Sucrose enters the phloem in poplar leaves through plasmodesmata.
Abstract
Sap is driven through phloem sieve tubes by an osmotically generated pressure gradient between source and sink tissues. In many plants, source pressure results from thermodynamically active loading in which energy is used to transfer sucrose (Suc) from mesophyll cells to the phloem of leaf minor veins against a concentration gradient. However, in some species, almost all trees, correlative evidence suggests that sugar migrates passively through plasmodesmata from mesophyll cells into the sieve elements. The possibility of alternate loading mechanisms has important ramifications for the regulation of phloem transport and source-sink interactions. Here, we provide experimental evidence that, in gray poplar (Populus tremula × Populus alba), Suc enters the phloem through plasmodesmata. Transgenic plants were generated with yeast invertase in the cell walls to prevent Suc loading by this route. The constructs were driven either by the constitutive 35S promoter or the minor vein-specific galactinol synthase promoter. Transgenic plants grew at the same rate as the wild type without symptoms of loading inhibition, such as accumulation of carbohydrates or leaf chlorosis. Rates of photosynthesis were normal. In contrast, alfalfa (Medicago sativa) plants, which have limited numbers of plasmodesmata between mesophyll and phloem, displayed typical symptoms of loading inhibition when transformed with the same DNA constructs. The results are consistent with passive loading of Suc through plasmodesmata in poplar. We also noted defense-related symptoms in leaves of transgenic poplar when the plants were abruptly exposed to excessively high temperatures, adding to evidence that hexose is involved in triggering the hypersensitive response.
In the mid-1930s, several laboratories discovered that sugar concentrations are higher in the phloem than in mesophyll cells, where the sugar is synthesized (Crafts, 1961). These findings led to the concept of thermodynamically active phloem loading, in which Suc and other transport compounds are transferred into the sieve tubes against a concentration gradient. The idea was rapidly accepted, in part because it was consistent with the pressure flow hypothesis proposed earlier by Münch (1930). Münch (1930) had suggested that sap is propelled through the sieve tubes by a pressure gradient between the leaves (sources) and sinks (Patrick, 2012; De Schepper et al., 2013; Stroock et al., 2014), and because elevated solute levels increase hydrostatic pressure, it was reasonable to assume that the energy used to load the phloem generates the pressure at the source end of the transport stream needed to drive long-distance transport.
However, it is important to note that the hypothesis by Münch (1930) predated the discovery of active phloem loading. Münch (1930) assumed that the upstream pressure is generated in the mesophyll cells and not the phloem and that carbohydrate is carried passively from the mesophyll into the sieve tubes (Münch, 1930). The two hypotheses, active and passive loading, lead to different perspectives on several important aspects of phloem physiology, including the regulated entry of ionic and molecular species into the transport system and the mechanisms of source-sink signaling.
We provide evidence here that phloem loading of Suc in poplar (Populus tremula × Populus alba) is passive, as envisioned by Münch (1930). The reason for choosing poplar for study is that there is correlative evidence consistent with a passive loading mechanism in this species. First, the mesophyll cells and minor vein phloem of poplar are linked by plasmodesmata that are much more dense than those at the same interfaces in plants known to load through the apoplast (Russin and Evert, 1985). Second, the osmotic potential of the sieve element-companion cell complex in the minor veins, estimated by plasmolysis, is lower than commonly found in herbaceous plants and in the same range as that of the mesophyll cells (Russin and Evert, 1985). In species that load actively, the osmotic potential in the phloem is generally, but not always, well above that in the photosynthetic cells.
Although these data are suggestive, they are only correlative and for several reasons, inconclusive (see “Discussion”). In the studies reported here, we experimentally tested the hypothesis of passive loading in poplar by introducing yeast invertase to the apoplast of transgenic plants. Invertase in the cell walls inhibits apoplastic loading by hydrolyzing Suc en route to the phloem (von Schaewen et al., 1990; Dickinson et al., 1991; Heineke et al., 1992). For comparison, we conducted the same experiments on alfalfa (Medicago sativa), which on the basis of low plasmodesmata numbers in the minor vein phloem (Gamalei, 1991), loads from the apoplast. Invertase-expressing alfalfa exhibited well-documented symptoms of loading inhibition: elevated foliar sugar and starch, leaf chlorosis, and slow growth. In contrast, transgenic poplar grew normally and accumulated little, if any, excess sugar and starch in the leaves, and it did so even under high light conditions, where sugar synthesis is most active and the loading mechanism is most challenged. The results are consistent with passive, symplastic (through plasmodesmata) phloem loading in poplar.
RESULTS
Effects of Cell Wall Invertase on Alfalfa
In previous studies, it has been shown that the proteinase inhibitor II signal peptide fused to yeast invertase (PI-INV) targets the translated enzyme to cell walls (von Schaewen et al., 1990; Dickinson et al., 1991; Heineke et al., 1992). A construct of this nature, using the constitutive Cauliflower mosaic virus 35S promoter to drive expression (35S::PI-INV), was used to generate transgenic alfalfa plants.
Cell wall invertase enzyme activity was assayed using a simple leaf disc procedure. Leaf discs (4.0-mm diameter) were washed extensively in buffer to remove contamination from cut cells and then placed individually on 200-µL drops of Suc (100 mm). Hexose in the drops was measured 30 min later. Total invertase was also measured by conventional methods in leaf tissue ground to a fine powder in liquid nitrogen.
