Figure 3.
Measurements of heterosis in the largest leaf from 15-DAS hybrid seedlings. A, Leaf blade area. B, Petiole length. C and D, PM cell size and estimated cell number in the leaf blade. Percentages above bars are levels from MPV (red dashed line). Red asterisks indicate significant differences from MPV, and black asterisks indicate above BPV (Student's t test P < 0.05). Statistical comparisons among the hybrids (uppercase letters) and among the parents (lowercase letters) are shown, and categories with different letters are statistically different from the others (Student's t test P < 0.05). E, Relative contribution of the increases in cell size and cell number to the larger leaves of the hybrid. F, Levels of cell size and cell number heterosis generated by different parental combinations. Values represent MPH. Green arrows, Evaluating maternal parent options for each paternal parent with the best performing maternal parent in each case highlighted in green. Blue arrows, Evaluating paternal parent options for each maternal parent with the best performing paternal parent in each case highlighted in blue. *, Different from MPV. **, Above BPV (Student's t test P < 0.05). G, Paclobutrazol treatment to reduce cell expansion. Reductions in rosette diameter compared with untreated values are shown as red percentage values. †, Significantly greater decrease in C24/Col compared with the other two hybrids (Student's t test P < 0.01). Black percentage values above columns indicate the level of heterosis in respective treatments. Red asterisks indicate significant differences from MPV; black asterisks indicate above BPV (Student's t test P < 0.01). Green carets indicate significant change in the level of heterosis between paclobutrazol treatment and untreated control. All error bars are sem. n = 16 to 26 in A to F, and n = 10 to 20 in G (Supplemental Table S11).
With the exception of cell size in WsxLer and cell number in C24xWs, the changes in cell number and cell size in the leaf blade of the hybrids resemble those occurring in the cotyledons. Increases in cell size contribute more to a larger mature cotyledon or leaf than an equivalent increase in the number of PM cells (see “Materials and Methods”). This factor was taken into account (see “Materials and Methods”) in calculating the relative importance of increased cell size and cell number to the enhanced cotyledon and leaf size of the hybrids (Figs. 2E and 3E). We inhibited cell expansion and expected that the reduction in leaf/rosette growth would differ between the hybrids depending on their different proportions of cell size and cell number increases. Hybrid plants were treated with paclobutrazol, which inhibits the biosynthesis of gibberellin, a hormone that promotes growth predominantly through cell expansion (Jiang et al., 2012). Paclobutrazol treatment caused reductions in rosette diameter in both the parents and hybrids (Fig. 3G). Of three hybrids, paclobutrazol treatment most affected C24/Col, which showed the greatest reduction (53%) in rosette diameter, making it the smallest of three hybrids in the treated group and decreasing its level of heterosis (31%–12%), consistent with it being the most dependent on cell size for its increased leaf growth (Fig. 3G). The C24/Ler and Col/Ler hybrids were less impacted by a reduction in cell size, with Col/Ler showing an increase in heterosis levels (14%–30%) to above BPV (Fig. 3G), which is consistent with it being the least dependent on cell expansion for its greater leaf growth. These data support the cellular morphological profiles of the hybrids that show that the hybrids share some common components to their heterotic growth (i.e. increased cell numbers) but that there are also unique factors (i.e. increased cell size) associated with some hybrids.
Genes That Control Leaf Size Show Altered Expression in 15-DAS Hybrid Seedlings
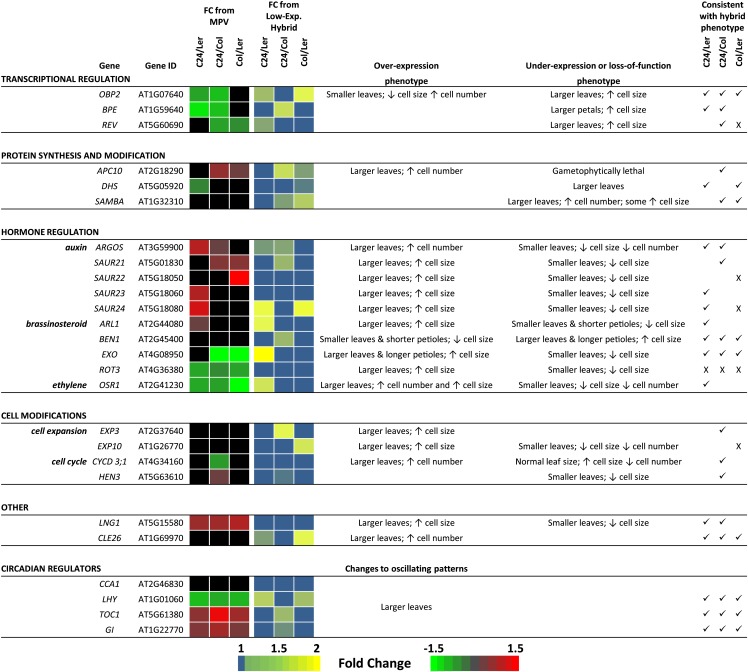
The increased rosette leaf size of the hybrids and the causal changes in cell size and cell number must be generated through alterations to the hybrid transcriptome. Hundreds to thousands of genes are differentially expressed in F1 Arabidopsis hybrids (Fujimoto et al., 2012; Meyer et al., 2012; Shen et al., 2012), many of which may have no direct impact on generating the increased vigor of the F1 plants. In this study, we focused on genes that have been identified as increasing leaf size when mutated or ectopically expressed together with a set of circadian clock genes that have been implicated in heterosis (Gonzalez et al., 2009; Ni et al., 2009; Breuninger and Lenhard, 2010); 71 genes associated with a broad range of functions, including transcriptional regulation, hormonal regulation, and cell modifications, were examined for changes in expression in the F1 hybrids (Supplemental Table S6).
Expression values for each gene were obtained from an mRNA-sequencing data set derived from aerial tissue of 15-DAS seedlings from C24, Ler, Col, and their reciprocal hybrid combinations (see “Materials and Methods”). Genes of interest were those with expression levels in the hybrid that deviated from MPV and/or those that were different between the hybrids, because either pattern of differential expression may contribute toward the different growth patterns of the hybrids. Of 71 genes, 26 genes fit these criteria (Fig. 4), with some of the genes expressed at levels different to MPV in all three hybrids, whereas others are different in only one or two of the hybrid combinations. For most of 26 genes, their reported effect on cell size or cell number and the required change in expression needed to obtain a larger leaf fit the altered expression and changes in cell size and cell number seen among the hybrids (Fig. 4). For instance, plants mutant for OBP2, a transcription factor that regulates indole glucosinolate biosynthesis, produce larger leaves through increased cell size (Skirycz et al., 2006). This gene is down-regulated compared with MPV in C24/Ler and C24/Col, the two hybrids that show enhanced cell size, whereas expression levels are at MPV and the highest level in the Col/Ler hybrid in which cell size is not increased (Fig. 4). In only five cases does the gene expression in the hybrids not fit the expected relationship between the known function of the gene and cellular morphological changes occurring in the hybrids (Fig. 4).
Figure 4.
Comparison of mRNA levels of 26 genes involved in determining leaf size through modulating cell size and cell number between C24, Ler, and Col and their hybrid offspring. Listed are the broad functional categories of the genes and their phenotypic effect on leaf organ development when their expression levels are changed. To the right are evaluations between the reported effects of these genes and consistencies (✓) or inconsistencies (X) with the hybrids. Full gene names and references to their reported functions are in Supplemental Table S6. Black shading indicates that the mRNA abundance is not significantly different between the given categories. FC, Fold change.
