Protein-protein interaction studies of R2R3 MYB transcription factors regulating glucosinolate biosynthesis and the analysis of multiple loss-of-function mutants and gain-of-function alleles demonstrated the specific role of an associated transcription factor complex in the transcriptional regulation of glucosinolate biosynthesis.
Abstract
By means of yeast (Saccharomyces cerevisiae) two-hybrid screening, we identified basic helix-loop-helix transcription factor05 (bHLH05) as an interacting partner of MYB51, the key regulator of indolic glucosinolates (GSLs) in Arabidopsis (Arabidopsis thaliana). Furthermore, we show that bHLH04, bHLH05, and bHLH06/MYC2 also interact with other R2R3-MYBs regulating GSL biosynthesis. Analysis of bhlh loss-of-function mutants revealed that the single bhlh mutants retained GSL levels that were similar to those in wild-type plants, whereas the triple bhlh04/05/06 mutant was depleted in the production of GSL. Unlike bhlh04/06 and bhlh05/06 mutants, the double bhlh04/05 mutant was strongly affected in the production of GSL, pointing to a special role of bHLH04 and bHLH05 in the control of GSL levels in the absence of jasmonic acid. The combination of two specific gain-of-function alleles of MYB and bHLH proteins had an additive effect on GSL levels, as demonstrated by the analysis of the double MYB34-1D bHLH05D94N mutant, which produces 20-fold more indolic GSLs than bHLH05D94N and ecotype Columbia-0 of Arabidopsis. The amino acid substitution D94N in bHLH05D94N negatively affects the interaction with JASMONATE-ZIM DOMAIN protein, thereby resulting in constitutive activation of bHLH05 and mimicking jasmonic acid treatment. Our study revealed the bHLH04, bHLH05, and bHLH06/MYC2 factors as novel regulators of GSL biosynthesis in Arabidopsis.
Plants produce an enormous variety of secondary metabolites that are known as defense products against microbes, fungi, or herbivores, but also serve as signaling molecules in plant-plant, plant-animal, and plant-microbe interactions. Approximately 200,000 to 1,000,000 metabolites have been predicted to exist in plants (Dixon and Strack, 2003; Wink, 2010; Mohanta, 2013), and tens of thousands have already been described, including 200 glucosinolates (GSLs; Clarke, 2010). The high natural diversity of gene products provides a high variability in secondary metabolites and their functions. However, the metabolic richness derives not only from the number of genes present (about 25,500 in Arabidopsis [Arabidopsis thaliana]), but also from multiple substrate specificities of enzymes (Schwab, 2003) and importantly, from the plethora of possibilities to specifically regulate these compounds in particular tissues under certain conditions. However, only a limited number of genes involved in the regulation of plant secondary metabolites (Broun, 2004; Grotewold, 2008; Wu and Chappell, 2008) and GSLs (Sønderby et al., 2010a) have been identified to date.
GSLs are known for their role in plant-herbivore interactions and for their cancer-preventive properties, which has intensified research on GSLs. Together with flavonoids (Lepiniec et al., 2006; Gonzalez et al., 2008; Li, 2014; Xu et al., 2014), GSLs have become model secondary metabolites (Sønderby et al., 2010b) in the field of natural plant products. The availability of genetic sequence information, large mutant collections, quantitative trait loci, and expression profiling tools have facilitated the identification of biosynthetic enzymes of GSLs (Supplemental Fig. S1) and led to the intensive characterization of a large number of genes for the production of GSL in Arabidopsis. However, knowledge concerning the factors that regulate GSL levels is scarce. For example, this is the case for the proteins controlling the levels of different GSLs in different plant tissues, which modulate their biosynthesis, transport, or breakdown in response to different biotic and abiotic factors. Therefore, understanding the regulatory mechanisms that modulate GSL concentrations in plants remains of great interest in plant science.