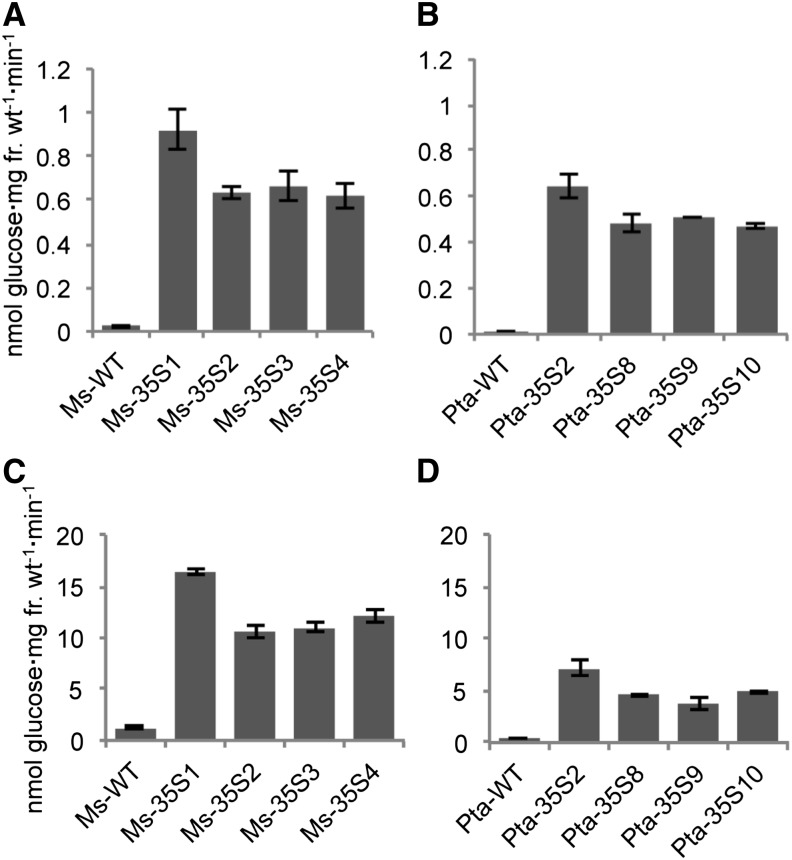
Of 35 alfalfa plants transformed with 35S::PI-INV, 17 plants had invertase activities higher than those measured in mature leaves of wild-type plants using both assay techniques (Fig. 1). The highest enzyme levels based on the leaf disc assay were approximately 42-fold greater than the wild type values (Fig. 1A). Alfalfa plants with the highest invertase levels in the leaf disc assay also had the highest total invertase levels (Fig. 1C), and both parameters correlated well with the amount of yeast invertase mRNA (Supplemental Fig. S1A). As expected if the transgenic invertase resides in the cell walls, the proportional increase in enzyme levels compared with the wild type was more pronounced in the leaf disc assay than in the assay measuring total invertase activity.
Figure 1.
Cell wall invertase activity in wild-type (WT) and transgenic (35S::PI-INV) alfalfa (A and C) and poplar (B and D) lines. Cell wall invertase in A and C is measured by the leaf disc assay. Total invertase activity in C and D is measured in pulverized leaf tissue. Error bars are sems (n = 10 in A and B; n = 3 in C and D). fr.wt, Fresh weight.
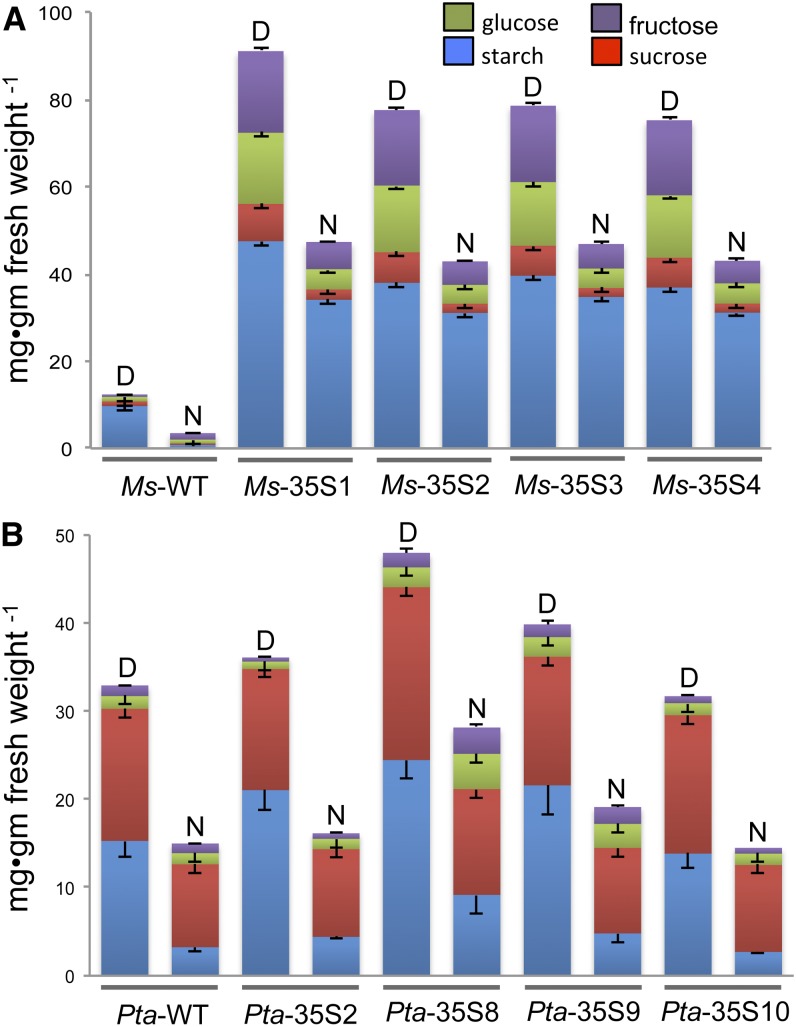
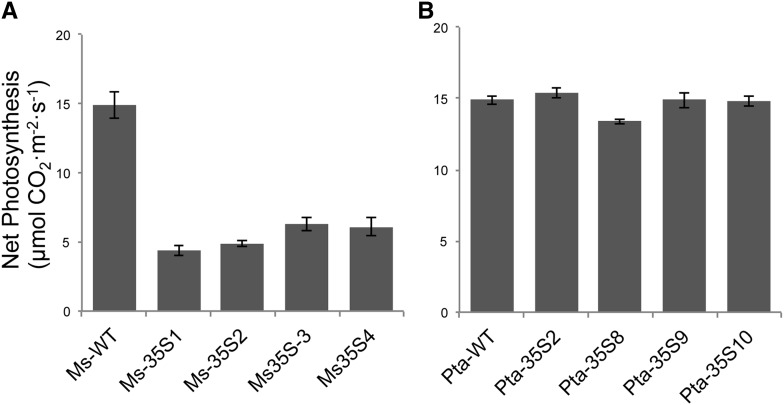
When grown in a greenhouse or a chamber under low (300 µmol photons m−2 s−1) or high (800 µmol photons m−2 s−1) light conditions, 13 of 35 transgenic alfalfa plants exhibited leaf chlorosis. Chlorosis is a phenotype associated with the inhibition of phloem loading. The alfalfa plants that did not develop chlorosis were those with the lowest invertase levels. As noted in other studies of this type (von Schaewen et al., 1990; Dickinson et al., 1991; Heineke et al., 1992), chlorosis developed basipetally in growing leaves in the same direction as the sink-source transition and became progressively more acute with leaf age. In transgenic alfalfa growing in a high light chamber (800 µmol photons m−2 s−1), the sugar and starch concentrations increased many fold compared with wild-type plants (Fig. 2A). Residual starch in leaves of alfalfa kept in the dark for 72 h was still visible after staining with iodine-potassium iodide (Fig. 3). Photosynthesis was also severely inhibited in the transgenic plants (Fig. 4A).
Figure 2.
NSCs in mature leaves of wild-type (WT) and transgenic (35S::PI-INV) alfalfa (A) and poplar (B) lines measured at the end of the day (D) and night (N). Error bars are sems (n = 3).
