Abstract
New research reveals unexpected targeting signals in a transmembrane domain of plasma membrane water channels.
Most proteins in plant cells are synthesized on cytoplasmic ribosomes and later get transported to their proper place of action. For proteins that function in the endomembrane system, at the plasma membrane, or outside the cell, translation occurs at the endoplasmic reticulum, and the resulting proteins are later trafficked via vesicular transport from there to their final destination. Proper sorting requires specific targeting signals that are recognized by the transport machinery, the coat proteins, which deform the membrane during transport vesicle formation while at the same time selecting the proteins that will be included in the bounding membrane of the vesicle (Bassham et al., 2008; Brandizzi and Barlowe, 2013). Thus, it is not astonishing that several signals for efficient protein export from the endoplasmic reticulum have been identified in the (usually short) cytoplasmic tails of proteins, which allow them be recognized by coat protein complex II (COPII; Gillon et al., 2012). Similarly, proteins that need to be recycled back to the endoplasmic reticulum or an earlier Golgi compartment interact via sequences in their cytoplasmic tails with subunits of the COPI coat for inclusion in retrograde transport vesicles (Gao et al., 2012). While a number of targeting signals have been identified for the early secretory pathway in plants (Gao et al., 2014), virtually nothing is known about the mechanisms that target membrane proteins to the plasma membrane.
The situation is further complicated for polytopic membrane proteins that have several transmembrane domains, such as many membrane transporters. These proteins have several cytoplasmic loops that could harbor targeting signals and can take on complex conformations that may influence the amino acid residues that are accessible to the coat proteins. For example, aquaporins, also known as major intrinsic proteins, are membrane channels that mediate the flux of water or other small hydrophilic molecules through membranes by forming a selective channel in the center between their six transmembrane domains (Wallace and Roberts, 2004). Aquaporins are found in all extant organisms from bacteria to multicellular eukaryotes and adopt a highly conserved protein structure (Fu et al., 2000; Sui et al., 2001; Törnroth-Horsefield et al., 2006). They function as tetramers and, in plants, are found in different membranes, most prominently in the plasma membrane (plasma membrane intrinsic proteins [PIPs]) and the vacuolar membrane (tonoplast intrinsic proteins). The signals that allow their proper targeting have not been identified. Curiously, not all PIPs are efficiently transported to the plasma membrane (Chaumont et al., 2000), suggesting that these PIPs differ in their targeting sequences. For example, a diacidic (DxE: Asp-any amino acid-Glu) endoplasmic reticulum export signal at the N terminus of ZmPIP2;5 of maize (Zea mays) is not present in ZmPIP1;2 (Zelazny et al., 2009). In this issue, Chaumont and colleagues (Chevalier et al., 2014) take the analysis of aquaporin targeting to the next level by systematically swapping all loop regions between ZmPIP1;2 and ZmPIP2;5 in order to test for additional targeting signals. Curiously, none of the exchanges altered the behavior of the hybrid proteins, suggesting that the loop regions are not involved in targeting. A series of additional hybrid fusions instead revealed transmembrane domain 3 as the crucial determinant for plasma membrane targeting of ZmPIP2;5 and, more specifically, two amino acids within this transmembrane domain (Leu-127 and Ala-131).
This discovery presents a new paradigm for the plasma membrane targeting of membrane proteins, since it identifies residues that are buried within the membrane as essential for normal transport (Fig. 1). How these residues are recognized by the transport machinery is not clear at this time. A direct interaction with coat proteins seems unlikely, unless the residues are somehow accessible on the cytoplasmic face of the membrane. It is possible, however, that other (integral or peripheral) membrane proteins mediate an indirect interaction between ZmPIP2;5 and the coat proteins, analogous to the vacuolar protein sorting74 system in yeast (Saccharomyces cerevisiae; Tu et al., 2008). Alternatively, the altered residues on the outer surface of the aquaporin tetramer might interact with different membrane lipids, which in turn could influence the recruitment of coat proteins (Matsuoka et al., 1998). Of course, a conformational change of the helix packing that secondarily affects the presentation of other protein segments on the membrane surface cannot be ruled out either. It is also not clear where along the secretory pathway this signal is recognized, as the authors only tested for arrival of the mutant proteins at the plasma membrane. Identification of the punctate structures in which the Leu-127Phe/Ala-131Met double mutant accumulates could give an indication of where the signal functions. Finally, even though Leu-127 and Ala-131 are necessary for the plasma membrane targeting of ZmPIP2;5, they were not sufficient to mediate a similar localization in ZmPIP1;2, suggesting that there are other signals that retain ZmPIP1;2 within the cell. It will be interesting to see whether similar mechanisms are at play in the PIN family of auxin transporters, of which some are retained in the endoplasmic reticulum while others function at the plasma membrane (Mravec et al., 2009). Clearly, we do not have all the answers yet, but the systematic study by Chevalier et al. (2014) highlights the usefulness of aquaporins as an experimental system that can lead to the discovery of novel plasma membrane targeting signals.
