The formation of arbuscules in the arbuscular mycorrhizal symbiosis is dependent on the plant hormone auxin.
Abstract
Most land plant species live in symbiosis with arbuscular mycorrhizal fungi. These fungi differentiate essential functional structures called arbuscules in root cortical cells from which mineral nutrients are released to the plant. We investigated the role of microRNA393 (miR393), an miRNA that targets several auxin receptors, in arbuscular mycorrhizal root colonization. Expression of the precursors of the miR393 was down-regulated during mycorrhization in three different plant species: Solanum lycopersicum, Medicago truncatula, and Oryza sativa. Treatment of S. lycopersicum, M. truncatula, and O. sativa roots with concentrations of synthetic auxin analogs that did not affect root development stimulated mycorrhization, particularly arbuscule formation. DR5-GUS, a reporter for auxin response, was preferentially expressed in root cells containing arbuscules. Finally, overexpression of miR393 in root tissues resulted in down-regulation of auxin receptor genes (transport inhibitor response1 and auxin-related F box) and underdeveloped arbuscules in all three plant species. These results support the conclusion that miR393 is a negative regulator of arbuscule formation by hampering auxin perception in arbuscule-containing cells.
Arbuscular mycorrhiza (AM) is a widespread symbiosis between soil fungi (Glomeromycota spp.) and most land plant species. The fungus colonizes the roots of its host plant, where it obtains carbohydrates (Bago et al., 2003). In exchange, it provides mineral nutrients to the plant, especially phosphate, that are taken up from the soil by its extraradical mycelium, thus considerably improving plant nutrition in soils of low fertility (Bago et al., 2003; Smith and Smith, 2011). After spore germination, the fungus forms a hyphopodium on the root surface, penetrates the rhizodermis through a prepenetration apparatus, colonizes the root tissue intercellularly, and eventually, forms highly ramified structures called arbuscules in cortical cells, where mineral nutrients are released to the host (Parniske, 2008; Harrison, 2012). Each branch of the arbuscule is surrounded by a plant-derived periarbuscular membrane that prevents fungal penetration of the root cell cytosol and controls nutrient and signal exchange between the symbionts. Arbuscule formation relies on drastic reorganization of the plant cell and the implementation of a plant genetic program that remains poorly known, because only few genes associated with this process have been identified so far (for review, see Delaux et al., 2013; Gutjahr and Parniske, 2013).
It is well established that plant hormones are key regulators of plant physiologic and developmental processes (Santner et al., 2009), and it has recently become apparent that some of them are also important for arbuscule development. For example, the abscisic acid-deficient mutant sitiens was impaired in arbuscule formation and viability, indicating an important function of abscisic acid in arbuscule maintenance (Herrera-Medina et al., 2007). Conversely, gibberellic acid (GA3) treatment or constitutive GA3 signaling in DELLA-deficient mutants suppresses arbuscule formation, and a reduction of GA3 signaling by stabilized DELLA proteins promotes arbuscule development (Floss et al., 2013; Foo et al., 2013). Auxin has also been suggested to play a role in AM symbiosis, although its exact role in this type of plant-microbe interaction remains elusive (Hause et al., 2007; Hanlon and Coenen, 2011). Although an increase in auxin content in mycorrhizal roots has been previously reported for Medicago truncatula, maize (Zea mays), and soybean (Glycine max; Ludwig-Müller et al., 1997; Kaldorf and Ludwig-Müller, 2000; Fitze et al., 2005; Ludwig-Müller and Güther, 2007), no change in auxin content in mycorrhizal roots of tobacco (Nicotiana tabacum) and leek (Allium porrum) has been observed (Torelli et al., 2000; Shaul-Keinan et al., 2002). Meixner et al. (2005) found that indole-3-acetic acid (IAA) levels were higher in mycorrhizal soybean roots. This increase of IAA content in mycorrhizal roots was lower in the mutant nark, which is deficient in autoregulation of nodulation, suggesting that IAA might play a role in the autoregulation of mycorrhization. Congruently, a strong decrease of AM colonization but with normal fungal structures was observed in both the auxin-resistant tomato (Solanum lycopersicum) mutant diageotropica and the auxin hypertransporting tomato mutant polycotyledon, indicating that, indeed, auxin could play a role in AM colonization (Hanlon and Coenen, 2011). It is, however, unknown whether reduced colonization was a direct consequence of the defective auxin signaling or transport or whether it was a consequence of cross talk with other hormone signaling or biosynthesis pathways (Hanlon and Coenen, 2011). Auxin signaling has also been implicated in legume root symbiosis with nitrogen-fixing rhizobia (Suzaki et al., 2012). However, disturbance of auxin signaling only had an impact on nodule development but not infection, which shares cell biological features with AM colonization (Mao et al., 2013; Turner et al., 2013).
Auxin was the first plant hormone to be described, possibly because of its paramount importance for most plant developmental processes. Among its many hormonal functions (Overvoorde et al., 2010), it is crucial to regulate cell elongation and cell and organ polarity (Lau et al., 2008; Kramer, 2009) and could potentially also exert such a role in plant cell developmental changes during symbiont accommodation. Auxin is perceived by nuclear-localized F-box domain-containing proteins (Dharmasiri et al., 2005; Kepinski and Leyser, 2005), and one important pathway linking auxin perception to gene expression is now well established. It involves the ubiquitination of Auxin (Aux)/IAA proteins by the transport inhibitor response1 (TIR1)/auxin-related F-box (AFB) proteins subunit of the Skp, Cullin, F-box containing complexTIR1/AFB ubiquitin ligase and the degradation of Aux/IAA proteins by the 26S proteasome. This degradation then releases the Aux/IAA-mediated inhibition of auxin response factors and allows these transcription factors to modulate the expression of their target genes (Hayashi, 2012). TIR1 and several AFB genes encoding auxin receptors are regulated posttranscriptionally by microRNA393 (miR393) during root development and response to pathogens (Navarro et al., 2006, 2008; Parry et al., 2009; Vidal et al., 2010).
miRNAs are small noncoding RNAs that regulate the expression of target genes having complementary sequences by cleaving their mRNAs or inhibiting their translation (Voinnet, 2009). miRNAs are involved in most biological processes, such as development, response to stresses, and interactions with microorganisms. To date, only a few miRNAs have been characterized with respect to their role in AM symbiosis. miR171h quantitatively regulates mycorrhizal root colonization by targeting the NODULATION SIGNALING PATHWAY2 (NSP2) transcription factor gene (Lauressergues et al., 2012), and miR396 regulates lateral root formation during fungal colonization (Bazin et al., 2013).
The suppression of auxin signaling by miR393 plays an important role in plant resistance to bacteria (Navarro et al., 2006). Extrapolating from it, we were interested to explore whether miR393 could also be involved in regulating AM interactions. We found that expression of miR393 was down-regulated in mycorrhizal roots, indicating that an active auxin perception might be needed during AM symbiosis. In parallel, treatment of plants with auxin analogs increased the quantity of arbuscules. Accordingly, the Direct repeat5 (DR5)-GUS promoter, which serves as an indicator of auxin response, was activated in mycorrhizal roots, specifically in arbuscule-containing cells. Overexpression of miR393 led to a down-regulation of auxin receptor expression and a concomitant strong defect in arbuscule formation. We showed this for three different plant species (tomato, M. truncatula, and rice [Oryza sativa]), indicating that auxin perception and/or auxin signaling are important for arbuscule development.