The network of proteins that controls the production of GSLs was recently characterized and comprises six R2R3 MYB transcription factors (TFs) belonging to subgroup 12 of the R2R3 MYB family, having a conserved [L/F]LN[K/R]VA motif (Stracke et al., 2001; Dubos et al., 2010). The MYB34, MYB51, and MYB122 proteins are involved in the transcriptional regulation of the Trp-derived (indole) GSL pathway (Celenza et al., 2005; Gigolashvili et al., 2007a; Malitsky et al., 2008; Frerigmann and Gigolashvili, 2014), and MYB28, MYB29, and MYB76 regulate the Met-derived (aliphatic) GSLs in Arabidopsis (Hirai et al., 2007; Sønderby et al., 2007, 2010a; Gigolashvili et al., 2007b, 2008; Beekwilder et al., 2008; Malitsky et al., 2008; Li et al., 2013). Only a few other nuclear-localized components have been reported, which affect the biosynthesis of GSL in Arabidopsis, but have additionally broader functions. These include IQ-DOMAIN1, DNA-BINDING-WITH-ONE-FINGER1.1, SULFUR LIMITATION1, and TERMINAL FLOWER2. The IQ-DOMAIN1 protein is a nuclear-localized calmodulin-binding protein and a positive regulator of GSL that was identified in a screen for mutants with increased GSL accumulation (Levy et al., 2005; Abel et al., 2013). The DNA-BINDING-WITH-ONE-FINGER1.1 protein (also known as OBP2) is a component of the regulatory network controlling GSL biosynthesis, which induces the transcription of at least cytochrome P450 CYP83B1 (Skirycz et al., 2006). The SULFUR LIMITATION1 protein represses the biosynthesis of GSLs and activates enzymes catabolizing GSL in response to sulfate deficiency. Finally, TERMINAL FLOWER2 (also known as TU8), which is involved in controlling heterochromatin structure (Kim et al., 2004; Bennett et al., 2005), affects the accumulation of GSL, but is also important in flowering, meristem formation, and leaf morphology. However, both qualitative and quantitative fluctuations in the accumulation of GSLs in different tissues during plant development (Petersen et al., 2002; Brown et al., 2003) or in response to environmental stimuli (Bodnaryk, 1992, 1994; Kiddle et al., 1994; Doughty et al., 1995; Brader et al., 2001; León et al., 2001; Pontoppidan, 2001; Kliebenstein et al., 2002; Mikkelsen et al., 2003; Farnham et al., 2004; Lorenzo et al., 2004; Mewis et al., 2005; Dombrecht et al., 2007; Yan and Chen, 2007; Erb et al., 2011; Verhage et al., 2011; Guo et al., 2013a) cannot be fully explained by activities of MYB or other nuclear-localized factors and more components of the GSL regulatory network are anticipated to exist.
Combinatorial interactions among TFs are central to the gene regulation of any given cellular process (Feller et al., 2011). Combinatorial regulation of transcription has several advantages, including the control of gene expression in response to a variety of signals from the environment and the use of a relatively limited number of TFs to create many combinations of regulators whose activities are modulated by diverse sets of conditions (Pilpel et al., 2001). Examining the expansion of TF families in plants, animals, and fungi revealed that TF families shared among these organisms have undergone a much more dramatic expansion in plants than in other eukaryotes (Shiu et al., 2005; Hanada et al., 2008). This elevated expansion of plant TFs is caused by not only higher duplication rates of plant genomes but also a higher degree of expansion of TFs compared with other plant genes. This diversity of possibilities to fine-tune plant responses is especially important for plants as sessile organisms, which require more sophisticated mechanisms of response to the environment, which will benefit the plant without being too costly.
Despite great advances made in GSL research, no combinatorial control of gene expression has been reported thus far. This study aimed to increase understanding of the molecular control of GSL synthesis by identifying further components of GSL regulation, acting together with MYBs in combinatorial manner.