The 26 genes range widely in their placement within the regulatory cascade, modulating cell size and cell number. At the lower levels of the hierarchy are genes such as CYCLIN D3;1 and HUA ENHANCER3, which are core members of the cell cycle and directly regulate cell proliferation; the expression of these two genes deviates from MPV in only the C24/Col hybrid, being down-regulated and up-regulated, respectively. The function of these genes and pattern of differential expression are consistent with the C24/Col hybrid relying less on cell proliferation and more on cell expansion for achieving its larger leaf size (Fig. 4). Quantitative reverse transcription (qRT) -PCR analysis of three additional cyclins (A2;3, B1;1, and D3;2) shows similar below-MPV expression patterns in the C24/Col hybrid (Supplemental Fig. S3). Expression levels of these genes are lowest in C24/Col and highest in Col/Ler (Supplemental Fig. S3), consistent with these two hybrids having the opposite cell size and cell number profiles for heterotic leaf growth. At the upper levels of the regulatory hierarchy are genes such as CIRCADIAN CLOCK ASSOCIATED1 (CCA1), LATE ELONGATED HYPOCOTYL1 (LHY), TIMING OF CAB EXPRESSION1 (TOC1), and GIGANTEA (GI), which as part of the circadian clock, regulate diurnal expression of approximately 30% of the transcriptome and have been implicated in heterosis (Chen, 2013). During the day-night cycles of the clock, CCA1 and LHY have an oscillating pattern opposite to TOC1 and GI. CCA1 and LHY have their lowest expression levels during the day and highest levels at night (Chen, 2013). At Zeitgeber time 8 (ZT8; ZT0 = dawn), CCA1 is at MPV, whereas the daytime-decreasing expression of LHY is below MPV in all hybrids with levels reduced to low parent in C24/Ler and Col/Ler and below low parent in C24/Col (Fig. 4; Supplemental Fig. S4); also, the daytime-increasing expression of TOC1 and GI at ZT8 is above MPV, at high parent in C24/Ler and Col/Ler, and above high parent in C24/Col (Fig. 4; Supplemental Fig. S4). The expression levels of these clock genes differ between the hybrids, with the lowest levels of LHY expression and highest levels of TOC1 and GI at ZT8 all occurring in C24/Col (Fig. 4; Supplemental Fig. S4). Differences in the expression levels of these clock genes from the parents and between hybrids are also observed at an earlier point in the day (ZT6) and an evening time point (ZT15; Supplemental Fig. S4). Together, these data suggest that the oscillating patterns of these clock genes may differ between the parents and hybrids and also, between the hybrids. For example, C24/Ler compared with C24/Col and Col/Ler expresses CCA1 at higher levels at ZT6, similar levels at ZT8, and then, higher levels at ZT15 (Supplemental Fig. S4). This suggests that C24/Ler has a delayed daytime repression but a more rapid evening increase of CCA1 compared with the two other hybrids. C24/Ler also shows a delay in both the daytime increase of TOC1 at ZT6 and its evening suppression at ZT15 compared with C24/Col and Col/Ler (Supplemental Fig. S4).
Some of the differential expression of genes is unique to one hybrid. This suggests that the hybrids may achieve their increases in cell size and/or cell number through different pathways or differences in the levels of change in a common pathway. For example, expression of AUXIN-REGULATED GENE INVOLVED IN ORGAN SIZE-LIKE (ARL) is different only in the C24/Ler hybrid, where it is up-regulated compared with MPV and expressed at levels greater than in C24/Col and Col/Ler (Fig. 4; Supplemental Fig. S3). ARL regulates petiole length and leaf blade size as part of the brassinosteroid pathway (Hu et al., 2006) along with EXORDIUM and BRI1-5 ENHANCED1, which are expressed at higher and lower levels, respectively, in C24/Ler compared with the other two hybrids (Fig. 4). Together, higher ARL, higher EXORDIUM , and lower BRI1-5 ENHANCED1 expression levels of C24/Ler are consistent with C24/Ler having the longer petioles (Figs. 3B and 4) and suggests that the larger cells of the C24/Ler hybrid may be promoted more so through this pathway. Increases in cell number among the hybrids may also be achieved through different pathways, with genes, such as AUXIN-REGULATED GENE INVOLVED IN ORGAN SIZE, up-regulated in C24/Ler and C24/Col compared with Col/Ler (Fig. 4; Supplemental Fig. S3), whereas other genes suggested to promote increased cell number, such as SAMBA and CLAVATA3/ESR 26, seem to be higher in the Col/Ler hybrid (Fig. 4).
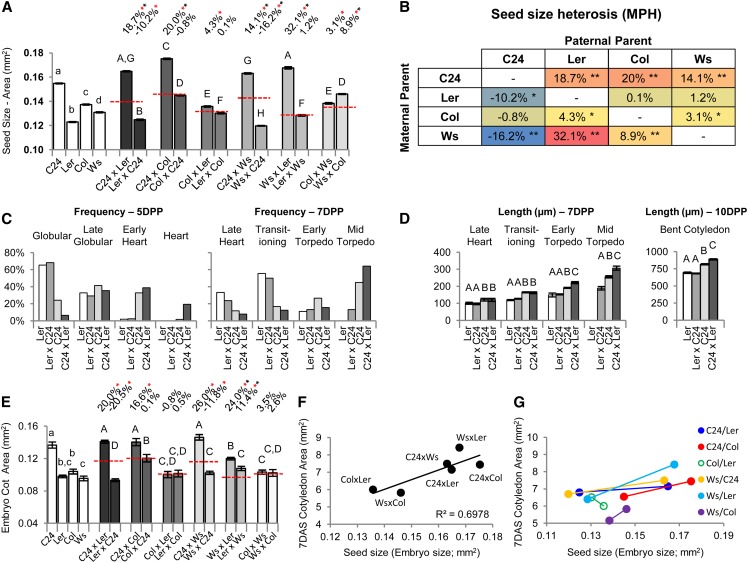
Heterosis Occurs in Embryogenesis but Varies between Reciprocal Crosses
The 7-DAS hybrid seedlings have larger cotyledons than their parents as a product of increased cell size and cell number. Because the cotyledons are an embryonic leaf structure, it is possible that these changes first occur during embryo development. Seed size was found to strongly correlate with mature embryo size for both parents and F1 hybrids (R = 0.97; Supplemental Fig. S5), and it was used as a proxy for assessing mature embryo heterosis across all of our hybrid combinations; 7 of 12 reciprocal hybrid combinations produce mature embryos that are larger than MPV, indicating that heterosis can first arise during embryogenesis (Fig. 5A). Unlike postgermination vegetative growth, where both reciprocal hybrid combinations show similar trends in their level of vigor, most hybrids differ in embryo size between the reciprocal combinations (Fig. 5A). Overall, the maternal parent is the major determinant of hybrid embryo size, with the paternal parent occasionally having an effect. C24 produces the largest embryos of the parental lines and supports BPH when present as the maternal parent (C24xLer, C24xCol, and C24xWs; Fig. 5, A and B). However, C24 has the opposite effect as the paternal donor, producing the two smallest and only hybrid embryos (LerxC24 and WsxC24) to show a decrease in size relative to the MPV (Fig. 5, A and B). Ws as the maternal parent promotes BPH, except when it is crossed with a C24 male, with WsxLer having the greatest relative increase above MPV of all the combinations (Fig. 5, A and B). Col and Ler as maternal parents have a minor influence on nonadditive embryo growth (the small LerxC24 embryos can be attributed to the C24 paternal effect; Fig. 5B), although Ler is the most restrictive maternal parent on embryo size across both the parental and hybrid lines (Fig. 5, A and B).
Figure 5.