Figure 3.
Photographs (A and C) and starch staining (B and D) of mature wild-type (A and B) and transgenic (C and D; 35S:PI-INV;Ms-35S1) alfalfa leaves. The plants were placed in the dark at the end of the light period, and leaves were photographed and stained 72 h later. Transgenic leaves retain their starch, even after this prolonged darkness. Note the chlorosis at the edges of the leaves in C. Bars = 1 cm.
Figure 4.
Net photosynthesis in mature leaves of wild-type (WT) and transgenic (35S::PI-INV) lines of alfalfa (A) and poplar (B). Error bars are sems (n = 3).
Effects of Cell Wall Invertase on Poplar
Poplar leaf tissue was transformed with 35S::PI-INV, and regenerated plants were screened by reverse transcription-PCR and the leaf disc assay. Invertase levels were lower in wild-type poplar than in wild-type alfalfa. Of more than 200 independently transformed poplar plants, approximately 50% exhibited invertase levels greater than the levels detected in wild-type plants. As with alfalfa, enzyme activities were elevated as much as 42-fold in many of the transgenics measured either by the leaf disc assay (Fig. 1B) or in ground tissue (Fig. 1D). Increases in invertase activities correlated well with yeast invertase mRNA levels (Supplemental Fig. S1B). To determine the cell types in which the 35S promoter is active, we transformed additional plants with 35S::GUS. As in other species, the 35S promoter is constitutive in poplar leaves, with higher activity levels in the veins (Supplemental Fig. S2).
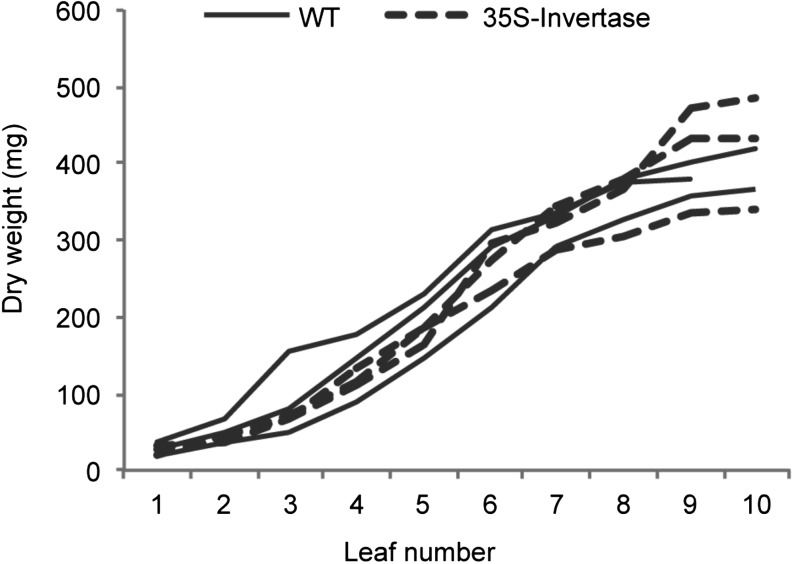
None of more than 200 poplar trees transformed with the 35S::PI-INV construct displayed evidence of leaf chlorosis when grown under stable conditions (see below), even in the greenhouse in the middle of the summer at photon flux densities exceeding 1,000 µmol photons m−2 s−1 in the middle of the day. For phenotypic characterization, four independent transgenic poplar lines with the highest levels of invertase activity were selected. There were no discernable differences in the overall morphology of the transgenic and wild-type plants. Heights were the same, and leaves reached the same dry weights over the same developmental periods (Fig. 5). Leaf initiation periods, determined by the leaf plastochron index (Erickson and Michelini, 1957), were the same (1.12 ± 0.07 [se; n = 4] and 1.14 ± 0.06 [se; n = 4] d/leaf) for wild-type and transgenic plants, respectively.