Yeast (Saccharomyces cerevisiae) two-hybrid analysis with MYB51 as a bait revealed several interacting proteins. After thorough selection and verification of these interactions, about 54 proteins belonging to the MYB51 interactome were identified. Within this group, one candidate basic helix-loop-helix transcription factor05 (bHLH05) and its homologous proteins (bHLH04, bHLH06, and bHLH28) were validated as bona fide interaction partners both in vitro and in planta and further analysis of these proteins was pursued in this study. Gene expression analysis in combination with chemotyping single and multiple bhlh loss-of-function mutations revealed that the bHLH proteins of the subgroup IIIe are novel regulators of GSL biosynthesis in Arabidopsis. Further analysis of double bHLH and MYB gain-of-function alleles revealed the specific role of combinatorial MYB-bHLH interactions in the transcriptional regulation of GSL biosynthesis.
RESULTS
Interactome of MYB51 Identified by Yeast Two-Hybrid Screening
To identify proteins that function in complex with MYB51, a yeast two-hybrid assay with MYB51 as a bait and an Arabidopsis complementary DNA (cDNA) library as a prey was performed. The full-length coding sequence of MYB51 and three different truncated forms of MYB51 containing 151, 251, and 351 amino acids, all containing the R2R3-MYB domain and a signature typical for the proteins regulating GSL biosynthesis, were initially tested for autoactivation. The full-length MYB51 coding sequence activated reporter gene expression without the need to interact with a prey and therefore was eliminated from the screen. Three other nonautoactivating clones of MYB51 fused to the GAL4 DNA-binding domain were assayed for their ability to bind other proteins fused to the GAL4 activation domain.
In total, 510 putative interacting candidates were obtained, which were further verified by retransformation into the prey vector via gap repair (Kelly and Hoffman, 2002) and by a second mating with yeast containing MYB51. This procedure eliminated false-positive interactors and reduced the number of putative MYB51-interacting candidates to 99. The final step of the assay, including the sequencing of these colonies, resulted in 47 positive candidate proteins presented in Table I, due to multiple copies of sequences of frequently interacting candidates.
Table I. List of proteins interacting with MYB51 in the yeast two-hybrid screen.
| AGI Code | Clones | Name | Description |
|---|---|---|---|
| n | |||
| At4g19700 | 17 | BOI | Has E3 ubiquitin ligase activity |
| IAP-LIKE PROTEIN | |||
| At1g47128 | 2 | RESPONSIVE TO DEHYDRATION21 | Has been shown to have peptide ligase activity and protease activity in vitro |
| At3g24800 | 2 | PROTEOLYSIS1 (PRT1) | Contains two ring finger domains and one ZZ-type zinc finger domain binding two zinc ions |
| At3g12920 | 2 | BRG3 | Encodes one of the BRGs involved in resistance to Botrytis cinerea |
| At5g46760 | 2 | ALTERED TRP REGULATION2 | MYC3 is a JAZ-interacting TF |
| MYC3, bHLH05 | |||
| At5g25480 | 1 | DNA METHYLTRANSFERASE2 | Encodes a DNA methyltransferase homolog |
| At1g52150 | 1 | CORONA and INCURVATA4 | Member of the class III homeodomain-leucine zipper protein family |
| At4g26930 | 1 | MYB DOMAIN PROTEIN97 (MYB97) | Encodes a putative TF |
| At1g08370 | 2 | DECAPPING1 (DCP1) | Encodes DCP1 involved in mRNA decapping |