RESULTS
The Expression of miR393 Is Down-Regulated during AM Symbiosis
To examine whether miR393 might be involved in the regulation of AM colonization, we assessed the expression of miR393 in tomato roots colonized by the AM fungus Rhizophagus irregularis. The precursors of miR393 in tomato were identified using the MIReNA software (Mathelier and Carbone, 2010) and the miR393 of Arabidopsis (Arabidopsis thaliana) as query. The tomato genome contains only one miR393 copy, for which the mature sequence is identical to the Arabidopsis miRNA (Supplemental Fig. S1; Lin et al., 2013). Tomato roots inoculated with R. irregularis were harvested and assessed for root colonization and the induction of a mycorrhiza-specific plant marker gene, phosphate transporter4 (PT4; Harrison et al., 2002; Paszkowski et al., 2002; Nagy et al., 2005; Supplemental Fig. S2). Root colonization (approximately 60%) was accompanied by a strong PT4 induction (Supplemental Fig. S2). Furthermore, we observed a decrease in microRNA precursor miR393 transcript level and mature miR393 accumulation (Fig. 1A; Supplemental Fig. S3). To know if the down-regulation of miR393 is a general feature of mycorrhization, we measured the expression of precursors of miR393 during mycorrhizal colonization in two phylogenetically distant species: the model plants M. truncatula and rice. The genome of both plants contains two precursors of miR393 according to miRbase (www.mirbase.org; Supplemental Fig. S1). We first monitored root length colonization and the expression of mycorrhiza-specific plant PT4 and PT11 in M. truncatula and rice, respectively (Supplemental Fig. S2; Harrison et al., 2002; Paszkowski et al., 2002; Nagy et al., 2005). As for tomato, the miR393 precursors of M. truncatula and rice accumulated to lower levels in colonized roots compared with noncolonized roots (Fig. 1, B and C). In a time course experiment, the down-regulation of miR393 in M. truncatula correlated with the onset of arbuscule formation, which was revealed by PT4 expression, at 3 weeks after inoculation and continued until 9 weeks postinoculation when the symbiosis was well established (Supplemental Fig. S4). This down-regulation of miR393 was not detected in roots treated with exogenous applications of Myc-LCOs and COs (Supplemental Fig. S5). These molecules are symbiotic molecular signals released by the fungus before colonization (Maillet et al., 2011; Genre et al., 2013). This suggests that down-regulation of miR393 specifically occurs later during root colonization. To support this hypothesis, the down-regulation of miR393 during mycorrhization was not observed in a doesn't make infection3 (dmi3) mutant, which is impaired in the formation of symbiotic structures (Supplemental Fig. S6).
Figure 1.
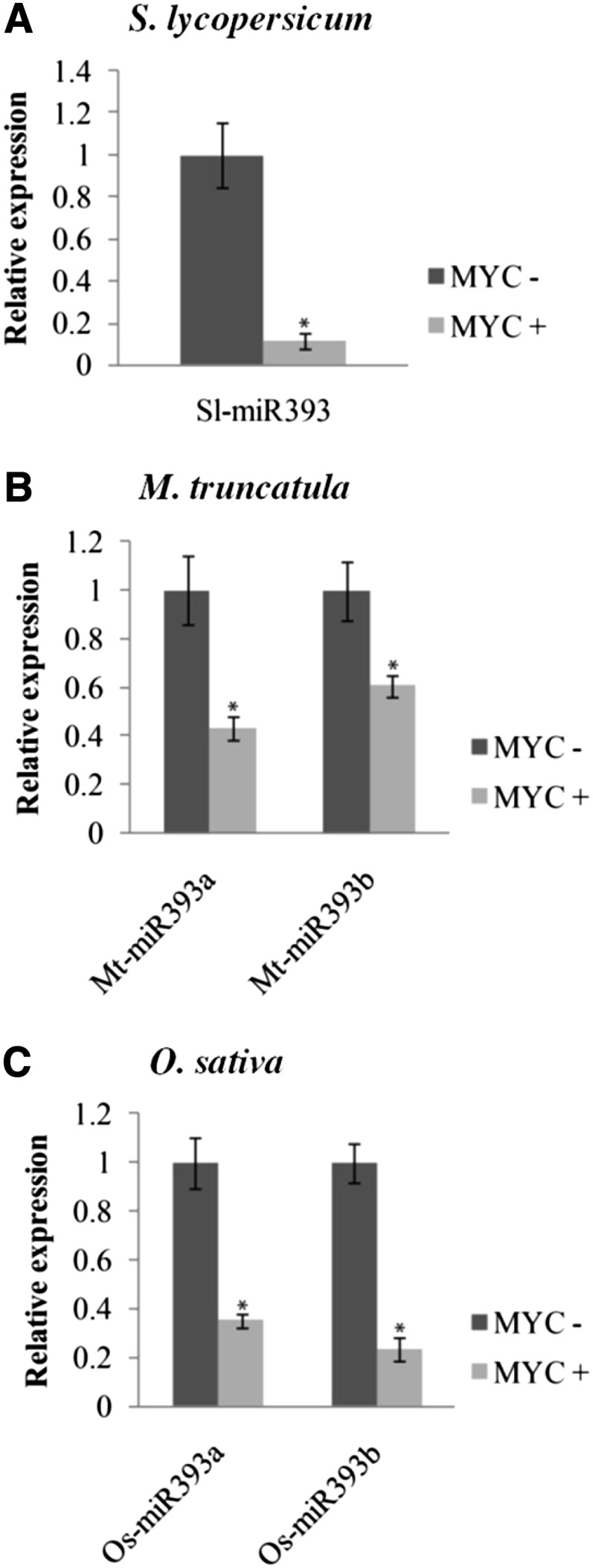
Down-regulation of premiR393 in AM symbiosis. Quantification by quantitative reverse transcription (qRT) -PCR of the expression of premiR393 in nonmycorrhizal (MYC−) and mycorrhizal (MYC+) roots of tomato (A), M. truncatula (B), and rice (C) colonized by R. irregularis. The measured transcripts were normalized to the relative expression value in nonmycorrhizal roots. Error bars represent sem. *, Significant difference between the two treatments according to the Kruskal-Wallis test (n = 6, P < 0.05).