Measurements of heterosis during the embryogenic phase. A, Seed size measurements as an indication of mature embryo heterosis. B, Levels of seed size heterosis (proxy for embryo heterosis) for the different parental combinations. Values are levels compared with MPV (Student's t test P < 0.01), shaded in a heat-map format; blue is the lowest value, and red is the highest value. *, Different from MPV. **, Above BPV or below low parent value. C, Rate of embryo development as indicated by a frequency distribution of embryos examined at 5 and 7 DPP and their developmental stage (depictions of developmental stages are in Supplemental Fig. S6A). D, Size of embryos at given developmental stages. Lettering indicates statistical comparisons between categories (Student's t test P < 0.01; 5 DPP in Supplemental Fig. S6B). E, Embryonic cotyledon area. F, Positive correlation between embryo size and cotyledon area. G, Relationship among reciprocal combinations between embryo size and cotyledon area showing that the reciprocal combination producing the larger embryo produces the larger cotyledons. Solid circles indicate significant difference in cotyledon area between reciprocal combinations (Fig. 2A). Percentages above bars are levels from MPV (red dashed line). Red asterisks indicate significant differences from MPV, and black asterisks indicate above BPV (Student's t test P < 0.01). Statistical comparisons among the hybrids (uppercase letters) and among the parents (lowercase letters) are shown (Student's t test P < 0.01). All error bars are sem. n = 201 to 1,242 in A, n = 39 to 101 in C and D, and n = 14 to 22 in E (Supplemental Table S11).
To characterize heterotic development during the embryogenic phase, the C24/Ler hybrid was examined given the large disparity in final embryo size between the reciprocal combinations (Fig. 5A). The differences between the Ler and C24 parents are evident early in embryogenesis, with C24 producing embryos with a more rapid rate of development by 5 d postpollination (DPP; χ2 P < 0.01; Fig. 5C) and becoming significantly larger than Ler by late heart stage at 7 DPP (Fig. 5D), which continues to the mature embryo. In the C24xLer hybrid, these traits are enhanced with heterosis first discernible at 5 DPP as a faster rate of development than the C24 maternal parent, which continues through 7 DPP (χ2 P < 0.01; Fig. 5C). The C24xLer hybrid embryo becomes significantly larger than C24 from early torpedo stage as the embryo enters its growth phase (Fig. 5D) and by the bent cotyledon stage, is 18% larger than MPV and 9% bigger than C24 (Fig. 5D), consistent with it maturing into a larger embryo (Fig. 5A). LerxC24 remained similar to its maternal smaller parent Ler (Fig. 5, C and D), consistent with it being undersized compared with MPV at maturity (Fig. 5A).
The growth of the embryonic cotyledons between the reciprocal combinations and between the hybrids resembles the patterns seen in cotyledons at 7 DAS (Figs. 2A and 5E). The C24xLer, C24xCol, C24xWs, and WsxLer hybrids produce the largest embryonic cotyledons (Fig. 5E; Supplemental Fig. S6, C and D). There was no obvious increase in cell size or cell number in the other hybrids (Supplemental Fig. S6, C and D). The smaller size of the LerxC24 and WsxC24 embryos is caused by reduced cell size (Fig. 5E; Supplemental Fig. S6, C and D). These results indicate that the alterations to cell size and cell number of the 7-DAS cotyledons begin during the embryogenic phase. However, it also indicates that the majority of the cellular morphological changes in the 7-DAS cotyledons (i.e. increased cell size in LerxC24, ColxC24, and WsxC24 and greater cell numbers in all hybrids) must be generated during the postgermination period. The extent of cell expansion and cell proliferation involved in postgermination cotyledon growth was estimated by comparing the embryogenic cotyledon and 7-DAS cotyledon data (Supplemental Table S7). The levels of postgermination cell expansion were increased above parental levels only in hybrids having C24 as a parent and WsxLer (Supplemental Table S7), with the greatest levels occurring in the LerxC24 and WsxC24 hybrids, the two combinations with the below MPV-sized embryos. The levels of cell proliferation were greater than parental levels in all hybrids (Supplemental Table S7).
More cells and/or increased cell size in the hybrid embryonic cotyledons suggest a greater potential for vegetative growth, which is realized through postgermination cell division and expansion. This seems to be true among the hybrids, with mature embryo size (of the larger of two reciprocal combinations) highly correlated with cotyledon area at 7 DAS (R = 0.84; Fig. 5F) and within reciprocal hybrids, where the genetic backgrounds are identical but the reciprocal combination with the larger embryo produces the bigger cotyledons by 7 DAS (Fig. 5G). Differences between reciprocal combinations are discernible as late as approximately 25 DAS in the C24/Ler and Ws/C24 hybrids (Supplemental Data Set S1), which have the largest differences in embryo size between reciprocal combinations. This suggests that embryo heterosis may have positive effects late into rosette development.
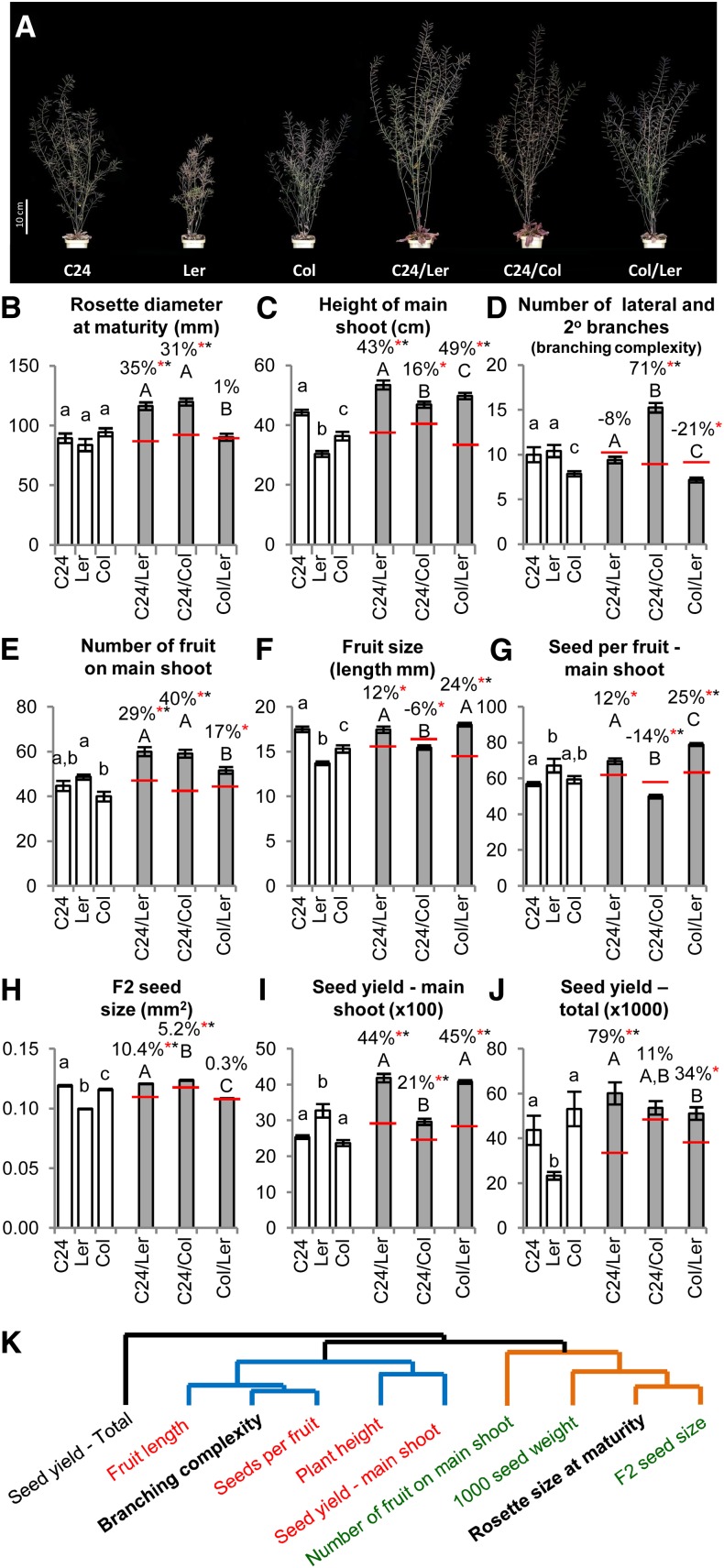
Shoot Architecture Influences the Level of Fruit and Seed Yield in the Hybrids
The rosette produces and mobilizes resources for the development of the reproductive shoot structure (Fig. 6A). At the time of flowering and at their maximum size, the rosettes of C24/Ler and C24/Col are larger than BPV, whereas the smaller Col/Ler rosette is above MPV and at MPV, respectively (Figs. 1B and 6B; Supplemental Data Set S1). At the termination of the main shoot, the three hybrids have larger shoot structures than their parental lines and differ in their architecture (Fig. 6, A–D; Supplemental Table S8). Each hybrid has a main shoot taller than MPV (Fig. 6C), with the height exceeding BPV in C24/Ler and Col/Ler. The C24/Col hybrid develops a complex branched architecture (i.e. a greater number of lateral and secondary branches), significantly increasing the size of its shoot structure over the other two hybrids (Fig. 6D). The Col/Ler hybrid has branching equivalent to that of the low parent, resulting in the least complex shoot structure of the three hybrids (Fig. 6D). Branching in the C24/Ler hybrid is at MPV and intermediate to C24/Col and Col/Ler (Fig. 6D).