| At3g62100 | 4 | INDOLE-3-ACETIC ACID INDUCIBLE30 (IAA30) | Encodes a member of the Aux/IAA family of proteins implicated in auxin signaling |
| At1g06620 | 1 | Encodes a protein whose sequence is similar to a 2-oxoglutarate-dependent dioxygenase | |
| At3g09700 | 6 | Chaperone DnaJ-domain superfamily protein | |
| At1g28210 | 1 | Homologous to Escherichia coli DnaJ (AtJ1) | DnaJ homolog |
| At5g17920 | 1 | MET SYNTHESIS1 | Encodes a cytosolic cobalamin-independent Met synthase |
| At5g05170 | 1 | CELLULOSE SYNTHASE3 | Encodes a cellulose synthase isomer |
| CONSTITUTIVE EXPRESSION OF VSP1 | |||
| At3g08530 | 1 | CLATHRIN H CHAIN2 | Clathrin, H chain |
| At5g41770 | 1 | Crooked neck protein (putative) and cell cycle protein (putative) | |
| At4g00180 | 1 | YABBY3 (YAB3) | YABBY gene family member, likely has TF activity |
| At5g11980 | 1 | Conserved oligomeric Golgi complex component related /conserved oligomeric Golgi complex component related | |
| At1g75940 | 1 | BETA GLUCOSIDASE20 | Encodes a protein similar to the BGL4 β-glucosidase from Brassica napus |
| At5g33290 | 1 | XYLOGALACTURONAN DEFICIENT1 | Acts as a xylogalacturonan xylosyltransferase within the xylogalacturonan biosynthesis pathway |
| At3g57550 | 1 | AGK2 | Guanylate kinase |
| At3g53620 | 1 | PYROPHOSPHORYLASE4 | Encodes a soluble protein with inorganic pyrophosphatase activity |
| At5g53120 | 2 | SPERMIDINE SYNTHASE3 | Encodes a novel spermine synthase |
| At2g24270 | 1 | ALDEHYDE DEHYDROGENASE11A3 | Encodes a protein with glyceraldehyde-3-phosphate dehydrogenase activity |
| At1g07960 | 2 | PROTEIN DISULFIDE ISOMERASE-LIKE 5-1 (PDIL5-1) | Encodes a PDIL protein |
| At1g21270 | 2 | WAK2 | Cytoplasmic Ser/Thr protein kinase induced by salicylic acid |
| At5g60340 | 1 | ADENYLATE KINASE6 | Encodes a nuclear adenylate kinase that interacts with a putative homolog of Rps14 |
| At5g48545 | 1 | HIS TRIAD NUCLEOTIDE-BINDING3 | Encodes a protein that has adenylylsulfate sulfohydrolase activity (E.C. 3.6.2.1) in vitro |
| At5g26190 | 1 | Cys/His-rich C1 domain family protein | |
| At5g22640 | 1 | EMBRYO DEFECTIVE1211 (EMB1211) | EMB1211 is involved in embryo development and chloroplast biogenesis |
| At4g32190 | 2 | Myosin H chain-related protein | |
| At2g39795 | 1 | Mitochondrial glycoprotein family protein | |
| At1g79280 | 2 | NUCLEAR PORE ANCHOR and TRANSLOCATED PROMOTER REGION | Encodes a 237-kDA protein with similarity to vertebrate translocated promoter region |
| At4g17330 | 1 | G2484-1 | Gene of unknown function expressed in seedlings, flower buds, and stems |
| At5g63200 | 1 | Tetratricopeptide repeat-containing protein | |
| At5g62090 | 1 | SEUSS-LIKE2 (SLK2) | Best Arabidopsis thaliana protein match is SLK1 (AT4G25520.1) |
| At5g51200 | 1 | EMB3142 | Protein of unknown function (DUF3414) |
| At5g48610 | 3 | Unknown protein | |
| At5g25757 | 1 | RNA polymerase I-associated factor PAF67 | |
| At4g17240 | 1 | Unknown protein | |
| At3g49570 | 1 | RESPONSE TO LOW SULFUR3 | Best Arabidopsis protein match is response to low sulfur2 (AT5G24660.1) |
| At3g45900 | 1 | RNase P protein subunit P38 related | |
| At3g11690 | 1 | Unknown protein | |
| At3g07780 | 2 | OBE1 | Encodes a nuclear Plant Homeo Domain finger protein that is functionally redundant with OBE2 |
| At3g02910 | 1 | AIG2-like (avirulence induced gene) family protein | |
| At1g21170 | 1 | SEC5B | Best Arabidopsis protein match is exocyst complex component sec5 (AT1G76850.1) |
AGI, Arabidopsis Genome Initiative.