Figure 5.
Leaf dry weight in wild-type (WT; solid lines) and transgenic (35S::PI-INV; dashed lines) poplar plants. Leaf number 1 is the oldest leaf with a petiole shorter than 1.0 cm. Three wild-type and transgenic lines were used in the measurements.
Sugars and starch were measured in mature leaves to determine if these nonstructural carbohydrates (NSCs) accumulate in the transgenics (Fig. 2B). In contrast to alfalfa, where NSC concentrations were far higher in transgenics than the wild type, NSC levels in transgenic poplar lines did not differ substantially from those in wild-type plants. Although starch levels tended to be slightly higher than in the wild type in some lines at the end of the day, this was not always the case (e.g. line Pta-35S10), and by the end of the night, starch in all lines except Pta-35S8 declined to the levels measured in wild-type plants. The rate of photosynthesis was the same in wild-type and transgenic poplar lines, except Pta-35S8, in which the rate was 10% lower than in wild-type leaves of the same age (Fig. 4B). In Pta-35S9 and Pta-35S10, two transgenic lines with similar levels of invertase activity as Pta-35S8, both photosynthesis and carbohydrate content were comparable with the wild type (Figs. 2B and 4B). Note the substantially higher concentration of foliar Suc in wild-type poplar (Fig. 2B) compared with wild-type alfalfa (Fig. 2A). In poplar, the difference in NSC at the end of the day and night is almost entirely accounted for by starch, whereas Suc concentrations remain almost constant over the 24-h period.
To determine whether overexpression of apoplastic invertase alters the composition of sugars transported in the phloem, mature leaves were exposed to 14CO2 for 15 min, and 45 min later, the petioles were collected for analysis. 14C-hexose proportions in the petioles were slightly elevated in the transgenics, but approximately 85% to 90% of the radiolabel was in the form of [14C]Suc (Supplemental Fig. S3).
To test the effect of introducing cell wall invertase directly to the companion cells of minor veins, additional poplar plants were transformed with the PI-INV gene fusion, which was driven in this case by the melon galactinol synthase promoter (CmGAS::PI-INV). The CmGAS promoter activates expression specifically in minor veins in the species that have been tested, and this is also the pattern in poplar (Slewinski et al., 2013). Two lines (Pta-GAS5 and Pta-GAS8) were determined to have significantly higher activities of apoplastic invertase than wild-type poplar. Invertase activities and mRNA levels in these lines were considerably lower than in plants transformed with the 35S-driven construct, which was expected given the restriction of the CmGAS promoter activity to minor vein phloem that constitutes approximately 1% of leaf tissue. Nonetheless, these levels were consistently higher than in the leaves of wild-type plants (Supplemental Fig. S4A). In these lines, there was no evidence of leaf chlorosis or a reduction in growth rate, and starch disappeared completely by the end of the night (Supplemental Fig. S5E). Rates of photosynthesis were indistinguishable from those of wild-type plants (Supplemental Fig. S4B). NSCs did not accumulate; indeed, Suc levels in Pta-GAS5 leaves (those with the highest invertase levels) were significantly lower than in wild-type plants at both the end of the day and the end of the night (Supplemental Fig. S4C).
Because the invertase levels in CmGAS::PI-INV plants were considerably lower than in those in which expression was driven by the 35S promoter, we transformed tobacco (Nicotiana tabacum), known to load apoplastically (von Schaewen et al., 1990), with the CmGAS::PI-INV construct. Again, cell wall invertase levels were only 2 to 4 times higher than background, comparable with the overexpression in poplar, but the transgenic tobacco plants displayed clear indications of transport inhibition, including chlorosis and carbohydrate overaccumulation in leaves (Supplemental Fig. S5B).
Hypersensitivity of Transgenic Poplar to High Temperature
As described above, transgenic poplar plants with the highest levels of apoplastic invertase grew normally, even under high photon flux densities in the greenhouse in the middle of the summer. However, on one occasion, we noted the appearance of severe chlorotic symptoms in the leaves of 35S::PI-INV transgenic but not wild-type or CmGAS::PI-INV transgenic poplar 2 d after a period of extreme greenhouse temperatures exceeding 40°C in the middle of the day. This chlorosis did not develop in the basipetal direction in maturing leaves. Rather, it appeared uniformly and rapidly in both immature and mature leaves. In subsequent control experiments, we were able to induce sudden necrosis in 35S::PI-INV transgenic but not wild-type or CmGAS::PI-INV transgenic plants by transferring them between growth chambers held at 28°C/23°C and 40°C/35°C day-night cycles. The leaves developed necrotic lesions within 2 d. When the temperature was lowered to a 28°C/23°C day-night cycle and held constant, the new leaves that developed in that temperature regime were normal in appearance.