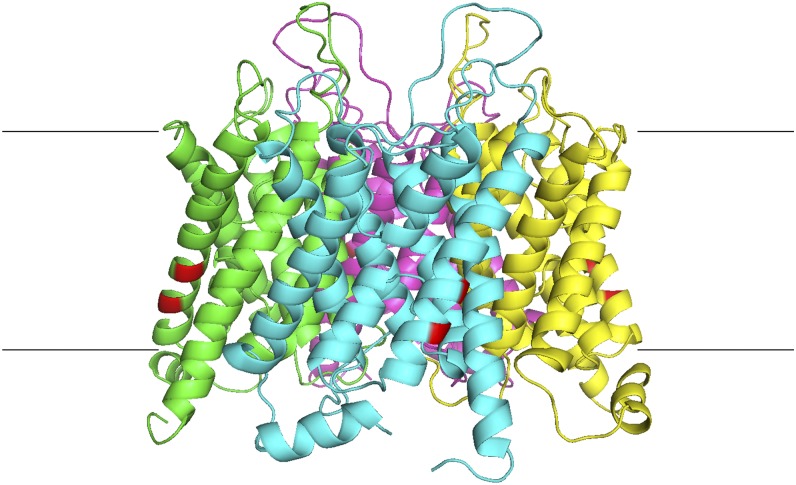
Figure 1.
Structure of the aquaporin tetramer with newly discovered targeting residues highlighted. The structure is based on bovine AQP1 (Protein Data Bank no. 1J4N; Sui et al., 2001). Individual aquaporin monomers are shown in different colors, and the residues involved in the plasma membrane targeting of ZmPIP2;5 are highlighted in red. Horizontal lines represent the approximate edges of the plasma membrane, with the cytoplasmic side at the bottom.
Acknowledgments
I thank David G. Robinson (University of Heidelberg) as well as Dan Roberts and Barry Bruce (University of Tennessee) for helpful comments.
References
- Bassham DC, Brandizzi F, Otegui MS, Sanderfoot AA. (2008) The secretory system of Arabidopsis. The Arabidopsis Book 6: e0116, /10.1199/tab.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Barlowe C. (2013) Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol 14: 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ. (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122: 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier AS, Bienert GP, Chaumont F. (2014) A new LxxxA motif in the transmembrane helix 3 of maize PIP2 aquaporins is required for their trafficking to the plasma membrane. Plant Physiol 166: 125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM. (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290: 481–486 [DOI] [PubMed] [Google Scholar]
- Gao C, Cai Y, Wang Y, Kang BH, Aniento F, Robinson DG, Jiang L. (2014) Retention mechanisms for ER and Golgi membrane proteins. Trends Plant Sci 19: 508–515 [DOI] [PubMed] [Google Scholar]
- Gao C, Yu CKY, Qu S, San MWY, Li KY, Lo SW, Jiang L. (2012) The Golgi-localized Arabidopsis endomembrane protein12 contains both endoplasmic reticulum export and Golgi retention signals at its C terminus. Plant Cell 24: 2086–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon AD, Latham CF, Miller EA. (2012) Vesicle-mediated ER export of proteins and lipids. Biochim Biophys Acta 1821: 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. (1998) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275 [DOI] [PubMed] [Google Scholar]
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Křeček P, Bielach A, Petrásek J, Zhang J, Gaykova V, Stierhof YD, et al. (2009) Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459: 1136–1140 [DOI] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414: 872–878 [DOI] [PubMed] [Google Scholar]
- Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. (2006) Structural mechanism of plant aquaporin gating. Nature 439: 688–694 [DOI] [PubMed] [Google Scholar]
- Tu L, Tai WCS, Chen L, Banfield DK. (2008) Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science 321: 404–407 [DOI] [PubMed] [Google Scholar]
- Wallace IS, Roberts DM. (2004) Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins: classification based on the aromatic/arginine selectivity filter. Plant Physiol 135: 1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F. (2009) An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J 57: 346–355 [DOI] [PubMed] [Google Scholar]