Among the proteins interacting with MYB51 were TFs (bHLH05, MYB97, and YAB3), regulatory proteins (GUANYLATE KINASE2 [AGK2],PYROPHOSPHORYLASE4 [PPA4], and OBERON1 [OBE1]), proteins involved in pathogen or wounding responses (BOTRYTIS SUSCEPTIBLE 1 INTERACTOR (BOI), BOI-RELATED GENE 3 (BRG3) and WALL-ASSOCIATED KINASE2 (WAK2)), and several with unassigned functions (At5g62090, At5g51200, At5g48610, At4g17240, and At3g11690). In this study, we thoroughly analyzed the interaction between MYB51 and bHLH05, because protein complexes consisting of MYBs and bHLHs have been shown to be important for many essential processes in plants (Baudry et al., 2004; Zimmermann et al., 2004; Feller et al., 2006; Butelli et al., 2008; Gonzalez et al., 2008).
The Spatiotemporal Activity of bHLH05 and MYB51 Overlaps and Meets the Requirement for Their Interactions in Vivo
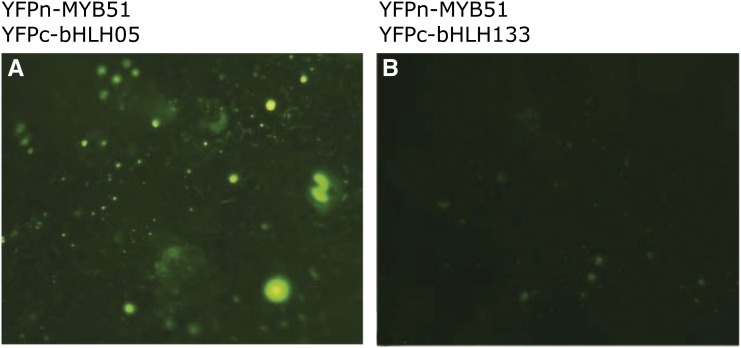
The interaction between R2R3-MYB and bHLH05 was confirmed via bimolecular fluorescence complementation (BiFC) in tobacco (Nicotiana benthamiana). We confirmed the interaction between MYB51 and bHLH05 in tobacco in three independent experiments (Fig. 1). Furthermore, we have uncovered the interactions of MYB34, MYB122, MYB28, and MYB29 with both bHLH05 and bHLH04 using BiFC (Supplemental Fig. S2).
Figure 1.
BiFC detection of the protein-protein interaction between MYB51 and bHLH05 in tobacco. BiFC assay in tobacco leaves infiltrated with A. tumefaciens containing constructs of interest. A, Interaction between N-terminal yellow fluorescent protein tagged (YFPn)-tagged MYB51 and C-terminal yellow fluorescent protein-tagged (YFPc)-tagged bHLH05 showing the interaction of both proteins. B, Interaction between YFPn-tagged MYB51 and YFPc-tagged bHLH133 (negative control) showing no interaction with MYB51. Supplemental Figure S2 shows the BiFC interaction of MYB51, MYB34, MYB122, MYB28, and MYB29 with bHLH05 and bHLH04.