Auxin Treatment Increases Arbuscule Abundance
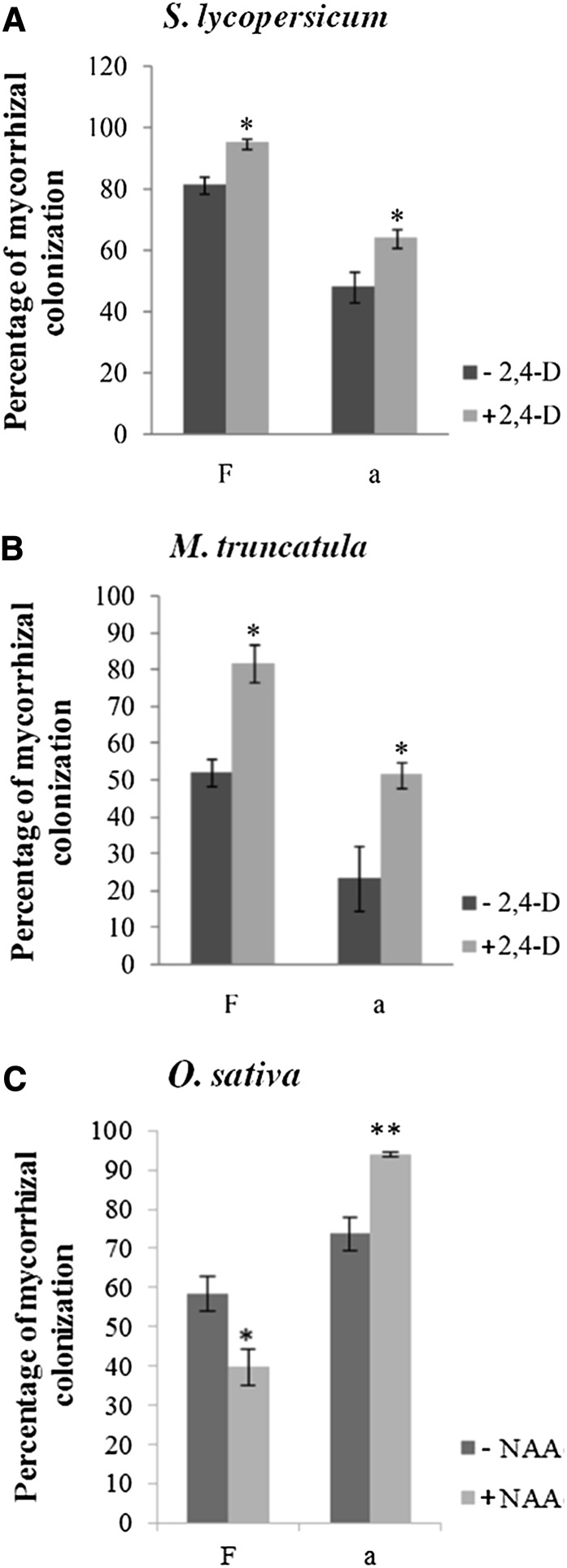
The previous experiments showing that miR393 was down-regulated in AM-colonized roots suggested that auxin might positively affect mycorrhizal colonization. To test this hypothesis, we treated tomato and M. truncatula plants with the synthetic auxin analog 2,4-dichlorophenoxyacetic acid (2,4-D; Song, 2013). Because high concentrations of 2,4-D are lethal to plants or can strongly influence root development, we first monitored the effect of 2,4-D concentration on root development. Because the tomato root system produced no lateral roots under our in vitro conditions, we tested the effect of several concentrations of 2,4-D on primary root length. For M. truncatula, we measured both root length and root branching. Concentrations less than 10−8 m influenced neither the root length of tomato plants nor the root length and root branching of M. truncatula (Supplemental Fig. S7). Therefore, we treated tomato and M. truncatula plants three times per week with 10−10 m 2,4-D during mycorrhiza development. Whereas the root development was not affected by prolonged watering with low concentrations of 2,4-D, treatment with 2,4-D resulted in a significant increase of tomato root length colonization (+16%) compared with treatment with water (Fig. 2A), and particularly, the proportion of arbuscules was significantly higher in 2,4-D-treated roots (+32%) compared with control roots (Fig. 2A). Treatment of M. truncatula roots led to comparable results (i.e. root length colonization [+57%] and arbuscule [+119%] abundance were increased by the 2,4-D treatment; Fig. 2B). Because monocotyledonous plants are hardly sensitive to 2,4-D, we used 10−10 m naphtyl acetic acid (NAA) to examine the effect of auxin treatment on colonization of rice. At this NAA concentration, the frequency of colonization was slightly reduced (−19%). Nevertheless, arbuscule abundance in the colonized areas was strongly increased (+ 20%; Fig. 2C). Taken together, these data showing a lower miR393 expression in mycorrhizal roots and a higher arbuscule formation in response to exogenous auxin treatments suggest that auxin signaling may be involved in arbuscule development.
Figure 2.
Frequency of colonization and arbuscule abundance increased in response to auxin treatment. Frequency (F) of mycorrhization and arbuscule abundance (a) in roots of tomato (A) and M. truncatula (B) in response to solvent control (−2,4-D) or 10−10 m 2,4-D (+2,4-D). C, F of mycorrhization and a in roots of rice in response to solvent control (−NAA) or 10−10 m NAA (+NAA). Error bars represent sem. Asterisks indicate significant difference between the two treatments according to the Kruskal-Wallis test (n = 6). *, P < 0.05. **, P < 0.01.
Auxin Response Is Activated in Arbuscule-Containing Cells
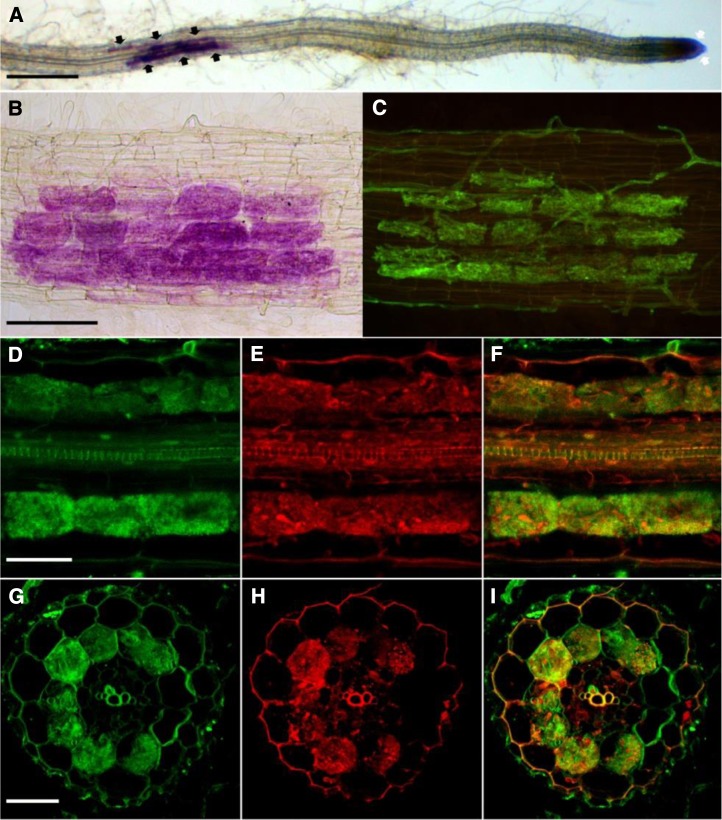
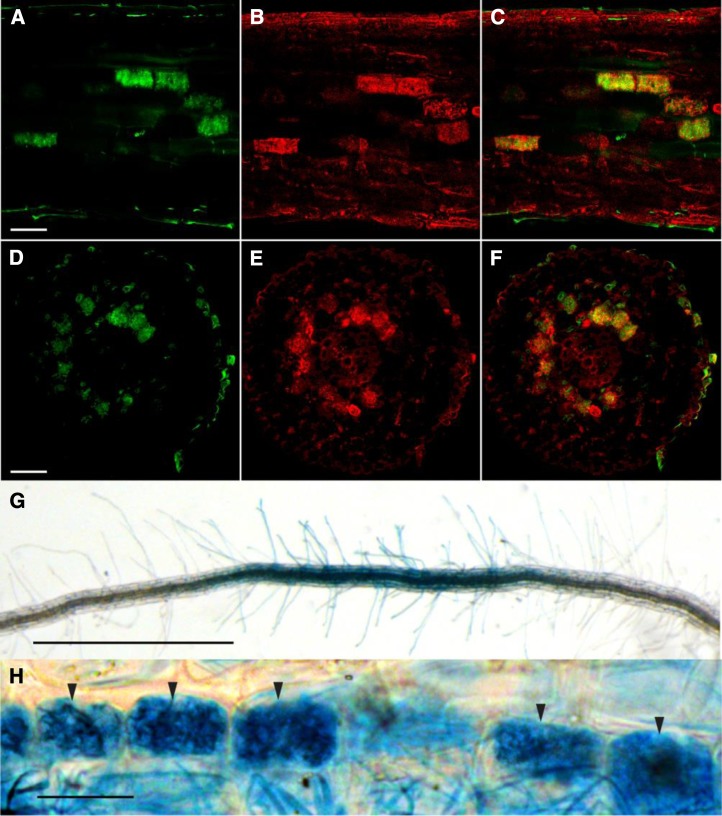
Auxin regulates the formation of lateral roots, and it has been shown that overexpression of miR393 leads to a decreased number of lateral roots (Vidal et al., 2010). It is also known that mycorrhizal root systems are generally more ramified (Oláh et al., 2005; Gutjahr et al., 2009; Mukherjee and Ané, 2011). Thus, the decreased expression of miR393 that we observed in mycorrhizal roots could be related to a stimulatory effect of the fungus on lateral root formation. However, auxin and auxin signaling could also play a more specific and direct role in the establishment of the symbiosis. To reveal the stage of mycorrhizal colonization at which auxin signaling intervenes, we attempted to identify cells within colonized roots that would display a higher auxin response. We visualized the activity of DR5, a synthetic auxin-inducible promoter, fused to the GUS reporter gene (Ulmasov et al., 1997; Chaabouni et al., 2009). We first characterized the DR5-GUS expression pattern in nonmycorrhizal tomato roots. GUS staining was detected in the root tips and lateral root primordia (Supplemental Fig. S8), which is a common pattern of DR5 activity in roots (Chaabouni et al., 2009). In mycorrhizal roots, strong additional GUS staining was present in larger patches localized in the cortex and apparently not linked to meristems (Fig. 3, A and B; Supplemental Fig. S9). To assess whether this staining corresponded to colonization units, we used specific fluorescent dyes: ImaGene Green, which is a fluorescent substrate of GUS (see “Materials and Methods”), and Uvitex2B or fluorescein-conjugated wheat germ agglutinin (WGA-FITC) to visualize the fungus (Diagne et al., 2011); each label was confirmed to be specific to cells expressing GUS or the fungus, respectively, except an unspecific labeling of lignified cell walls by ImaGene Green (Supplemental Fig. S10). Interestingly, dual labeling revealed that the nonmeristematic regions displaying GUS activity corresponded to root cortical cells containing arbuscules (Fig. 3, D–I). To investigate whether this specific localization of GUS activity could be generalized to other plant species, similar experiments were performed on stable transgenic M. truncatula and rice DR5-GUS lines (Scarpella et al., 2003; Herrbach et al., 2014). For both species, GUS staining was observed in root meristems (data not shown) and arbuscule-containing cells. However, whereas the GUS labeling in M. truncatula roots was highly specifically correlated to the presence of arbuscules (Fig. 4, A–F), the GUS staining in rice was more diffuse across all tissue layers including root hairs. However (apart from root meristems), its highest intensity was restricted to arbuscule-containing root portions (Fig. 4, G and H).