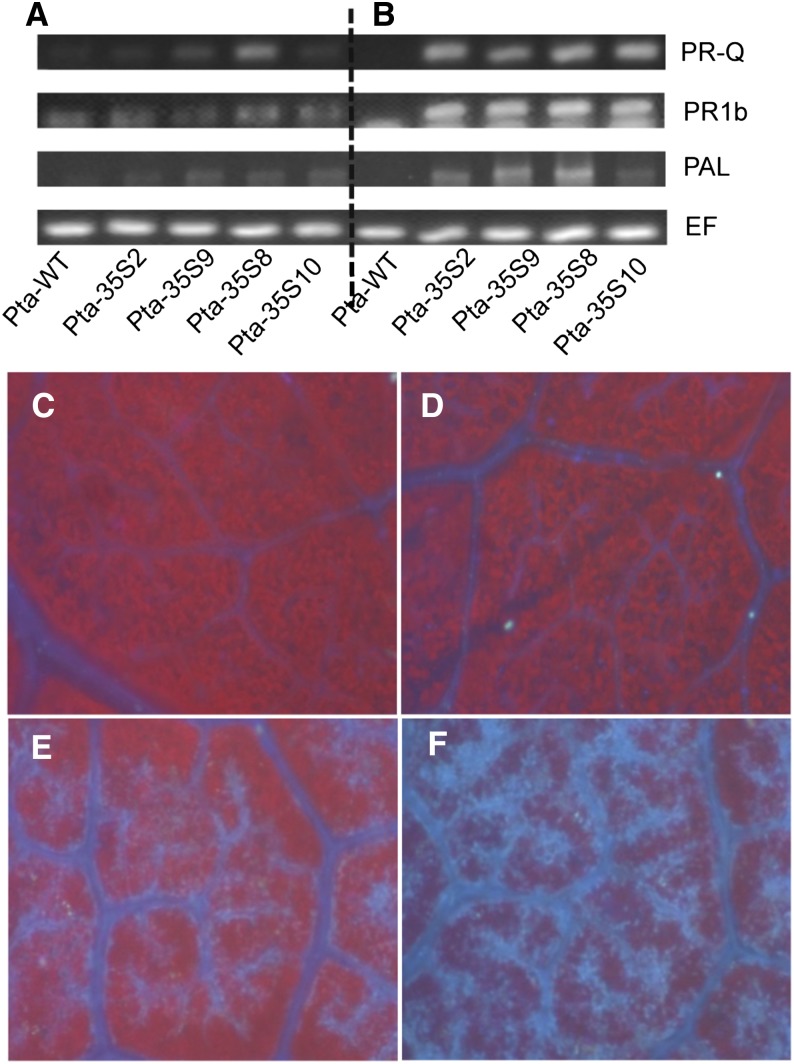
These observations suggested to us that the transgenic plants had suffered from the activation of defense-related responses as described by Herbers et al. (1996) in invertase-expressing tobacco. Other studies have also shown that constitutive expression of apoplast-localized invertase may activate the defense status of the plant (von Schaewen et al., 1990; Roitsch and González, 2004; Berger et al., 2007). To test this hypothesis, we analyzed the expression of three pathogenesis-related (PR) genes (PR-Q, PR-1b, and Phe ammonia lyase [PAL]) in leaf tissue harvested from plants growing under high temperature or normal temperature in both wild-type and transgenic plants (Fig. 6, A and B). The PR genes were expressed in all transgenic plants, even those growing under stable conditions (28°C/23°C day-night cycle), but expression was considerably higher in the leaves of plants exposed to an abrupt transfer to high temperature (40°C/35°C day-night cycle).
Figure 6.
Responses of wild-type and transgenic (35S::PI-INV) poplar to elevated temperatures. A and B, Expression of defense-related genes in leaves of the wild type (Pta-WT) and four transgenic lines. Plants were grown at a 28°C/23°C day-night cycle and then exposed for 4 d to either the same temperature regime (A) or a40°C/35°C day-night cycle (B). C–F, Callose staining in leaf tissue of the wild type (C and D) and the transgenic line Pta-35S2 (E and F). Plants were grown at a 28°C/23°C day-night cycle and then exposed for 4 d to either the same temperature regime (C and E) or a 40°C/35°C day-night cycle (D and F). Tissue is stained for callose (blue) against a background of chlorophyll fluorescence (red). PR-Q, PR-1b, and PAL are defense-related genes (in the text). EF, Elongation factor.
Because callose accumulates in various tissues of plants under stress, we stained leaf tissue of plants grown under different conditions with the callose-specific stain aniline blue. Callose staining was stronger in veins of 35S::PI-INV transgenic plants (Fig. 6E) than in wild-type plants (Fig. 6C) when grown under stable conditions (28°C/23°C day-night cycle). Four days after transfer to 40°C/35°C day-night cycle, staining was increased (Fig. 6, D and F), especially in the transgenic tissue (Fig. 6F).
DISCUSSION
Thermodynamically active Suc loading by the phloem can take two forms: one apoplastic and the other symplastic. In apoplastic loading, a proton gradient drives Suc from the cell wall space into the minor vein phloem through transporter proteins located on the plasma membranes (Patrick, 2012; De Schepper et al., 2013; Stroock et al., 2014). In polymer trapping, Suc is hydrolyzed in the companion cells, allowing it to enter the phloem through plasmodesmata down its concentration gradient; the synthesis of larger molecules, raffinose and stachyose, prevents backflow into the mesophyll (De Schepper et al., 2013). Polymer trapping is also an active loading mechanism in the sense that energy used to synthesize raffinose and stachyose elevates phloem sugar concentrations to levels above those in the mesophyll.
On the basis of plasmodesmatal numbers, it is reasonable to postulate a symplastic phloem loading pathway in poplar (Russin and Evert, 1985). However, poplar does not use the polymer trap mechanism; it transports raffinose and stachyose but in much lower concentrations relative to Suc than polymer trap species (Zimmermann and Ziegler, 1975). Also, the plasmodesmata linking the mesophyll to the phloem, although numerous, do not have the characteristic asymmetric branching pattern seen in the cucurbits and other plants that load by polymer trapping (Russin and Evert, 1985).
If poplar loads symplastically but does not have a trapping mechanism, it is reasonable to postulate that the process is passive in the sense that Suc diffuses or flows through plasmodesmata into the phloem without a concentration step. This hypothesis is supported by plasmolysis data indicating that the osmotic potentials of the mesophyll and the phloem are approximately the same (500–600 mosmol L−1; Russin and Evert, 1985). In active phloem loaders, the osmotic potential of the minor vein phloem approaches or exceeds 1000 mosmol L−1 (Beebe and Evert, 1992). A passive loading mechanism, driven by high Suc levels, is also consistent with the much greater standing concentration of Suc in poplar leaves than alfalfa (Fig. 2), a concentration that changes little throughout the day and night.