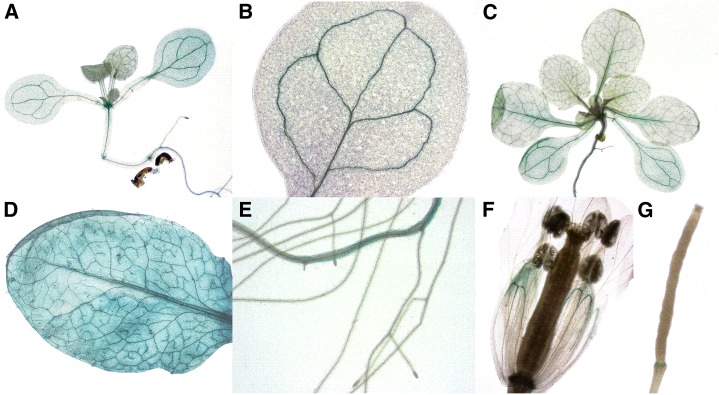
To gain further information on the importance of MYB51-bHLH05 complexes in the regulation of indolic glucosinolates (IGs), the expression patterns of bHLH05 were analyzed via fusions of its promoter to the uidA (GUS) reporter in stable transgenic plants (for details, see the “Materials and Methods”). A relevant interaction of both proteins and thus their combined effect on GSL biosynthesis in planta is only possible if two TFs are coexpressed in the same organs or tissues and at the sites of GSL production. As presented in Figure 2, bHLH05 showed strong expression in both roots and shoots of 7-d-old seedlings and mainly in the vasculature. In adult plants (Fig. 2, C, D, and H), GUS staining was observed in most organs of the plant, including stems, siliques, flowers, and roots. The reproductive organs, including flowers and siliques, displayed weak promoter-driven GUS activity. Taken together, the promoter of bHLH05 showed a similar expression pattern to that previously found for its interacting partner MYB51 (Gigolashvili et al., 2007a) and GSL biosynthesis genes (Mikkelsen et al., 2000; Grubb et al., 2004; Schuster et al., 2006; Gigolashvili et al., 2009). Thus, bHLH05 and MYB51 are coexpressed under normal growth conditions and the interaction of their gene products is therefore possible in planta. Notably, the expression pattern of bHLH04 has also been reported to overlap with the expression of bHLH05 and sites of GSL biosynthesis and regulation (Fernández-Calvo et al., 2011).
Figure 2.
The bHLH05 promoter is active at sites of GSL production. Histochemical GUS staining of ProbHLH05-uidA plants: 7-d-old seedling (A), cotyledon (B), 3-week-old plant (C), mature rosette leaf (D), main and lateral roots (E), flower (F), and young silique (G).
Multiple Interactions of R2R3-MYB GSL Regulators with bHLHs Are Revealed by the Luminescence-Based Mammalian Interactome Assay
To extend the yeast two-hybrid and BiFC assays and to systematically analyze possible interactions between R2R3-MYB and bHLHs proteins, protein-protein interaction studies by the luminescence-based mammalian interactome (LUMIER) assay (Barrios-Rodiles et al., 2005) in human HEC293TN cells were conducted. For the LUMIER assay, protein fusions to protein A or to luciferase were expressed in human HEC293TN cells and protein A-tagged protein was immunoprecipitated with IgG beads and quantified by luciferase assay.
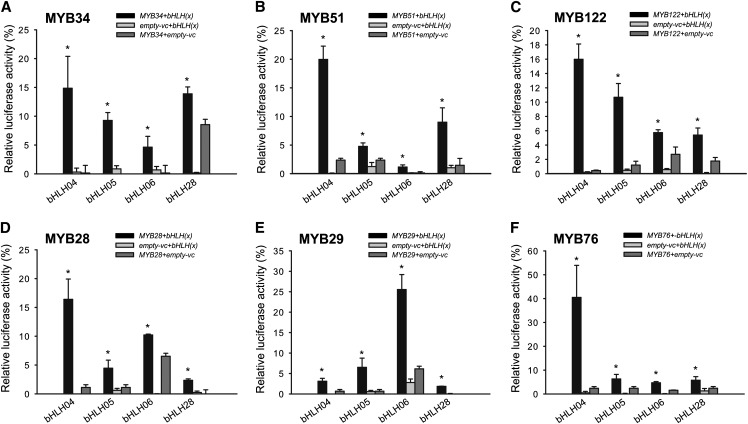
Using this approach, the interaction between all known R2R3-MYB regulators of GSL biosynthesis (MYB34, MYB51, MYB122, MYB28, MYB29, and MYB76 with bHLH04, bHLH05, bHLH06, and bHLH28) was addressed. The LUMIER experiments confirmed an interaction of MYB51 with bHLH05 that was observed by BiFC and additionally uncovered the complex interactions of all GSL-regulating MYBs with bHLHs belonging to the subgroup IIIe. As summarized in Figure 3, all analyzed R2R3-MYBs interacted with bHLH04, bHLH05, bHLH06, and bHLH28, suggesting that these bHLH proteins represent an important functional component