Figure 3.
DR5:GUS expression in arbuscule-containing cells in roots of tomato colonized by R. irregularis. A, DR5:GUS staining (5-bromo-6-chloro-3-indolyl-β-d-glucuronic acid) of root tips (white arrows) and colonized root tissue (black arrows) by R. irregularis. Bar = 500 µm. B, Higher magnification of DR5:GUS staining of a colonized root. Bar (for B and C) = 100 µm. C, Fungal staining of the same root segment using WGA-FITC. D to F, Longitudinal root confocal section containing arbuscules. Bar = 50 µm. G to I, Confocal root cross section containing arbuscules. Bar = 50 µm. D and G, Fungal staining using Uvitex2B. E and H, DR5:GUS staining using ImaGene Green. F and I, Overlaps of images D and E and images G and H, respectively.
Figure 4.
DR5:GUS expression in arbuscule-containing cells of M. truncatula and rice roots colonized by R. irregularis. A to C, Longitudinal M. truncatula root confocal section containing arbuscules. Bar = 50 µm. D to F, Confocal M. truncatula root cross section containing arbuscules. Bar = 50 µm. A and D, Fungal staining using Uvitex2B. B and E, DR5-GUS staining using ImaGene Green. C and F, Overlaps of images A and B and images D and E, respectively. G, DR5:GUS staining (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, cyclohexylammonium salt) shows a colonized root of rice by R. irregularis. Bar = 1 mm. H, Higher magnification of DR5-GUS staining of a colonized root of rice. Black arrows show the arbuscule-containing cells. Bar = 5 µm.
These data suggest that arbuscule development or functioning is accompanied by an auxin response.
Overexpression of miR393 Causes Inhibition of Arbuscule Development
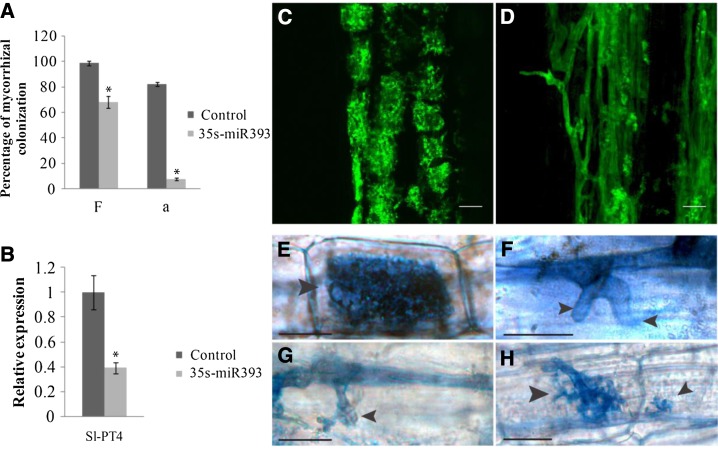
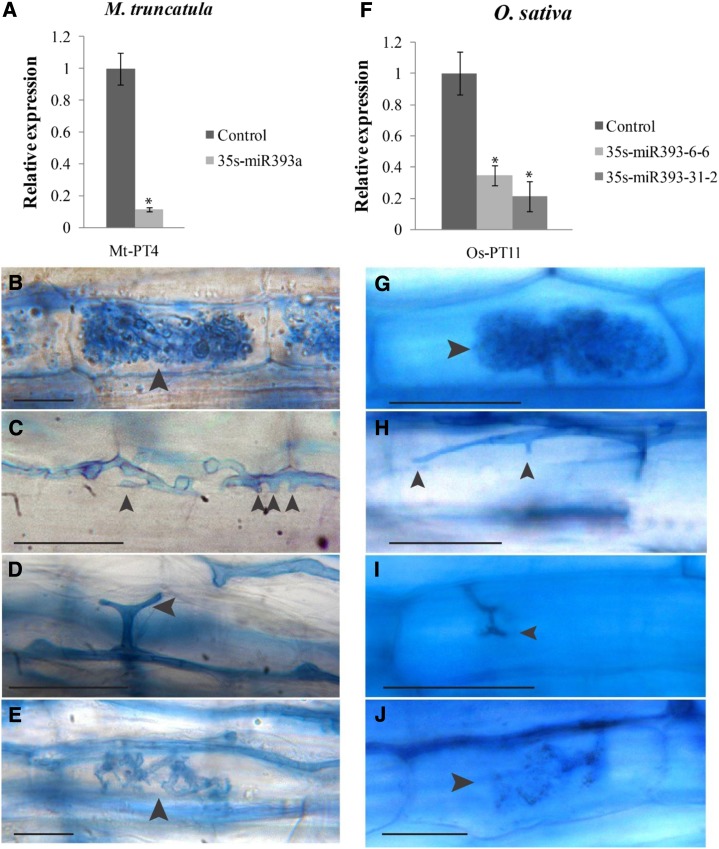
To specifically investigate the impact of altered auxin signaling on mycorrhiza development, we transformed tomato roots using Agrobacterium rhizogenes with a vector to overexpress the precursor of miR393 under the control of the 35S promoter. As expected, these transgenic roots significantly overexpressed the precursor of miR393 and the mature miR393 (Supplemental Fig. S11). Accordingly, transcripts of miR393 target genes were detected at lower levels than in control roots (Supplemental Fig. S12). The three potential target genes of miR393 in tomato had been identified by using psrnatarget (Dai and Zhao, 2011) and according to their homology, with the TIR1-AFB gene family members of Arabidopsis (Supplemental Figs. S13 and S14). We analyzed the transformed roots of 15 chimeric plants and repeated the experiment three times. Although the overall colonization of miR393-overexpressing roots was slightly decreased (Fig. 5A), arbuscule formation was strongly reduced (Fig. 5, A and D). Reduction in arbuscule formation caused by miR393 overexpression was confirmed by the expression level of the gene PT4, which is exclusively expressed in arbuscule-containing cells and therefore, an unequivocal marker for arbuscule abundance (Fig. 5B). Observation of arbuscule morphology revealed that miR393-overexpressing roots only allowed the formation of stunted arbuscules with coarse, lower-order branches and no fine branches (Fig. 5, D and H). Moreover, we observed finger-like hyphal protrusions into cortical cells, indicating that, in some cases, arbuscule formation was already blocked at the stage of cell penetration (Fig. 5, F and G).
Figure 5.