However, these data are correlative only; they do not prove that loading is passive. The physical presence of plasmodesmata does not necessarily mean that they are open to the passage of Suc, because plasmodesmata are highly regulated and, in some circumstances, closed to small molecules (Maule et al., 2011). Also, although the osmotic potentials of the mesophyll cells and the minor vein phloem cells are approximately the same in poplar, this does not rule out active loading. There could still be an uphill concentration gradient of Suc between the mesophyll cell cytosol and the phloem that is not detected by the plasmolysis technique, which only measures total osmotic potential. Even if the Suc concentration gradient is shown to be downhill into the phloem, this does not necessarily rule out active loading, which can take place no matter the concentration on the proximal side of the transporter.
Apoplastic loading has to be ruled out by experimentation. One strategy that has been used to test the apoplastic loading hypothesis is to down-regulate or eliminate Suc transporter function, which results in symptoms of transport inhibition, including hyperaccumulation of carbohydrate, foliar chlorosis, and slow growth (Riesmeier et al., 1994; Kühn et al., 1996; Bürkle et al., 1998; Gottwald et al., 2000; Hackel et al., 2006; Slewinski et al., 2009; Srivasava et al., 2009). The difficulty in using this strategy in poplar is that six Suc transporters have been identified in the poplar genome, and their sites of expression and the functions of the proteins are only partially understood (Payyavula et al., 2011; Mahboubi et al., 2013). To show convincingly that transporters are not directly involved in loading, it would be necessary to down-regulate all of them simultaneously to avoid the possibility of redundancy. Given that down-regulating even the vacuolar transporter Suc transporter4 (SUT4) results in the reduction of Suc export in source leaves (Payyavula et al., 2011), it seems very likely that this approach would result in pleotropic effects.
Another strategy that has been used to test the apoplastic loading model is to express invertase in the apoplast. In the presence of invertase, Suc is hydrolyzed as it crosses the cell walls, depriving the Suc transporter of its substrate. This experimental approach has been used in tobacco, potato (Solanum tuberosum), and tomato (Solanum lycopersicum), and in each case the plants showed obvious symptoms of transport inhibition as described above (von Schaewen et al., 1990; Dickinson et al., 1991; Heineke et al., 1992).
We used the invertase strategy to test the concept of apoplastic loading in alfalfa and poplar using the same construct described in the above studies in tobacco, potato, and tomato. Yeast invertase, which is active over a broad pH range, was targeted to the apoplast by a potato proteinase inhibitor II signal peptide.
We included alfalfa as a control, because it is a putative apoplastic loader based on limited symplastic continuity between the mesophyll and minor vein phloem (Gamalei, 1991). Studying alfalfa also extends the range of plant families analyzed by the invertase strategy beyond the two families (Solanaceae spp. and Brassicaceae spp.) used to date. As expected, transformed alfalfa plants expressing yeast invertase in the apoplast suffered classical symptoms of loading inhibition.
Strikingly, phloem loading was not inhibited in transgenic poplar plants, even those with the highest levels of yeast invertase. Two lines of evidence indicate that yeast invertase activity was restricted to the cell walls of transgenic poplar. First, exogenous Suc was readily hydrolyzed to hexose when leaf discs were floated on Suc solution (Fig. 1). Second, if the exogenous Suc provided in the leaf disc assay was actually cleaved inside the leaf cells to come back out as hexose, then the endogenous Suc in the cells, present in high concentrations, should do the same. However, almost no hexose was detected in the leaf disc assay unless exogenous Suc was provided.
As long as the transgenic poplar plants were kept in stable environmental conditions, they grew normally, even in high light, with little or no evidence of abnormal carbohydrate accumulation and without chlorosis. Photosynthesis was not inhibited. The same results were obtained whether the construct was driven by the constitutive 35S or the CmGAS promoter. We included experiments using the GAS promoter to be certain that cell wall invertase is not inhibited specifically in the minor vein phloem, a conclusion that is difficult to make with constructs driven by the constitutive 35S promoter. Enzyme activity was detected in the leaf disc assay, but this activity did not result in inhibition of phloem loading. A similar up-regulation of cell wall invertase activity in tobacco minor vein phloem using the same construct did inhibit loading.
It has been reported that overexpression of yeast invertase in the companion cell cytosol of potato results in the synthesis and transport of a novel trisaccharide, 6-kestose (Zuther et al., 2004). To test whether overexpression of cell wall invertase modifies the transport sugars in poplar, we analyzed sugars extracted from the petioles of leaves exposed to 14CO2. No 6-kestose was identified, and the experiment confirmed that Suc is still the major transported sugar in the transgenic poplar.
Although the transgenic poplar plants grew normally, they nonetheless reacted in certain ways to the overexpression of invertase. This is to be expected, because any transgenic modification has pleotropic potential. When we provided 14CO2 to leaves, we noted more 14C-hexose in the leaf blades and petioles of transgenic plants than the wild type. Although it is difficult to trace the origin of the additional radiolabeled hexose, it could have resulted from hydrolysis of [14C]Suc in the apoplast. Suc is constantly being leaked into and retrieved from the apoplast (Srivastava et al., 2008), and it is presumably cleaved to hexoses when it encounters the abundant invertase present in the cell walls of the transgenics.