in the regulation of GSL biosynthesis. Notably, the interaction of MYBs was stronger with bHLH04 and bHLH05 and less strong with bHLH06 (Fig. 3), implying that these strong interactions may play a special role in the regulation of GSL. Furthermore, a strong interaction between bHLH28 and either MYB51 or MYB34 was observed, despite the inability of bHLH28 to interact with JASMONATE-ZIM DOMAIN (JAZ) proteins (Fernández-Calvo et al., 2011; Niu et al., 2011). JAZ proteins are negative regulators of jasmonate (JA) signaling that also interact with bHLH proteins, thus inhibiting the activation of target genes of bHLH proteins (Chini et al., 2009). Thus, if bHLH28 is able to influence the production of GSL in plants, its effect on GSL biosynthesis should be independent of JA signaling. However, a strong interaction of bHLH28 with MYBs could be also independent from its importance in GSL biosynthesis and related to structural properties of bHLH proteins representing a negative correlation in the interaction strength of bHLH-JAZ proteins pairs versus bHLH-MYB. Notably, the interaction strength of bHLH06 with MYBs was low, with the exception of the combination bHLH06 and MYB29, which supports the important role of MYB29 in JA signaling (Hirai et al., 2007; Gigolashvili et al., 2008). Moreover, less interaction of bHLH06 with MYBs could be an additional mechanism to fine-tune the GSL production in cells having a relatively high abundance of bHLH06 mRNA compared with that of bHLH04 and bHLH05 (Supplemental Fig. S3).
Figure 3.
Pull-down experiments revealed an interaction of MYB TFs regulating GSL biosynthesis with bHLH04, bHLH05, bHLH06, and bHLH28. LUMIER assays to analyze the interaction of bHLH05, bHLH04, bHLH06, and bHLH28 with the six MYB TFs regulating IGs (MYB34, MYB51, and MYB122 in A–C) and AGs (MYB28, MYB29, and MYB76 in D–F). Interaction was assessed using fusion proteins of the MYBs with R. reniformis luciferase and of bHLHs with protein A. The proteins were coexpressed in human cells (HEK293TN) and coimmunoprecipitated with IgG Dynabeads. Values marked with asterisks are significantly different from both empty vector controls (empty-vc; Student’s t test; P < 0.05).
Simultaneous Loss of Function of BHLH05 and MYB51 Causes a Reduction in IG Content in Arabidopsis Seedlings
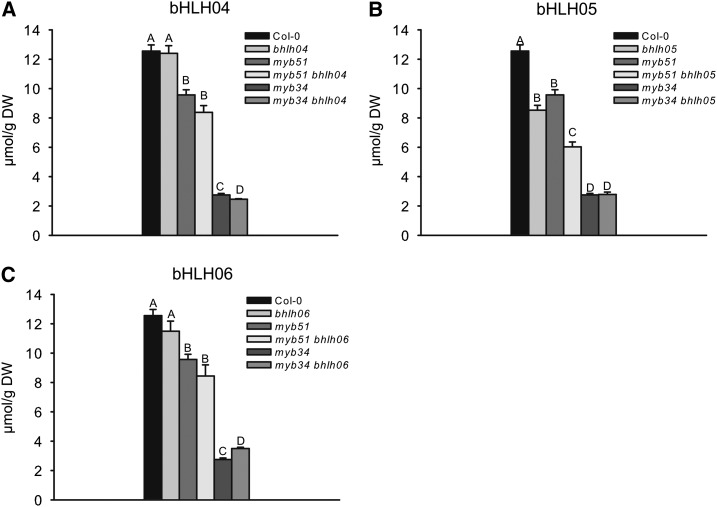
To evaluate the function of bHLH05 in IG biosynthesis, the publicly available insertional mutants bhlh05 (GK445B11), bhlh04 (GK491E10), and bhlh06 (jin1.9) were isolated and double mutants with the main indolic GSL regulators myb51 and myb34 (Frerigmann and Gigolashvili, 2014) were generated (for details, see the “Materials and Methods”). These mutants were examined for the accumulation of IG (Fig. 4; Supplemental Fig. S4) and aliphatic glucosinolates (AGs; Supplemental Fig. S4) in Arabidopsis seedlings. As shown in Figure 4, IG biosynthesis was not affected in the bhlh04 and bhlh06 mutants compared with the wild type, and was similar in double myb51 bhlh04 and myb51 bhlh06 compared with the myb51 mutant (Fig. 4, A and C).