Overexpression of miR393 in tomato roots inhibits formation of arbuscules. A, Frequency (F) of mycorrhization and arbuscule abundance (a) in control and miR393-overexpressing roots of tomato. B, Expression measured by qRT-PCR of mycorrhiza-specific plant PT4 in control and miR393-overexpressing roots. The measured transcripts were normalized to the relative expression value in control (empty vector-transformed) roots. Error bars represent sem. *, Significant differences between the genotypes according to the Kruskal-Wallis test (n = 6, P < 0.05). C and D, Confocal microscopy images showing mycorrhizal colonization stained with WGA-FITC of control (C) and miR393-overexpressing (D) roots. Bars = 25 µm. E to H, Arbuscules (arrows) images in control (E) and miR393-overexpressing roots (F–H) stained with ink. Bars = 25 µm.
To investigate whether miR393 overexpression would perturb arbuscule development in other plant species, we transformed M. truncatula roots with a vector containing the p35S-miR393 cassette. M. truncatula hairy roots transformed with the p35S-miR393 construct showed higher transcript levels of miR393 and lower transcript levels of the miR393 target genes TIR1, AFB2, and AFB4 (Supplemental Figs. S12 and S13). They were also less sensitive to auxin treatment, which was determined by root elongation assays and DR5-GUS expression (Supplemental Fig. S15). As for tomato, the three potential target genes of miR393 in M. truncatula were identified by BLAST homology with the TIR1-AFB protein family members of Arabidopsis (Supplemental Figs. S13 and S14). Similar to tomato, the expression of the arbuscule marker PT4 was decreased compared with control roots (Fig. 6A), and arbuscule formation was defective: roots overexpressing miR393 contained many hyphal protrusions into cortical cells that did not develop into arbuscules, and arbuscules had a lower magnitude of branching compared with control arbuscules (Fig. 6, B–E). Two independent stable transgenic lines of the monocot rice, overexpressing the miR393 (Xia et al., 2012), revealed a similar phenotype. The roots or 35S-miR393-transformed plants did not contain any mature arbuscules like in control roots (Fig. 6G) but instead, numerous abortive or poorly branched arbuscules (Fig. 6, H–J). The arbuscule phenotype was confirmed by decreased transcript accumulation of the arbuscule marker gene PT11 in miR393-overexpressing roots compared with control roots (Fig. 6F). In summary, miR393 overexpression hampers arbuscule development in three distantly related plant species.
Figure 6.
Overexpression of miR393 in M. truncatula and rice roots inhibits formation of arbuscules. A and F, Expression, measured by qRT-PCR, of mycorrhiza-specific plant PT4 (A) and PT11 (F) in control roots (transformed with an empty vector) compared with miR393-overexpressing roots of M. truncatula (A) and rice (F). The measured transcripts were normalized to the relative expression value in control roots. Error bars represent sem. *, Significant differences between the genotypes according to the Kruskal-Wallis test (n = 6, P < 0.05). Fungal structures (arrows) observed in control (B and G) and miR393-overexpressing (C–E and H–J) roots of M. truncatula (B–E) and rice (G–J) inoculated with R. irregularis. Fungal structures are stained with ink (B–E) or trypan blue (G–J). Bars in B to E = 25 µm. Bars in G to J = 5 µm.
DISCUSSION
Previous studies in several plant species had shown a different auxin level in mycorrhizal roots compared with nonmycorrhizal roots but without any consensual role (Jentschel et al., 2006; Campanella et al., 2007). Some tomato mutants with pleiotropic phenotypes related to impaired auxin signaling or transport exhibited a defect in mycorrhizal colonization but without any arbuscule defect (Hanlon and Coenen, 2011). Here, we collected evidence that auxin perception is required for arbuscule development, because (1) the miR393, which is known to target auxin receptor transcripts, was down-regulated in mycorrhizal roots, (2) treatments of roots with low concentrations of 2,4-D or NAA increased arbuscule abundance, (3) the expression of the DR5-GUS reporter for auxin response was mainly restricted to arbuscule-containing cells, and (4) overexpression of miR393 strongly impaired arbuscule development. These phenomena were observed in three plant species, including monotyledons and dicotyledons, indicating that the requirement of auxin perception for arbuscule development is conserved, at least across the angiosperms. To date, only two miRNAs have been reported to be regulated and play a role during AM symbiosis (Lauressergues et al., 2012; Bazin et al., 2013). We show here that miR393 is another miRNA regulated in AM symbiosis with a potential negative impact on arbuscule formation.
Several fungi, such as plant pathogens (Reineke et al., 2008) or ectomycorrhizal fungi (Tranvan et al., 2000; Felten et al., 2009), are able to synthesize auxin. Our data on DR5-GUS activity show that the auxin response increased in roots colonized by R. irregularis, mainly in cells containing arbuscules. This increase in auxin response could be caused by increased auxin accumulation in arbuscule-containing cells. Although a previous study has shown that AM fungi alone do not produce auxin (Jentschel et al., 2006), we cannot exclude that they are capable of producing this hormone in planta to improve their colonization success.
Fu and Harberd (2003) have shown that, in Arabidopsis, auxin stimulates GA3-mediated DELLA protein destabilization. In this context, the promoting effect of auxin signaling on mycorrhiza formation seems not to be in agreement with the study by Floss et al. (2013) showing that DELLA proteins, by repressing GA3 signaling, are positive regulators of arbuscule formation. However, DELLA expression in the vasculature was sufficient to drive arbuscule formation in the cortex (Floss et al., 2013), although we have observed auxin responses in arbuscule-containing cortical cells. It is, therefore, possible that DELLA and auxin act in different cell types. Furthermore, we have seen that DELLA gene expression is decreased in M. truncatula roots with reduced sensitivity to auxin (Supplemental Fig. S16).
Arbuscule development is preceded in the cortical cell by the formation of a prepenetration apparatus corresponding to cytoplasmic aggregations that organize the apoplastic space in which the arbuscule will develop (Genre et al., 2008). The fungus can then penetrate the cell by producing an arbuscular trunk from which coarse and later, fine hyphal branches will emerge to form the mature arbuscule (Gutjahr and Parniske, 2013). This process is severely hampered in cortical cells of miR393-overexpressing roots, which only display arbuscular trunks or stunted arbuscules with highly reduced and disorganized ramifications. This is reminiscent of the phenotype of vapyrin mutants, which are defective in a protein of unknown function but proposed to be an executor of intracellular accommodation (Feddermann et al., 2010; Pumplin et al., 2010; Gutjahr and Parniske, 2013). Continuous arbuscule branching is accompanied by the formation in cortical cells of a periarbuscular membrane (PAM), which corresponds to an exocytosis-mediated massive extension of the plasma membrane surrounding each fine arbuscular branch. Interestingly, mutants altered in the exocytotic machinery also show arbuscule branching defects (Ivanov et al., 2012; Lota et al., 2013), indicating that exocytosis is required for PAM extension and arbuscule formation. The PAM contains a distinct set of proteins and can be considered as a unique membrane domain that differs from the peripheral plasma membrane (Pumplin and Harrison, 2009; Kobae and Hata, 2010; Zhang et al., 2010). Arbuscule development requires the polarization of individual cortical cells within the tissue context, and vesicle trafficking for PAM construction is likely to be cytoskeleton dependent (Brandizzi and Wasteneys, 2013). Polarization during arbuscule development is accompanied by rearrangement of the actin and microtubule cytoskeleton, such that, finally, actin filaments run along arbuscule branches and microtubules form a basket-like structure around the arbuscule (Genre and Bonfante, 1998). Auxin is regarded to be a crucial signaling substance for development and maintenance of cell polarity in plants (Yang, 2008), and artificial elevation of auxin concentration in epidermal cells leads to a reorganization of actin filaments and microtubules (Holweg et al., 2004; Nick et al., 2009). Furthermore, local auxin maxima lead to a cell-specific repolarization of membrane-localized pinoid proteins, which are auxin efflux carriers, in a TIR1-dependent manner (Sauer et al., 2006). Taking together these published data, it is tempting to speculate that the malformation of arbuscules in roots overexpressing miR393 (i.e. with altered expression of auxin receptors and thus, auxin signaling) could result from a defect in cytoskeletal rearrangement and cell polarization. AUXIN BINDING PROTEIN1 (ABP1) is another plasmamembrane and endoplasmic reticulum-localized type of auxin receptor (Sauer and Kleine-Vehn, 2011) that, in Arabidopsis, has been implicated in regulating leaf pavement cell polarity through activation of ρ-like guanosine triphosphatases and cytoskeletal changes (Xu et al., 2011). It will be interesting to investigate whether ABP1 also plays a role in AM development.