Figure 6.
Measurement of reproductive architecture and yield of mature hybrid plants. A, Examples of mature plants of C24, Ler, and Col and their hybrid offspring. B to D, The three key structural traits of the mature plant. E to J, Various yield traits. Percentages above bars are levels from MPV (red dashed line). Red asterisks indicate significant differences from MPV, and black asterisks indicate above BPV (Student's t test P < 0.05). Statistical comparisons among the hybrids (uppercase letters) and among the parents (lowercase letters) are shown (Student's t test P < 0.01). K, A dendogram depicting the relationship between the major source component (rosette size) and the major sink component (branching complexity) on various yield traits in the hybrids. There are two major clades identified in blue and orange. Traits in green are positively correlated with rosette size at maturity. Traits in red are negatively correlated with increasing number of branches. Total seed production sits as an outgroup in this relationship of traits, consistent with it being the cumulative outcome of the other traits. All error bars are sem. n = 15 to 28 in B to E and I, n = 40 to 105 in F and G, n = 337 to 6,926 in H, and n = 5 to 6 in J (Supplemental Table S11).
Because reproductive shoot development is largely fed by nutrients remobilized from the rosette (Bennett et al., 2012), it is reasonable to assume that the larger the vegetative biomass, the greater the growth and output of the reproductive structure. Correlations between the three key structural traits of (1) mature rosette size, (2) main shoot height, and (3) branching complexity reveal that mature rosette size does not correlate with plant height in the hybrids (Supplemental Table S9); however, mature rosette size does positively correlate with branch number (r = 0.76), whereas branch number negatively correlates with plant height (r = −0.69; Supplemental Table S9). These patterns imply that, on a gross structural level, the larger rosette of the hybrids can support a greater reproductive structure but that resource sinks in the form of branches affect resource allocation for main shoot development.
Because resource levels and distribution are also important for yield, the association between rosette size (resource), branch numbers (sinks), and a number of yield traits was examined in the hybrids (Fig. 6, E–J; Supplemental Tables S8 and S9). The large rosette size and intermediate branching complexity of the C24/Ler hybrid result in the highest resource to sink ratio of the three hybrids. Consistently, the C24/Ler hybrid is the best yielding hybrid with improved traits, including BPH for number of fruit on the main shoot (23%), F2 seed size (1.3%), seed yield from the main shoot (28%), and total seed yield (38%). C24/Ler also shows BPV for fruit size and seeds per fruit (Fig. 6, E–J; Supplemental Table S8).
Significant differences in yield were observed between the C24/Col and Col/Ler hybrids, which exhibited the extremes of rosette size and branching complexity. Despite having, by far, the smallest mature rosette size, the reduced branching of the Col/Ler hybrid improves its resource to sink ratio, which is consistent with it showing MPH for number of fruit on the main shoot and BPH for seeds per fruit (17%), fruit size (17%), and seed yield (25%) from the main shoot (Fig. 6, E–G and I; Supplemental Table S8). However, the Col/Ler still only ranks as the second best yielding hybrid of the three hybrids, with seed size only at MPV and total seed production not as great as in the C24/Ler hybrid (Fig. 6, H and J; Supplemental Table S8).
The increased resource drain of the larger number of branches supported by the C24/Col rosette correlates with significant reductions in seeds per fruit and fruit size to levels below low parent and MPV, respectively (Fig. 6, F and G). A BPH increase in fruit number (32%) enabled the C24/Col hybrid to yield a modest BPH increase in seed production from the main shoot (21%) and MPV for total production, with seed size slightly larger than BPV (Fig. 6, E, H, and I). However, C24/Col produced the lowest yields compared with MPV of the three hybrids, despite having an equivalently large rosette to that of C24/Ler and presumably greater amounts of available photosynthate compared with Col/Ler. Overall, the correlations of the various yield traits cluster into two main groups (Fig. 6K). Seed size and number of fruit on the main shoot positively associate with mature rosette size, whereas the resource drain of increased branch numbers negatively impacts fruit size, seeds per fruit, and seed yield from the main shoot. Total seed production sits as an outgroup in the relationship of these traits, consistent with it being the cumulative outcome of the other traits (Fig. 6K).
DISCUSSION
The parental accessions C24, Ler, Col, and Ws have similar genomes with relatively few genetic differences but more pronounced epigenetic variation likely associated with their different geographical origins (for review, see Cao et al., 2011; Schmitz and Ecker, 2012; Weigel, 2012; Schmitz et al., 2013). Together, these properties result in the phenotypic differences in vegetative growth and reproductive yield observed between the parental accessions. The F1 hybrids between these accessions have unique combinations of these (epi)allelic variants and show different patterns of growth in discrete phases throughout their lifecycle.
Early in seedling development, all six hybrid systems had sizeable increases in vegetative biomass exceeding the better parent. In the subsequent vegetative growth phases, only two hybrids, C24/Ler and Ws/C24, continued an almost linear trend of rosette growth greater than the better parent, with the heterotic growth in the other hybrids fluctuating during development. Two hybrids, Col/Ler and Ws/Col, despite showing early vigor, finished with a mature vegetative size similar to MPV. Our data are consistent with reports that different combinations of Arabidopsis accessions produce hybrids with different levels of mature vegetative heterosis (Barth et al., 2003; Meyer et al., 2004; Moore and Lukens, 2011). In addition, the data show that heterotic phenotypes occur at many stages in the lifecycle of the plant at levels that can vary between stages and hybrid combinations.
Delayed flowering time is considered to contribute to increasing vegetative growth (Aarssen and Clauss, 1992; Pigliucci and Schlichting, 1995; Pigliucci and Hayden, 2001). This may apply to late flowering hybrids, such as C24/Col and Ws/C24 (Moore and Lukens, 2011); however, effects seem limited to late in development, because increases in size before flowering (i.e. phases 1 and 2) are unperturbed by accelerating flowering time (Fujimoto et al., 2012). Factors other than delayed flowering time must also be contributing to late-phase vegetative heterosis, because hybrids with similar prolonged vegetative phases (e.g. C24/Col and Ws/C24) can exhibit substantial differences in size (also seen in other hybrid combinations; Barth et al., 2003).