The other more profound effect of invertase overexpression is that the transgenic plants express defense-related genes to a limited extent and are primed to react to adverse environmental conditions by further up-regulating these genes accompanied by severe leaf chlorosis and necrosis. In apoplastic loaders, no such environmental shock is required: chlorosis is induced under even mild and stable conditions. Herbers et al. (1996) ascribed this response in tobacco to the activation of defense-related genes by hexose sensing. Indeed, invertase has long been implicated in plant-pathogen interactions (Keunen et al., 2013). Subsequent to the article by Herbers et al. (1996), Essmann et al. (2008) asked whether cell wall invertase “is of any significance for successful plant defense in cases where the apoplastic system is not involved in the allocation of carbohydrates” (Essmann et al., 2008). Our work with poplar provides an answer to that question: cell wall invertase is still part of the defense response in symplastic loaders, but the plants do not react as readily as apoplastic loaders. Presumably, some Suc leaks to the apoplast from the phloem and other cells, which it does in all plants (see above). In transgenic poplar, this leads to a minor up-regulation of defense-related genes and limited callose deposition in veins, although loading is unimpaired. Although the plants appear normal, they are poised on the edge of the hypersensitive response and can be driven to it by an external shock, such as sudden exposure to high temperature. It is possible that a high-temperature shock causes leaf chlorosis by inducing the deposition of callose, which in turn, blocks phloem transport, but this hypothesis will require additional testing.
In conclusion, the expression of cell wall invertase in poplar impacts sugar metabolism, at least to a minimal extent, and primes defense responses, but the plants grow normally, with no measurable effects on photosynthesis or phloem transport. The results, especially when considered against the backdrop of abundant plasmodesmatal connections between cells and similar osmotic potential along the loading pathway, are incompatible with an apoplastic mode of phloem loading. The data support the concept that, in poplar, Suc migrates passively through plasmodesmata from the mesophyll cells to the minor vein sieve tubes.
MATERIALS AND METHODS
Plant Materials
Wild-type and transgenic hybrid poplar (Populus tremula × Populus alba) and alfalfa (Medicago sativa) plants were grown in either a greenhouse at 800 to 1,400 μmol photons m−2 s−1 or a growth chamber on 12-h/12-h day-night cycles under two light conditions: low light (approximately 350 μmol photons m−2 s−1) or high light (approximately 800 μmol photons m−2 s−1) with day-night temperatures of 28°C/23°C. Individual poplar or alfalfa plants were propagated by cuttings to make clonal plants used as technical replicates.
Apoplastic Invertase Vector Construction and Plant Transformation
All plasmid constructions were by standard procedures. Enzymes and reagents were from New England Biolabs, Invitrogen, and TaKaRa and used according to the manufacturers' instructions. The Cornell BioResource Center performed sequencing reactions. Detailed procedures for construction of the GAS-PI-INV and 35S-PI-INV plasmids are in Supplemental Text S1. These constructs were used for plant transformation to generate plants with elevated invertase in the cell wall area of poplar and alfalfa. Poplar transformation followed the procedure in Han et al., 2000. Alfalfa transformation followed the procedure in Austin et al., 1995.
Invertase mRNA and Activity Measurement
To measure the mRNA expression of exogenous apoplast yeast invertase, three samples were collected from each line for both wild-type and transgenic alfalfa and poplar. Primers used for quantitative analysis are listed in Supplemental Table S1.
Total invertase activity in ground tissue was measured by standard methodology (Dickinson et al., 1991). Liberated hexose was measured by the bicinchoninate assay (Waffenschmidt and Jaenicke, 1987). To measure cell wall invertase activity, 10 leaf discs (4.0-mm diameter) were randomly punched from mature leaf tissue with a cork borer, avoiding major veins. The discs were washed three times for a total of 10 min in sodium acetate buffer (100 mm) at pH 5.0 to remove the contents of damaged cells and placed individually on 200-µL drops of Suc (100 mm) in the same buffer. Hexose was measured by the bicinchoninate assay after 30 min of incubation in a shaker (37°C) at 50 rpm.
Photosynthesis Measurement, Sugar Analysis, and Starch Staining
A LI-COR 6400 (LI-COR Biotechnology) was used to analyze CO2 uptake in the first three fully expanded leaves of each plant at 800 μmol m−2 s−1 with a CO2 concentration of 360 μmol m−2 s−1. Sugars and starch were analyzed as in Li et al., 2012. To stain the starch in the leaves of alfalfa and poplar, the harvested leaves were cleared in 70% (v/v) ethanol and stained by Lugol’s iodine.
Semiquantitative Analysis of the Expression of PR Gene and Callose Staining
To analyze the expression of PR genes (i.e. PR-Q, PR-1B, and PAL), total RNA from leaves was extracted from both wild-type and transgenic poplar plants growing in a growth chamber with the constant growth condition described as above or in a greenhouse. The primers used for quantification are listed in Supplemental Table S2. Callose staining followed the procedure in Slewinski et al., 2012.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Semiquantitative gene expression analysis of yeast invertase in wild-type and transgenic alfalfa and poplar.
Supplemental Figure S2. GUS staining driven by the 35S promoter in wild-type and transgenic poplar.
Supplemental Figure S3. Distribution of radiolabel in different sugars in the leaf blades and petioles of wild-type and transgenic poplar after exposure of the leaf blades to 14CO2.
Supplemental Figure S4. Cell wall invertase, photosynthesis, and sugar concentrations in leaves of poplar plants transformed with the CmGAS::PI-INV construct.
Supplemental Figure S5. Starch-stained leaves of tobacco and poplar plants transformed with the CmGAS::PI-INV construct at the end of the day and night.
Supplemental Table S1. Primers used for the quantification of yeast invertase gene expression in transgenic alfalfa and poplar.
Supplemental Table S2. Primers used for the quantification of the expression of defense-related genes.
Supplemental Text S1. Construction of the GAS-PI-INV and 35S-PI-INV plasmids.
Acknowledgments
We thank Caiping Ma for providing poplar explants, Dr. Lailiang Cheng for critical discussions, and Dr. André Jagendorf and Xin Li for technical assistance.