The mechanisms underlying the establishment of AM symbiosis are far from being fully understood. Nevertheless, showing additional evidence that they are likely conserved across plant kingdom, at least with regard to the requirement for an auxin signaling, provides new leads toward deciphering this highly complex developmental process.
MATERIALS AND METHODS
Biological Materials
Medicago truncatula ‘Jemalong’ genotype A17 and tomato (Solanum lycopersicum ‘MicroTom’) seeds were surface sterilized by bleach and water for 2 to 5 min and then rinsed five times in sterile water. M. truncatula and tomato plants were cultivated in 250-mL pots filled with Oil-Dri US special substrate (Damolin) for 8 and 12 weeks, respectively, in a growth chamber (M. truncatula: 16-h/8-h at 22°C/20°C day-night cycle, 400 µmol m−2 s−1; tomato: 16-h/8-h at 25°C/25°C day-night cycle, 600 µmol m−2 s−1) and watered every 2 d with modified Long Ashton medium containing a low concentration (7.5 µm) of phosphate (Balzergue et al., 2011). Rhizophagus irregularis (formerly named Glomus intraradices) DAOM197198 sterile spores were purchased from Agronutrition. Tomato and M. truncatula roots were inoculated with 400 spores of R. irregularis per plant. M. truncatula DR5-GUS plants were provided by Sandra Bensmihen (Laboratoire des Interactions Plantes-Microorganismes; Herrbach et al., 2014). For auxin treatment, plants were watered three times a week with Long Ashton medium supplemented or not with 10−10 m 2,4-D during mycorrhiza development. Fifteen plants (for mycorrhization) and six plants (for quantitative PCR) per experiment were used for all experiments with three different biological replicates. One representative of three independent experiments is shown.
Control and miR393 overexpressing seeds of Oryza sativa ssp. ‘Japonica’ ZH11 were provided by Mingyong Zhang (Chinese Academy of Science; Xia et al., 2012). The O. sativa ssp. ‘Japonica’ Taichung 65 DR5-GUS line (Scarpella et al., 2003) was provided by Pieter B.F. Ouwerkerk (University of Leiden, Leiden, The Netherlands). Rice seedlings pregerminated for 4 d in the dark were inoculated with 500 spores of R. irregularis (SYMPLANTA) in 128-mL pots filled with quartz sand (16-h/8-h at 26°C/26°C day-night cycle, 223 µmol m−2 s−1). They were fertilized two times a week with 10 mL of one-half-strength Hoagland solution containing 25 µm phosphate and 0.001% (w/v) Sequestrene rapid (Syngenta). For auxin treatment, 10−10 m NAA was added to the fertilizer or water and supplied three times a week. Rice roots were harvested 6 weeks postinoculation. Six plants were used in each experiment. The root system of each plant (n = 6) was divided into two equal parts: one-half was used for AM quantification (n = 3), and one-half was used for RNA extraction (n = 3, quantitative PCR analysis).
For root length measurement, composite plants were transferred to a modified Fahraeus medium containing 2,4-D (0.2 µm) or solvent control. Root apices were directly marked on the petri dishes at time point zero to monitor the root elongation. At 14 d, root elongation was scored from digital images of petri dishes using ImageJ software.
Plasmid Construction
Precursor of miR393 was amplified using Pfu polymerase (Promega), and the primers are shown in Supplemental Table S1. They were cloned using XhoI and NotI restriction enzymes into the pPEX-discosoma RED (DsRED) plasmid (Combier et al., 2008) for overexpression under the control of the strong constitutive Cauliflower mosaic virus 35S promoter.
Plant Transformation
Root transformations of tomato and M. truncatula were performed with Agrobacterium rhizogenes as described by Boisson-Dernier et al. (2001). Transformed roots were selected by observation of DsRED fluorescence using a fluorescence binocular (Leica). Control roots corresponded to roots transformed with A. rhizogenes carrying the empty vector pPEX-DsRED.
Expression Analyses
Total RNA was extracted using a Plant RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Total RNA was treated by DNase I (Promega) to remove any genomic DNA contamination. Reverse transcription was performed using M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant (Promega) on 500 ng of total plant RNA. For each experiment, six independent plants or transformants were analyzed. Quantitative PCR amplifications were conducted on a Roche LightCycler 480 System (Roche Diagnostics) under the following conditions: 95°C for 5 min and then 45 cycles of 95°C for 15 s and 60°C for 1 min. The various primer sets used are described in Supplemental Table S1. The measured transcripts were normalized to the relative expression value in nonmycorrhizal roots. For the miR393 overexpressing lines inoculated with R. irregularis, expression of genes of interest was normalized to the relative expression value of mycorrhizal control roots. Actin, ubiquitin, and cyclophilin (Supplemental Table S1) were used as reference genes for normalization of gene expression of tomato, M. truncatula, and rice, respectively.
Northern-blot analyses were performed as described by Lauressergues et al. (2012).
Identification of Target Genes and Phylogenic Tree
Putative miR393 target genes in M. truncatula and tomato were found by using psRNAtarget (Dai and Zhao, 2011). The protein sequences of putative targets were extracted, and we performed a phylogenetic analysis. Amino acid alignments were made using Tcoffee, and phylogenetic trees were performed using Mega5 (maximum likelihood, bootstrap = 100; Tamura et al., 2011; Supplemental Figs. S13 and S14).
Histochemical Staining and Microscopy Studies
5-Bromo-6-chloro-3-indolyl-β-d-GlcA cyclohexyl ammonium salt GUS staining was performed as described by Combier et al. (2008). GUS expression at the cellular/tissue level was detected by treating the transgenic tissue in 50 µm ImaGene Green C12FDGlcU substrate (ImaGene Green GUS Gene Expression Kit; Invitrogen) in phosphate buffer (pH 7) at 37°C for 2 h in the dark. GUS activity was detected by fluorescence microscopy using the Leica SP2 Confocal Microscope.
Fungal structures were visualized by staining with 0.01% (w/v) Uvitex2B in phosphate buffer (pH 7) for 30 min at room temperature (Diagne et al., 2011). Root sections (50 µm) were made using the vibratome VT1000S from samples embedded in 4% (w/v) agarose. For root mycorrhizal phenotyping, roots were cleared in 10% (w/v) KOH, rinsed in sterile water, treated for 30 min with WGA-FITC (Invitrogen), which binds fungal chitin, washed three times for 10 min in PBS, and observed using an inverted light microscope or a confocal microscope (Leica). Alternatively, they were stained with Schaeffer black ink as described by Vierheilig et al. (1998). Quantification of mycorrhizal colonization was performed as described by Trouvelot et al. (1986): the frequency of mycorrhiza in the root system and the arbuscule abundance (percentage) were calculated in the colonized root sections using Mycocalc software (http://www2.dijon.inra.fr/mychintec/Mycocalc-prg/download.html). Fifteen root systems of tomato and M. truncatula and two mutant lines (6-6 and 31-2) of rice were analyzed, and each experiment was repeated three times. Trypan blue staining of rice roots was performed as described (Gutjahr et al., 2008)
Statistical Analyses
The mean values for relative gene expression (n = 6) or mycorrhization rates (n = 15) were compared using the Kruskal-Wallis test, and when significant, a pairwise comparison was made using the nonparametric Mann-Whitney test. In the figures, asterisks indicate significant differences compared with the control (P < 0.05 or P < 0.01), and error bars represent the sem.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Conservation of mature miR393.