In the hybrids, all the leaves of the rosette show differences in their overall dimensions, shape, and thickness, with the leaf blades generally larger than the parents. The larger leaves of the hybrids were associated with increases in size and/or number of the photosynthetic PM cells, with relative levels and ratios of change differing between the hybrids. Variation in the growth processes augmenting the leaf growth of the hybrids was reflected in the different expression levels of genes known to control leaf size. In general, greater leaf cell numbers are associated more frequently with heterosis than are increases in cell expansion (for review, see Birchler et al., 2010; Blum, 2013). Accordingly, all but one of the hybrids had more cells in their leaves. However, increases in cell size were found to contribute proportionally more in generating a larger leaf (or cotyledon), with this feature promoted by the C24 parent.
The greater leaf area of the hybrids provides a greater capacity for photosynthesis and energy production important for heterotic growth and yield (for review, see Blum, 2013). This feature applies to hybrids in maize, cotton (Gossypium hirsutum), and Arabidopsis (Meyer et al., 2004; Li et al., 2007; Fujimoto et al., 2012; Zeng et al., 2012). In Arabidopsis hybrids, the greater capacity for photosynthesis is more prominent under high light intensities and seems common to hybrids having C24 as a parent (Meyer et al., 2004; Fujimoto et al., 2012). This feature coincides with our observations that C24 factors promote increased expansion of the photosynthetic PM leaf cells in the hybrids. These two traits seem developmentally related in that chloroplast number per cell is positively correlated with increased cell size (Pyke and Leech, 1991; Fujimoto et al., 2012) and that artificially increasing cell size induces more chloroplast divisions (Pyke, 1997). Chloroplast-targeted genes are increased in expression in the C24/Col hybrid but not in the moderately heterotic Col/Ler hybrid that does not show increased cell size (Fujimoto et al., 2012). The increased expression of the chlorophyll gene LIGHT-HARVESTING CHLOROPHYLL A/B-BINDING is associated with larger cells (Meehan et al., 1996), and impairment of chloroplasts by Norflurazon treatment causes a reduction in cell size (Meehan et al., 1996) and loss of heterosis in C24/Col hybrids (Fujimoto et al., 2012) but not Col/Ler hybrids (Groszmann M, unpublished data).
The level of energy production required to support the greater growth of the hybrids may be provided by alterations to the circadian clock. The circadian clock regulates key processes, such as daytime photosynthesis and nighttime starch use, and its oscillating patterns vary among parental accessions (Michael et al., 2003; Dodd et al., 2005), with changes in these patterns implicated in heterosis (Ni et al., 2009). In 15-DAS hybrid seedlings, we found that key regulatory genes of the circadian clock show patterns of expression levels that differ between the hybrids and also, from the parents. Similar changes in clock gene expression have been reported in Arabidopsis Recombinant Inbred Lines, F1 hybrids, and allopolyploids (Swarup et al., 1999; Michael et al., 2003; Ni et al., 2009; Miller et al., 2012; Shen et al., 2012) and are associated with changes in photosynthetic capacity and energy production (Ni et al., 2009; Miller et al., 2012). The circadian clock is under epigenetic control (Kurihara et al., 2008; Ni et al., 2009; Shen et al., 2012), suggesting that the differential expression of these clock regulators may be a consequence of the changes to the hybrid epigenome. These clock genes are not differentially expressed in young hybrid seedlings (Fujimoto et al., 2012; Meyer et al., 2012) but are by 15 DAS (this study), and they show greater differences by 25 DAS (Ni et al., 2009; Miller et al., 2012). Progressive changes in key regulatory mechanisms may contribute to the different patterns and levels of growth vigor in the hybrids over their life history and may be a common aspect of the hybrid F1 generation. Consistently, some of the altered epigenetic states affecting gene expression in the hybrids progressively develop throughout the course of the F1 generation (Greaves et al., 2014).
Heterosis during Embryogenesis
We observed changes in growth during embryogenesis in the hybrids, resulting in some combinations developing larger mature F1 embryos. Embryo heterosis initially is seen as a faster rate of development and then, an increase in size after the embryo enters the growth phase. Differences between reciprocal hybrids revealed that parent-of-origin effects influence embryo heterosis. Such effects seem to determine levels of seed size heterosis in other Arabidopsis F1 hybrids (Alonso-Blanco et al., 1999; Meyer et al., 2004; House et al., 2010; de Jong et al., 2011). The extremes of this effect occurred with C24 and Ler, which contrary to their roles as maternal parents, repress and promote embryo heterosis, respectively, as paternal parents.
Although both parents influence F1 hybrid embryo size, the maternal genotype is the major determinant, indicating that the maternally dominant tissues (endosperm and sporophytic seed coat) and other factors, such as maternal nutrient supply, may contribute to embryo heterosis. The hybrids with increased F1 embryo size may be supported by greater nutrition supplied by the maternal parent and hybrid endosperm and possibly, additional room to grow provided by a less restrictive seed coat growth. The opposite effects may restrict the potential for increased hybrid embryo size, which was seen when Ler is a maternal parent, a potential denoted by the marked increases in growth vigor after Ler maternal hybrids germinate.
In crop species, embryo heterosis has been reported in hybrids of rice (Akita et al., 1990), fava bean (Vicia faba; Dieckmann and Link, 2010), and maize (Meyer et al., 2007; Jahnke et al., 2010). In nonhybrid crops, larger embryo and seed size are desired traits selected by breeders, because they often confer greater early seedling vigor and larger leaf area (for review, see Lopez-Castaneda et al., 1996; Richards, 2000). Consistently, the embryogenic phase of heterosis was found to have a positive effect on the postgermination growth vigor of the hybrids, including increased size of the photosynthetic cotyledons and the initial set of leaves, and potentially, farther into vegetative development.
Early Vegetative Phase of Heterosis
Shortly after germination, the young hybrid seedlings transition from heterotrophic to autotrophic growth, and it is at this time that heterotic growth intensifies. The changes to cell size and cell number that determine the mature hybrid cotyledon size are most extensive during the early postgerminative period. In Arabidopsis and other higher plants, postembryonic growth of cotyledons occurs predominantly through light-induced cell expansion, with minor contribution from cell division (Tsukaya et al., 1994; De Veylder et al., 2002; Stoynova-Bakalova et al., 2004). Surprisingly, all the hybrids showed substantial increases in postgermination cell proliferation in generating their larger cotyledons, whereas only hybrids having C24 as a parent and WsxLer had greater postgermination cell expansion.
The patterns of change in the cotyledons are similar to those in the later developing rosette leaves of the hybrid. This suggests that some of the mechanisms determining later vegetative heterosis are set up early in hybrid development. Accordingly, the increases in photosynthetic capacity, metabolic rate, and metabolite stores are already present at this early stage (Fujimoto et al., 2012; Meyer et al., 2012). They are influenced by the maternal parent (Meyer et al., 2012), which agrees with our assessment that the predominantly maternal-influenced embryogenic phase of heterosis contributes to early seedling vigor.
Yield Heterosis
Vegetative heterosis differed among the hybrids and was dependent on growth characteristics from embryogenesis right through to the mature rosette of the plant. As Arabidopsis, like other annual plants, enters into the reproductive stage, the resources accumulated in the rosette during the photosynthetic period are remobilized into the developing fruit and seeds (Bennett et al., 2012). The larger vegetative biomass was expected to result in an equivalent increase in fruit and seed yield. However, the relationship seems more complex, with increases in fruit and seed yield relying on productive resource allocation into the reproductive structure. The better yielding hybrids, like C24/Ler, will be those with the most favorable resource to sink ratio produced by having a large rosette and simple branching structure. Pruning experiments in parental lines point to similar conclusions (Bennett et al., 2012), suggesting that manipulating the shoot architecture of hybrids, especially those having greatly enlarged vegetative structures, could improve yields.