Glossary
- NSC
nonstructural carbohydrate
- PAL
Phe ammonia lyase
- PR
pathogenesis-related
Footnotes
This work was supported by the U.S. Department of Agriculture-National Institute of Food and Agriculture (postdoctoral fellowship no. 2011–67012030774 to T.L.S.) and the National Science Foundation (grant no. IOS–1121254 to R.T.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Austin S, Bingham ET, Mathews DE, Shahan MN, Will J, Burgess RR. (1995) Production and field performance of transgenic alfalfa (Medicago sativa L.) expressing alpha-amylase and manganese-dependent lignin peroxidase. Euphytica 85: 381–393 [Google Scholar]
- Beebe DU, Evert RF. (1992) Photoassimilate pathway(s) and phloem loading in the leaf of Moricandia arvensis (L.) DC. (Brassicaceae). Int J Plant Sci 153: 61–77 [Google Scholar]
- Berger S, Sinha AK, Roitsch T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 58: 4019–4026 [DOI] [PubMed] [Google Scholar]
- Bürkle L, Hibberd JM, Quick WP, Kühn C, Hirner B, Frommer WB. (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts AS. (1961) Translocation in Plants. Holt, Rinehart and Winston, New York [Google Scholar]
- De Schepper V, De Swaef T, Bauweraerts I, Steppe K. (2013) Phloem transport: a review of mechanisms and controls. J Exp Bot 64: 4839–4850 [DOI] [PubMed] [Google Scholar]
- Dickinson CD, Altabella T, Chrispeels MJ. (1991) Slow-growth phenotype of transgenic tomato expressing apoplastic invertase. Plant Physiol 95: 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RO, Michelini FJ. (1957) The plastochron index. Am J Bot 44: 297–305 [Google Scholar]
- Essmann J, Schmitz-Thom I, Schön H, Sonnewald S, Weis E, Scharte J. (2008) RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol 147: 1288–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalei Y. (1991) Phloem loading and its development related to plant evolution from trees to herbs. Trees (Berl West) 5: 50–64 [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C. (2006) Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 45: 180–192 [DOI] [PubMed] [Google Scholar]
- Han KH, Meilan R, Ma C, Strauss SH. (2000) An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids (genus Populus). Plant Cell Rep 19: 315–320 [DOI] [PubMed] [Google Scholar]
- Heineke D, Sonnewald U, Büssis D, Günter G, Leidreiter K, Wilke I, Raschke K, Willmitzer L, Heldt HW. (1992) Apoplastic expression of yeast-derived invertase in potato: effects on photosynthesis, leaf solute composition, water relations, and tuber composition. Plant Physiol 100: 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Metraux JP, Sonnewald U. (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen E, Peshev D, Vangronsveld J, VAN DEN Ende W, Cuypers A. (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36: 1242–1255 [DOI] [PubMed] [Google Scholar]
- Kühn C, Quick WP, Schulz A, Riesmeier JW, Sonnewald U, Frommer WB. (1996) Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ 19: 1115–1123 [Google Scholar]
- Li M, Feng F, Cheng L. (2012) Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 7: e33055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboubi A, Ratke C, Gorzsás A, Kumar M, Mellerowicz EJ, Niittylä T. (2013) Aspen SUCROSE TRANSPORTER3 allocates carbon into wood fibers. Plant Physiol 163: 1729–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule AJ, Benitez-Alfonso Y, Faulkner C. (2011) Plasmodesmata-membrane tunnels with attitude. Curr Opin Plant Biol 14: 683–690 [DOI] [PubMed] [Google Scholar]
- Münch E. (1930) Die Stoffbewegungen in der Pflanze. Gustav Fischer, Jena, Germany [Google Scholar]
- Patrick JW. (2012) Fundamentals of phloem transport physiology. In Thompson GA, van Bel AJE, eds, Phloem: Molecular Cell Biology, Systemic Communication, Biotic Interactions. John Wiley and Sons, New York, pp 30–60 [Google Scholar]
- Payyavula RS, Tay KH, Tsai CJ, Harding SA. (2011) The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J 65: 757–770 [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, González MC. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9: 606–613 [DOI] [PubMed] [Google Scholar]
- Russin WA, Evert RF. (1985) Studies on the leaf of Populus deltoides (Salicaceae): ultrastructure, plasmodesmatal frequency, and solute concentrations. Am J Bot 72: 1232–1247 [Google Scholar]
- Slewinski TL, Baker RF, Stubert A, Braun DM. (2012) Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves. Plant Physiol 160: 1540–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL, Meeley R, Braun DM. (2009) Sucrose transporter1 functions in phloem loading in maize leaves. J Exp Bot 60: 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL, Zhang C, Turgeon R. (2013) Structural and functional heterogeneity in phloem loading and transport. Front Plant Sci 4: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AC, Dasgupta K, Ajieren E, Costilla G, McGarry RC, Ayre BG. (2009) Arabidopsis plants harboring a mutation in AtSUC2, encodong the predominant sucrose/proton symporter necessary for efficient phloem tansport, are able to complete their life cycle and produce viable seed. Anal Bot 104: 1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG. (2008) Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol 148: 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroock AD, Pagay VV, Zwieniecki MA, Holbrook NM. (2014) The physicochemical hydrodynamics of vascular plants. Annu Rev Fluid Mech 46: 615–642 [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L. (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waffenschmidt S, Jaenicke L. (1987) Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal Biochem 165: 337–340 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Ziegler H. (1975) List of sugars and sugar alcohols in sieve-tube exudates. In Zimmermann MH, Milburn JA, eds, Encyclopedia of Plant Physiology, Vol 1 Transport in Plants 1: Phloem Transport. Springer, New York, pp 480–503 [Google Scholar]
- Zuther E, Kwart M, Willmitzer L, Heyer AG. (2004) Expression of a yeast-derived invertase in companion cells results in long-distance transport of a trisaccharide in an apoplastic loader and influences sucrose transport. Planta 218: 759–766 [DOI] [PubMed] [Google Scholar]