Supplemental Figure S2. Expression of mycorrhiza-induced plant phosphate transporter and root length colonization.
Supplemental Figure S3. Expression of tomato mature miR393.
Supplemental Figure S4. Expression of M. truncatula precursor of miR393 during mycorrhization.
Supplemental Figure S5. Relative expression of M. truncatula precursor of miR393 in response to mycorrhizal-lipochito-oligosaccharides and chito-oligosaccharides.
Supplemental Figure S6. Expression of M. truncatula precursor of miR393 during mycorrhization in wild-type and dmi3 mutant.
Supplemental Figure S7. Effect of different concentration of 2,4-d on root development.
Supplemental Figure S8. Expression of the DR5-GUS auxin response marker in tomato roots.
Supplemental Figure S9. Expression of the DR5-GUS auxin response marker in mycorrhizal tomato and M. truncatula roots.
Supplemental Figure S10. Specificity of Uvitex2B and ImaGene Green root staining.
Supplemental Figure S11. miR393 is overexpressed in 35S miR393 roots.
Supplemental Figure S12. Expression of miR393 targets in miR393-overexpressing roots.
Supplemental Figure S13. Phylogenetic analysis of putative target genes of miR393.
Supplemental Figure S14. Identity of mature miR393 and targets.
Supplemental Figure S15. Overexpression of miR393 reduces the sensitivity to exogenous application of 2,4-d in M. truncatula.
Supplemental Figure S16. Expression of a DELLA gene in tomato mycorrhized roots.
Supplemental Table S1. List of primers used in this study.
Acknowledgments
We thank the Plateforme Imagerie-Fédération de Recherche Agrobiosciences, Interactions et Biodiversité facility for microscopy analyses and technical advice on the histologic analyses; the MetaToul Platform, Saïda Danoun, and Sylvie Fournier (Laboratoire de Recherche en Sciences Végétales, France) for access to the gas chromatography-mass spectrometry facility; Jean-Marie Prospéri (Centre de Ressources Biologiques M. truncatula, Unité Mixte de Recherche Amélioration Génétique et Adaptation des Plantes 1334, Montpellier, France) for M. truncatula A17 seeds; Sandra Bensmihen and Violaine Herrbach (Laboratoire des Interactions Plantes-Microorganismes, France) for providing DR5-GUS M. truncatula seeds; Mingyong Zhang (Chinese Academy of Science, China) for the O. sativa ssp. ‘Japonica’ ZH11 lines overexpressing miR393; and Pieter B.F. Ouwerkerk (Leiden University) for the O. sativa ssp. ‘Japonica’ Taichung 65 DR5-GUS line.
Glossary
- AM
arbuscular mycorrhiza
- 2,4-D
2,4-dichlorophenoxyacetic acid
- IAA
indole-3-acetic acid
- miR
microRNA
- NAA
naphtyl acetic acid
- PAM
periarbuscular membrane
- qRT
quantitative reverse transcription
- WGA-FITC
fluorescein-conjugated wheat germ agglutinin
Footnotes
This work was supported by the French Agence Nationale pour la Recherche (ANR) Project miRcorrhiza (grant no. ANR–12–JSV7–0002–01), the French Laboratory of Excellence Project Vers une Théorie Unifiée des Interactions Biotiques: Rôle des Perturbations Environnementales (grant nos. ANR–10–LABX–41 and ANR–11–IDEX–0002–02), the Fédération de Recherches 3450 Institute, and the French-Bavarian University Center (Bayerisch-Französisches Hochschulzentrum/Centre de Coopération Universitaire Franco-Bavarois; collaboration between C.G. and J.-P.C.).
The online version of this article contains Web-only data.
References
- Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, Douds DD, Lammers PJ, Shachar-Hill Y. (2003) Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol 131: 1496–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzergue C, Puech-Pagès V, Bécard G, Rochange SF. (2011) The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot 62: 1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin J, Khan GA, Combier JP, Bustos-Sanmamed P, Debernardi JM, Rodriguez R, Sorin C, Palatnik J, Hartmann C, Crespi M, et al. (2013) miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J 74: 920–934 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG. (2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14: 695–700 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Wasteneys GO. (2013) Cytoskeleton-dependent endomembrane organization in plant cells: an emerging role for microtubules. Plant J 75: 339–349 [DOI] [PubMed] [Google Scholar]
- Campanella JJ, Smith SM, Leibu D, Wexler S, Ludwig-Müller J. (2007) The auxin conjugate hydrolase family of Medicago truncatula and their expression during the interaction with two symbionts. J Plant Growth Regul 27: 26–38 [Google Scholar]
- Chaabouni S, Jones B, Delalande C, Wang H, Li Z, Mila I, Frasse P, Latché A, Pech JC, Bouzayen M. (2008) Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J Exp Bot 60: 1349–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combier JP, de Billy F, Gamas P, Niebel A, Rivas S. (2008) Trans-regulation of the expression of the transcription factor MtHAP2-1 by a uORF controls root nodule development. Genes Dev 22: 1549–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhao PX. (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 39: W155–W159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaux PM, Séjalon-Delmas N, Bécard G, Ané JM. (2013) Evolution of the plant-microbe symbiotic ‘toolkit’. Trends Plant Sci 18: 298–304 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Diagne N, Escoute J, Lartaud M, Verdeil JL, Franche C, Kane A, Bogusz D, Diouf D, Duponnois R, Svistoonoff S. (2011) Uvitex2B: a rapid and efficient stain for detection of arbuscular mycorrhizal fungi within plant roots. Mycorrhiza 21: 315–321 [DOI] [PubMed] [Google Scholar]
- Feddermann N, Muni RRD, Zeier T, Stuurman J, Ercolin F, Schorderet M, Reinhardt D. (2010) The PAM1 gene of petunia, required for intracellular accommodation and morphogenesis of arbuscular mycorrhizal fungi, encodes a homologue of VAPYRIN. Plant J 64: 470–481 [DOI] [PubMed] [Google Scholar]
- Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, Ditengou FA, Legué V. (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151: 1991–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitze D, Wiepning A, Kaldorf M, Ludwig-Müller J. (2005) Auxins in the development of an arbuscular mycorrhizal symbiosis in maize. J Plant Physiol 162: 1210–1219 [DOI] [PubMed] [Google Scholar]
- Floss DS, Levy JG, Lévesque-Tremblay V, Pumplin N, Harrison MJ. (2013) DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 110: E5025–E5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Jones WT, Reid JB. (2013) Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann Bot (Lond) 111: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Harberd NP. (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743 [DOI] [PubMed] [Google Scholar]
- Genre A, Bonfante P. (1998) Actin versus tubulin configuration in arbuscule-containing cells from mycorrhizal tobacco roots. New Phytol 140: 745–752 [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Balzergue C, Puech-Pagès V, Novero M, Rey T, Fournier J, Rochange S, Bécard G, Bonfante P, Barker DG. (2013) Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol 198: 190–202 [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Faccio A, Barker DG, Bonfante P. (2008) Prepenetration apparatus assembly precedes and predicts the colonization patterns of arbuscular mycorrhizal fungi within the root cortex of both Medicago truncatula and Daucus carota. Plant Cell 20: 1407–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Banba M, Croset V, An K, Miyao A, An G, Hirochika H, Imaizumi-Anraku H, Paszkowski U. (2008) Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20: 2989–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Casieri L, Paszkowski U. (2009) Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol 182: 829–837 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Parniske M. (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 29: 593–617 [DOI] [PubMed] [Google Scholar]
- Hanlon MT, Coenen C. (2011) Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol 189: 701–709 [DOI] [PubMed] [Google Scholar]
- Harrison MJ. (2012) Cellular programs for arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol 15: 691–698 [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Dewbre GR, Liu J. (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14: 2413–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Mrosk C, Isayenkov S, Strack D. (2007) Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 68: 101–110 [DOI] [PubMed] [Google Scholar]
- Hayashi K. (2012) The interaction and integration of auxin signaling components. Plant Cell Physiol 53: 965–975 [DOI] [PubMed] [Google Scholar]