CONCLUSION
Heterosis ultimately depends on the unique interactions of alleles and epialleles provided by the parents. This accounts for different hybrids exhibiting differing levels and patterns of heterotic growth, even within the same species. Increases in biomass and yield can be achieved through different patterns of heterotic growth driven by varied complements of altered gene expression. Various omic studies on the developmental stages highlighted here may identify key biological networks and their initial triggers that generate heterosis. After they are identified, common networks and hybrid-specific exploits could be manipulated to capture aspects of heterosis in not only nonhybrid crops but also, hybrids to further enhance their vigor and yields.
MATERIALS AND METHODS
Plant Growth
Seeds were surface sterilized and sown on 150-mm diameter plates containing Gamborg’s B-5 Basal Media (G5893-10L; Sigma) balanced at pH 7.0 using KOH and supplemented with 0.6% (w/v) agar. Plates were sectioned into quarters consisting of three hybrid lines, with the maternal parent in common sown alongside the maternal parent at a density of six to eight plants per genotype. Plates were placed at 4°C for 2 d to stratify the seeds and then transferred to a growth room (0 DAS) with conditions of a 22°C/18°C day-night cycle and a 16-h light/8-h dark photoperiod under Philips Cool Daylight TLD 58 W/840 fluorescent tubes providing a photosynthetic photon flux density of 160 to 180 μmol m−2 s−1 as measured using a quantum meter (model MQ-200 calibrated for electric light source; Apogee). At 18 DAS, the seedlings were each transferred to 60-mm2 × 70-mm deep pots containing Debco Seed Raising Mix supplemented with 1 g L−1 of Osmocote Exact Mini controlled release fertilizer pellets; 20 pots were placed in each tray at a density of four genotypes × five plants per tray, with positioning and combinations of genotypes randomized across the trays. Trays were placed in a larger growth room at a 22°C/18°C day-night cycle and a 16-h light/8-h dark photoperiod at 170 to 180 μmol m−2 s−1. Clear plastic covers were placed on each tray for the first 3 d to assist in the continued healthy growth of the seedling after the transfer. The positioning of both plates and trays on the shelves was rotated and shifted daily to minimize possible positional effects on growth. Plants on soil were watered at a rate of 800 mL per tray as needed (approximately every 3–4 d).
Hybrid Crosses
Seed Stocks
Both F1 hybrid and parental control seeds were generated through hand pollination and restricting silique numbers on the mother plant as suggested by Meyer et al. (2004).
Crosses for Examining Hybrid Embryo Development at 5, 7, and 10 DPP
For each sample (i.e. genotype and DPP), six siliques from three plants (i.e. two crosses per plant) were harvested and placed in a methanol:acetic acid:water (50%:10%:40% v/v/v) fixative solution and stored at 4°C. For consistency, developing seeds were harvested from the middle two-thirds of the silique to minimize possible apical basal bias caused by differences in pollen tube growth rates through ovary to ovule between genotypes. Developing seeds were mounted and cleared in a chloral hydrate solution (4 g of chloral hydrate:2 mL of deionized water:1 mL of glycerol), and embryos were visualized using a Zeiss Axioimager upright motorized microscope mounted with an Axiocam camera using differential interference contrast optics and images captured using Axiovision software (http://microscopy.zeiss.com/). Embryo sizes were measured using ImageJ.
Expression Analysis
RNA Sequencing
Tissue for RNA extraction was harvested from aerial tissues from 15-DAS seedlings from C24, Ler, Col, and their reciprocal hybrid offspring. Two biological replicates were used for each sample, with each replicate consisting of a pool of 15 seedlings. Total RNA was extracted using the QIAGEN RNeasy Plant MiniKit with on-column DNA digestion using the QIAGEN RNAse Free DNAse Set. Libraries were prepared and sequenced by a service provider using the Illumina True-Seq Kit and sequenced on an Illumina Hi-Seq as mRNA sequencing pair-ended 100-nucleotide runs; 65 to 70 million reads were sequenced per sample and mapped (>85% of sequenced reads) to The Arabidopsis Information Resource 10 reference genome using BioKanga align (http://code.google.com/p/biokanga/) with default settings and additionally implementing parameters -A5000 and -M5. Mapped reads were allocated to genomic features (as per The Arabidopsis Information Resource 10 annotations) using BioKanga map loci on default settings. Reads per genomic feature were standardized across libraries using the normalization procedure in DESEQ (Anders and Huber, 2010; http://bioconductor.org/packages/2.13/bioc/html/DESeq.html). Negative binomial tests implementing the fitonly variance dispersion model in DESEQ were used to determine significant differences in mRNA levels between samples (Supplemental Table S6).
qRT-PCR
RNA was extracted as above, and complementary DNA was synthesized using Superscript III reverse transcriptase (Invitrogen). Real-time quantitative PCR was performed using SYBR green and Platinum Taq DNA polymerase (Invitrogen) as per the manufacturer’s instructions. Reactions were carried out in a 7900HT Fast Real-Time PCR System (Applied Biosystems). Absolute transcript quantity was obtained using standards of Arabidopsis (Arabidopsis thaliana) complementary DNA from each sample. Three biological replicates (each consisting of a pool of aerial tissue from 10 × 15 DAS seedlings) and between two and four technical replicates were performed for each sample, and expression levels were normalized against Ag4g26410 (Czechowski et al., 2005).
Rosette Diameters, Leaf Dimensions, and PM Cell Measurements
Rosette Diameters
Rosette sizes (diameters) were obtained by imaging plants from an aerial perspective using a stage mounted Nikon DX3 camera using a Nikon 60-mm lens. Rosette diameters were determined using ImageJ software (http://rsb.info.nih.gov/ij/).
Measurements of Individual Cotyledons and Leaves
Seedlings were harvested, submerged in a methanol:acetic acid:water (50%:10%:40% v/v/v) fixative solution, and stored at 4°C. Samples were transferred to 50% (v/v) ethanol, and cotyledons or leaves were dissected, run through an ethanol-water series (50%, 40%, 30%, 20%, 10%, and 0% v/v), cleared in a chloral hydrate solution (4 g of chloral hydrate:2 mL of deionized water:1 mL of glycerol), and mounted for visualization. Whole-mount images of cotyledons and leaves were taken under a Leica MZFLIII dissector microscope mounted with an Axiocam camera, and images were captured using the Axiovision software. Length, width, and area measurements were determined using ImageJ software.
PM Cell Measurements
Images of PM cells were captured using a Zeiss Axioimager upright motorized microscope using differential interference contrast optics at ×20 magnification mounted with an Axiocam camera using Axiovision software. All PM images were taken at a similar location on the cotyledons or leaves for all samples (distal from the midpoint of the leaf and either side of the midvein). Average cell size was determined by the number of cells in a 500-µm2 quadrant, and total cell numbers were estimated by extrapolating the average cell size across the area of the given cotyledon or leaf blade. Cotyledons and leaf blade thickness were measured using the vertical-stage movement readings (micrometer sensitivity) of the software-controlled Zeiss Axioimager upright motorized microscope.