- Herrbach V, Rembliere C, Gough C, Bensmihen S. (2014) Lateral root formation and patterning in Medicago truncatula. J Plant Physiol 171: 301–310 [DOI] [PubMed] [Google Scholar]
- Herrera-Medina MJ, Steinkellner S, Vierheilig H, Ocampo Bote JA, García Garrido JM. (2007) Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol 175: 554–564 [DOI] [PubMed] [Google Scholar]
- Holweg C, Süsslin C, Nick P. (2004) Capturing in vivo dynamics of the actin cytoskeleton stimulated by auxin or light. Plant Cell Physiol 45: 855–863 [DOI] [PubMed] [Google Scholar]
- Ivanov S, Fedorova EE, Limpens E, De Mita S, Genre A, Bonfante P, Bisseling T. (2012) Rhizobium-legume symbiosis shares an exocytotic pathway required for arbuscule formation. Proc Natl Acad Sci USA 109: 8316–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentschel K, Thiel D, Rehn F, Ludwig-Müller J. (2007) Arbuscular mycorrhiza enhances auxin levels and alters auxin biosynthesis in Tropaeolum majus during early stages of colonization. Physiol Plant 129: 320–333 [Google Scholar]
- Kaldorf M, Ludwig-Müller J. (2000) AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol Plant 109: 58–67 [Google Scholar]
- Kepinski S, Leyser O. (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Kobae Y, Hata S. (2010) Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol 51: 341–353 [DOI] [PubMed] [Google Scholar]
- Kramer EM. (2009) Auxin-regulated cell polarity: an inside job? Trends Plant Sci 14: 242–247 [DOI] [PubMed] [Google Scholar]
- Lau S, Jürgens G, De Smet I. (2008) The evolving complexity of the auxin pathway. Plant Cell 20: 1738–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauressergues D, Delaux PM, Formey D, Lelandais-Brière C, Fort S, Cottaz S, Bécard G, Niebel A, Roux C, Combier JP. (2012) The microRNA miR171h modulates arbuscular mycorrhizal colonization of Medicago truncatula by targeting NSP2. Plant J 72: 512–522 [DOI] [PubMed] [Google Scholar]
- Lin D, Yang Y, Khalil R, Xian Z, Hu G, Li Z. (2013) SlmiR393 controls the auxin receptor homologous genes expression,and regulates sensitivity to auxin in tomato root growth. Sci Hortic (Amsterdam) 162: 90–99 [Google Scholar]
- Lota F, Wegmüller S, Buer B, Sato S, Bräutigam A, Hanf B, Bucher M. (2013) The cis-acting CTTC-P1BS module is indicative for gene function of LjVTI12, a Qb-SNARE protein gene that is required for arbuscule formation in Lotus japonicus. Plant J 74: 280–293 [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J, Güther M. (2007) Auxins as signals in arbuscular mycorrhiza formation. Plant Signal Behav 2: 194–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J, Kaldorf M, Sutter EG, Epstein E. (1997) Indole- ‘3-butyric acid (IBA) is enhanced in young maize (Zea mays L.) roots colonized with the arbuscular mycorrhizal fungus Glomus in traradices. Plant Sci 125: 153–162 [Google Scholar]
- Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, et al. (2011) Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469: 58–63 [DOI] [PubMed] [Google Scholar]
- Mao G, Turner M, Yu O, Subramanian S. (2013) miR393 and miR164 influence indeterminate but not determinate nodule development. Plant Signal Behav 9: 26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelier A, Carbone A. (2010) MIReNA: finding microRNAs with high accuracy and no learning at genome scale and from deep sequencing data. Bioinformatics 26: 2226–2234 [DOI] [PubMed] [Google Scholar]
- Meixner C, Ludwig-Müller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H. (2005) Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 222: 709–715 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Ané JM. (2011) Germinating spore exudates from arbuscular mycorrhizal fungi: molecular and developmental responses in plants and their regulation by ethylene. Mol Plant Microbe Interact 24: 260–270 [DOI] [PubMed] [Google Scholar]
- Nagy R, Karandashov V, Chague V, Kalinkevich K, Tamasloukht M, Xu G, Jakobsen I, Levy AA, Amrhein N, Bucher M. (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J 42: 236–250 [DOI] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD. (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312: 436–439 [DOI] [PubMed] [Google Scholar]
- Navarro L, Jay F, Nomura K, He SY, Voinnet O. (2008) Suppression of the microRNA pathway by bacterial effector proteins. Science 321: 964–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick P, Han MJ, An G. (2009) Auxin stimulates its own transport by shaping actin filaments. Plant Physiol 151: 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh B, Brière C, Bécard G, Dénarié J, Gough C. (2005) Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J 44: 195–207 [DOI] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2: a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6: 763–775 [DOI] [PubMed] [Google Scholar]
- Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M. (2009) Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA 106: 22540–22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski U, Kroken S, Roux C, Briggs SP. (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 99: 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Harrison MJ. (2009) Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiol 151: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Mondo SJ, Topp S, Starker CG, Gantt JS, Harrison MJ. (2010) Medicago truncatula Vapyrin is a novel protein required for arbuscular mycorrhizal symbiosis. Plant J 61: 482–494 [DOI] [PubMed] [Google Scholar]
- Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Basse CW. (2008) Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol Plant Pathol 9: 339–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner A, Calderon-Villalobos LI, Estelle M. (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5: 301–307 [DOI] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinöhl V, Friml J, Benková E. (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Kleine-Vehn J. (2011) AUXIN BINDING PROTEIN1: the outsider. Plant Cell 23: 2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Rueb S, Meijer AH. (2003) The RADICLELESS1 gene is required for vascular pattern formation in rice. Development 130: 645–658 [DOI] [PubMed] [Google Scholar]
- Shaul-Keinan O, Gadkar V, Ginzberg I, Grünzweig JM, Chet I, Elad Y, Wininger S, Belausov E, Eshed Y, Ben-Tal Y, et al. (2002) Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol 154: 501–507 [DOI] [PubMed] [Google Scholar]
- Smith SE, Smith FA. (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62: 227–250 [DOI] [PubMed] [Google Scholar]
- Song Y. (2014) Insight into the mode of action of 2,4-Dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56: 106–113 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M. (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli A, Trotta A, Acerbi L, Arcidiacono G, Berta G, Branca C. (2000) IAA and ZR content in leek (Allium porrum L.), as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant Soil 226: 29–35 [Google Scholar]
- Tranvan H, Habricot Y, Jeannette E, Gay G, Sotta B. (2000) Dynamics of symbiotic establishment between an IAA-overproducing mutant of the ectomycorrhizal fungus Hebeloma cylindrosporum and Pinus pinaster. Tree Physiol 20: 123–129 [DOI] [PubMed] [Google Scholar]
- Trouvelot A, Kough JL, Gianinazzi-Pearson V. (1986) Mesure du taux de mycorhization VA d’un syste`me radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. INRA Press, Paris [Google Scholar]
- Turner M, Nizampatnam NR, Baron M, Coppin S, Damodaran S, Adhikari S, Arunachalam SP, Yu O, Subramanian S. (2013) Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiol 162: 2042–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutiérrez RA. (2010) Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci USA 107: 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piché Y. (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64: 5004–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O. (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136: 669–687 [DOI] [PubMed] [Google Scholar]
- Xia K, Wang R, Ou X, Fang Z, Tian C, Duan J, Wang Y, Zhang M. (2012) OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS ONE 7: e30039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Nagawa S, Yang Z. (2011) Uniform auxin triggers the Rho GTPase-dependent formation of interdigitation patterns in pavement cells. Small GTPases 2: 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (2008) Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol 24: 551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Blaylock LA, Harrison MJ. (2010) Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell 22: 1483–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]