Cell Size and Cell Number Contributions to Increased Leaf Organ Area over MPV
Percentage increases over MPV for cell number and increases over MPV for cotyledon (or leaf) area were plotted for hybrids that showed no increases in PM cell size (i.e. Col/Ler, Ws/Col, and LerxWs). A liner regression model was applied to these data, whereby the coefficient of x was considered the relative increase in cotyledon or leaf area caused by increases in cell numbers. The linear regression equation was used to estimate the amount of cotyledon or leaf area in the C24/Ler, C24/Col, C24/Ws, and WsxLer that was caused by increased cell number, with the remaining area allocated as being caused by increased cell size. The cell size portion of growth was plotted, and a regression model was applied to obtain an estimate of the contribution of cell size to increased area of the cotyledons or leaves. Increase in cell size over MPV contributes to increased cotyledon area 1.27× (leaf area is 1.42×) more than the equivalent increase over MPV in cell number.
Paclobutrazol Application
The experiment consisted of two replicate sowings, each consisting of five plants per treatment for both parental and hybrids lines. Plants were grown on agar plates (as described above) for 13 DAS and transferred to a hydroponic system consisting of GroWool (Horticultural Systems) placed on Osmocote Exact Mini Controlled Release Fertilizer Pellets. At the time of transfer, a single dose of 5 × 10−6 m paclobutrazol was administered to the treated group. Rosette diameter was assayed at 29 DAS (as described above).
Yield Data
All but total seed yield data were measured when the shoot apical meristem was terminated. Siliques 5 to 15 on the main shoot were used for seed number and fruit length measurements. Total seed counts per main shoot were estimated by extrapolating the average number of seeds per silique across all fruit produced on the main shoot. Silique (or fruit) lengths were measured from the distal end of the gynophore to the base of the style (i.e. ovary region) using vernier calipers (Kincrome Tools). A dendogram of the relationship between reproductive traits was generated by using hclust in R (http://www.R-project.org) and inputting the pairwise Pearson’s correlation coefficients values between traits.
Seed Size and Embryo Measurements
Seed Measurements
Groups of separated seeds were imaged using the Leica MZFLIII dissector microscope, and area measurements were taken using ImageJ.
Embryo Measurements
Mature seeds were imbibed for 1 h, and embryos were excised and mounted such that the embryos were fully spread, revealing the entire structure. Embryos were imaged using Zeiss Axioimager, and size (area) was measured using ImageJ.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Timing of developmental events associated with germination and emergence in parental and hybrid lines.
Supplemental Figure S2. Leaf elongation rates in parental and hybrid lines.
Supplemental Figure S3. qRT-PCR expression analysis.
Supplemental Figure S4. Expression of circadian regulators CCA1, LHY, TOC, and GI at three time points during the light period (ZT0 = dawn).
Supplemental Figure S5. Correlation between hybrid embryo size and seed size, indicating that seed size can be used as a noninvasive proxy measurement for assessing embryo size and heterosis.
Supplemental Figure S6. Additional measurements assessing embryo heterosis.
Supplemental Table S1. Analysis of flowering time in the C24/Ler hybrid across several independent sowings.
Supplemental Table S2. A comparison of the differentiation rate of leaves from the apical meristem in parental and hybrids lines.
Supplemental Table S3. Cotyledon thickness and whole-seedling fresh weight of 7-DAS seedlings.
Supplemental Table S4. Additional leaf blade measurements.
Supplemental Table S5. Aerial fresh weights.
Supplemental Table S6. Comparison of mRNA levels between C24, Ler, and Col and their hybrid offspring of 71 genes involved in determining leaf size through modulating cell size and cell number.
Supplemental Table S7. The extent of cell expansion and cell proliferation involved in postgermination cotyledon growth.
Supplemental Table S8. Reproductive trait measurements.
Supplemental Table S9. Pearson’s correlation coefficients values for the pairwise comparison between reproductive traits.
Supplemental Table S10. Primer sequences for qRT-PCR reactions.
Supplemental Table S11. Detailed sample sizes for figures in the text.
Supplemental Data Set S1. Rosette diameter measurements over the vegetative developmental period.
Supplemental References S1. References for functions of genes listed in Supplemental Table S6.
Acknowledgments
We thank Melanie Hand and Maria Alonso-Peral for commenting on the article; Limin Wu, Bjorg Sherman, Aihua Wang, and Carl Davies for technical assistance; and the heterosis group for stimulating discussions.
Glossary
- BPH
better parent heterosis
- BPV
better parent value
- Col
Columbia
- DAS
d after sowing
- DPP
d postpollination
- Ler
Landsberg erecta
- MPH
midparent heterosis
- MPV
midparent value
- PM
palisade mesophyll
- qRT
quantitative reverse transcription
- Ws
Wassilewskija
- ZT
Zeitgeber time
Footnotes
This work was supported by the Science and Industry Endowment Fund.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Aarssen LW, Clauss MJ. (1992) Genotypic variation in fecundity allocation in Arabidopsis-thaliana. J Ecol 80: 109–114 [Google Scholar]
- Akita S, Blanco L, Katayama K. (1990) Physiological mechanism of heterosis in seedling growth of Indica-F1 rice hybrids. Jpn J Crop Sci 59: 548–556 [Google Scholar]
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. (1999) Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc Natl Acad Sci USA 96: 4710–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranwal VK, Mikkilineni V, Zehr UB, Tyagi AK, Kapoor S. (2012) Heterosis: emerging ideas about hybrid vigour. J Exp Bot 63: 6309–6314 [DOI] [PubMed] [Google Scholar]
- Barth S, Busimi AK, Friedrich Utz H, Melchinger AE. (2003) Heterosis for biomass yield and related traits in five hybrids of Arabidopsis thaliana L. Heynh. Heredity (Edinb) 91: 36–42 [DOI] [PubMed] [Google Scholar]
- Beemster GTS, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, Galichet A, Gruissem W, Inzé D, Vuylsteke M. (2005) Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol 138: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, Roberts JA, Wagstaff C. (2012) Manipulating resource allocation in plants. J Exp Bot 63: 3391–3400 [DOI] [PubMed] [Google Scholar]
- Birchler JA, Yao H, Chudalayandi S, Vaiman D, Veitia RA. (2010) Heterosis. Plant Cell 22: 2105–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. (2013) Heterosis, stress, and the environment: a possible road map towards the general improvement of crop yield. J Exp Bot 64: 4829–4837 [DOI] [PubMed] [Google Scholar]
- Breuninger H, Lenhard M. (2010) Control of tissue and organ growth in plants. Curr Top Dev Biol 91: 185–220 [DOI] [PubMed] [Google Scholar]
- Cao J, Schneeberger K, Ossowski S, Günther T, Bender S, Fitz J, Koenig D, Lanz C, Stegle O, Lippert C, et al. (2011) Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43: 956–963 [DOI] [PubMed] [Google Scholar]
- Chen ZJ. (2013) Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet 14: 471–482 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TJ, Hermans CM, van der Veen-van Wijk KC. (2011) Paternal effects on seed mass in Arabidopsis thaliana. Plant Biol (Stuttg) (Suppl 1) 13: 71–77 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, de Almeida Engler J, Ormenese S, Maes S, Naudts M, Van Der Schueren E, Jacqmard A, Engler G, et al. (2002) Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J 21: 1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann S, Link W. (2010) Quantitative genetic analysis of embryo heterosis in faba bean (Vicia faba L.). Theor Appl Genet 120: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309: 630–633 [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Fujimoto R, Taylor JM, Shirasawa S, Peacock WJ, Dennis ES. (2012) Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc Natl Acad Sci USA 109: 7109–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SA. (2011) A unifying theory for general multigenic heterosis: energy efficiency, protein metabolism, and implications for molecular breeding. New Phytol 189: 923–937 [DOI] [PubMed] [Google Scholar]
- Gonzalez N, Beemster GTS, Inzé D. (2009) David and Goliath: what can the tiny weed Arabidopsis teach us to improve biomass production in crops? Curr Opin Plant Biol 12: 157–164 [DOI] [PubMed] [Google Scholar]
- Greaves I, Groszmann M, Dennis ES, Peacock WJ. (2012) Trans-chromosomal methylation. Epigenetics 7: 800–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves IK, Groszmann M, Wang A, Peacock WJ, Dennis ES. (2014) inheritance of trans chromosomal methylation patterns from Arabidopsis F1 hybrids. Proc Natl Acad Sci USA 111: 2017–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszmann M, Greaves IK, Albert N, Fujimoto R, Helliwell CA, Dennis ES, Peacock WJ. (2011a) Epigenetics in plants-vernalisation and hybrid vigour. Biochim Biophys Acta 1809: 427–437 [DOI] [PubMed] [Google Scholar]
- Groszmann M, Greaves IK, Albertyn ZI, Scofield GN, Peacock WJ, Dennis ES. (2011b) Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc Natl Acad Sci USA 108: 2617–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszmann M, Greaves IK, Fujimoto R, Peacock WJ, Dennis ES. (2013) The role of epigenetics in hybrid vigour. Trends Genet 29: 684–690 [DOI] [PubMed] [Google Scholar]
- House C, Roth C, Hunt J, Kover PX. (2010) Paternal effects in Arabidopsis indicate that offspring can influence their own size. Proc Biol Sci 277: 2885–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Poh HM, Chua NH. (2006) The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J 47: 1–9 [DOI] [PubMed] [Google Scholar]
- Jahnke S, Sarholz B, Thiemann A, Kühr V, Gutiérrez-Marcos JF, Geiger HH, Piepho HP, Scholten S. (2010) Heterosis in early seed development: a comparative study of F1 embryo and endosperm tissues 6 days after fertilization. Theor Appl Genet 120: 389–400 [DOI] [PubMed] [Google Scholar]
- Jiang X, Li H, Wang T, Peng C, Wang H, Wu H, Wang X. (2012) Gibberellin indirectly promotes chloroplast biogenesis as a means to maintain the chloroplast population of expanded cells. Plant J 72: 768–780 [DOI] [PubMed] [Google Scholar]
- Kaeppler S. (2012) Heterosis: many genes, many mechanisms - end the search for an undiscovered unifying theory. ISRN Botany 2012: 682824 [Google Scholar]
- Kurihara Y, Matsui A, Kawashima M, Kaminuma E, Ishida J, Morosawa T, Mochizuki Y, Kobayashi N, Toyoda T, Shinozaki K, et al. (2008) Identification of the candidate genes regulated by RNA-directed DNA methylation in Arabidopsis. Biochem Biophys Res Commun 376: 553–557 [DOI] [PubMed] [Google Scholar]
- Li X, Ding ZS, Li LL, Wang MY, Zhao M. (2007) Heterosis of photosynthetic performance of maize. Chinese J Appl Ecol 18: 1049–1054 [PubMed] [Google Scholar]
- Lopez-Castaneda C, Richards RA, Farquhar GD, Williamson RE. (1996) Seed and seedling characteristics contributing to variation in early vigor among temperate cereals. Crop Sci 36: 1257–1266 [Google Scholar]
- Meehan L, Harkins K, Chory J, Rodermel S. (1996) Lhcb transcription is coordinated with cell size and chlorophyll accumulation (studies on fluorescence-activated, cell-sorter-purified single cells from wild-type and immutans Arabidopsis thaliana). Plant Physiol 112: 953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RC, Törjék O, Becher M, Altmann T. (2004) Heterosis of biomass production in Arabidopsis: establishment during early development. Plant Physiol 134: 1813–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RC, Witucka-Wall H, Becher M, Blacha A, Boudichevskaia A, Dörmann P, Fiehn O, Friedel S, von Korff M, Lisec J, et al. (2012) Heterosis manifestation during early Arabidopsis seedling development is characterized by intermediate gene expression and enhanced metabolic activity in the hybrids. Plant J 71: 669–683 [DOI] [PubMed] [Google Scholar]
- Meyer S, Pospisil H, Scholten S. (2007) Heterosis associated gene expression in maize embryos 6 days after fertilization exhibits additive, dominant and overdominant pattern. Plant Mol Biol 63: 381–391 [DOI] [PubMed] [Google Scholar]
- Michael TP, Salomé PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, Alonso JM, Ecker JR, McClung CR. (2003) Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302: 1049–1053 [DOI] [PubMed] [Google Scholar]
- Miller M, Zhang C, Chen ZJ. (2012) Ploidy and hybridity effects on growth vigor and gene expression in Arabidopsis thaliana hybrids and their parents. G3 (Bethesda) 2: 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Lukens L. (2011) An Evaluation of Arabidopsis thaliana Hybrid Traits and Their Genetic Control. G3 (Bethesda) 1: 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Kim ED, Ha M, Lackey E, Liu J, Zhang Y, Sun Q, Chen ZJ. (2009) Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 457: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M, Hayden K. (2001) Phenotypic plasticity is the major determinant of changes in phenotypic integration in Arabidopsis. New Phytol 152: 419–430 [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Schlichting CD. (1995) Reaction norms of Arabidopsis (brassicaceae). 3. Response to nutrients in 26 populations from a worldwide collection. Am J Bot 82: 1117–1125 [Google Scholar]
- Pyke K. (1997) The genetic control of plastid division in higher plants. Am J Bot 84: 1017–1027 [PubMed] [Google Scholar]
- Pyke KA, Leech RM. (1991) Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heynh. Plant Physiol 96: 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA. (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J Exp Bot 51: 447–458 [DOI] [PubMed] [Google Scholar]
- Schmitz RJ, Ecker JR. (2012) Epigenetic and epigenomic variation in Arabidopsis thaliana. Trends Plant Sci 17: 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Schultz MD, Lewsey MG, O’Malley RC, Urich MA, Libiger O, Schork NJ, Ecker JR. (2011) Transgenerational epigenetic instability is a source of novel methylation variants. Science 334: 369–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Schultz MD, Urich MA, Nery JR, Pelizzola M, Libiger O, Alix A, McCosh RB, Chen H, Schork NJ, et al. (2013) Patterns of population epigenomic diversity. Nature 495: 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable PS, Springer NM. (2013) Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol 64: 71–88 [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Finnegan EJ, Rouse DT, Tadege M, Bagnall DJ, Helliwell CA, Peacock WJ, Dennis ES. (2000) The control of flowering by vernalization. Curr Opin Plant Biol 3: 418–422 [DOI] [PubMed] [Google Scholar]
- Shen H, He H, Li J, Chen W, Wang X, Guo L, Peng Z, He G, Zhong S, Qi Y, et al. (2012) Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell 24: 875–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J, et al. (2006) DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47: 10–24 [DOI] [PubMed] [Google Scholar]
- Stoynova-Bakalova E, Karanov E, Petrov P, Hall MA. (2004) Cell division and cell expansion in cotyledons of Arabidopsis seedlings. New Phytol 162: 471–479 [Google Scholar]
- Swarup K, Alonso-Blanco C, Lynn JR, Michaels SD, Amasino RM, Koornneef M, Millar AJ. (1999) Natural allelic variation identifies new genes in the Arabidopsis circadian system. Plant J 20: 67–77 [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Tsuge T, Uchimiya H. (1994) The cotyledon - a superior system for studies of leaf development. Planta 195: 309–312 [Google Scholar]
- Weigel D. (2012) Natural variation in Arabidopsis: from molecular genetics to ecological genomics. Plant Physiol 158: 2–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B, Xu X, Zhou S, Zhu C, Tang C. (2012) Effects of temperature and light on photosynthetic heterosis of an upland cotton hybrid cultivar. Crop Sci 52: 282–291 [Google Scholar]