Figure 4.
Levels of IGs in seedlings of double myb bhlh mutants. Double loss-of-function mutant combinations of MYB51 and MYB34 with bHLH04 (A), MYB51 and MYB34 with bHLH05 (B), and MYB51 and MYB34 with bHLH06 (C). For GSL analysis, 3-week-old seedlings were harvested and the sum of the three major IGs (I3M, 1MO-I3M, and 4MO-I3M) is shown. Results are means ± se from three independent experiments with four biological replicates each (n = 12). Values marked with letters are significantly different from other letters (Student’s t test; P < 0.05). DW, Dry weight.
By contrast, seedlings of the bhlh05 transfer DNA (T-DNA) insertion line were moderately but significantly affected in IG accumulation, highlighting the importance of bHLH05 in IG regulation in Arabidopsis. Moreover, the concentration of IGs in the double myb51 bhlh05 mutant was substantially lower than that of single myb51 mutants (Fig. 4B), suggesting the importance of bHLH05 in the combinatorial regulation of IGs together with MYB51.
In contrast with myb51 bhlh05, the IG level in myb34 bhlh05 seedlings was not significantly lower than that of the single myb34 mutant. Because MYB34 is known to be the main regulator and rate-limiting step of IG biosynthesis in young Arabidopsis seedlings, loss of bHLH05 function in double myb34 bhlh05 seedlings did not further reduce IG production in this double mutant. If we would have analyzed the IG content at a developmental stage when MYB51 instead of MYB34 was rate limiting (e.g. in leaves of adult plants), a different IG level (decreased levels of IG in myb34 bhlh05 versus myb34) would be anticipated.
The Combination of Gain-of-Function Alleles of bHLH05 and MYB34 Leads to a Dramatic Increase in GSL Levels in Arabidopsis
In the following experiment, we focused the analysis of GSL biosynthesis on plants either overexpressing both R2R3-MYB and bHLH proteins or containing gain-of-function alleles for both TFs. The MYB51-1D (HIG1-1D) activation-tagged line and 35S:MYB51 overexpression plants were available from a previous study (Gigolashvili et al., 2007a) and bHLH05 overexpressors were generated in this study. Whereas the overexpression of MYB51 leads to the increased production of IG, the overexpression of bHLH05 did not cause an increased accumulation of GSL in plants (Supplemental Fig. S5). The fact that bHLH05 overexpression did not induce the production of GSL indicates that bHLH05 additionally requires other regulatory proteins to activate the transcription of GSL biosynthesis genes. These findings are in agreement with transactivation analysis of bHLH05 in cultured Arabidopsis cells (see below), which revealed no ability of bHLH05 to activate GSL biosynthetic genes in trans when produced in wild-type cells.
To generate transgenic plants that stably overexpressed both TFs, we transformed the homozygous activation-tagged line MYB51-1D (also known as HIG1-1D) with 35S::bHLH05 (Supplemental Fig. S5) using Agrobacterium tumefaciens-mediated plant transformation and also crossed MYB51-1D to homozygous 35S:bHLH05 overexpression plants. Several hundred independent transgenic lines that should contain both MYB51 and bHLH05 transgenes were analyzed. However, simultaneous overexpression of bHLH05 and MYB51 always led to silencing of at least one of the transgenes in the subsequent generation, most probably because of counter selection of double overexpression transgenes with potentially very high levels of GSLs.
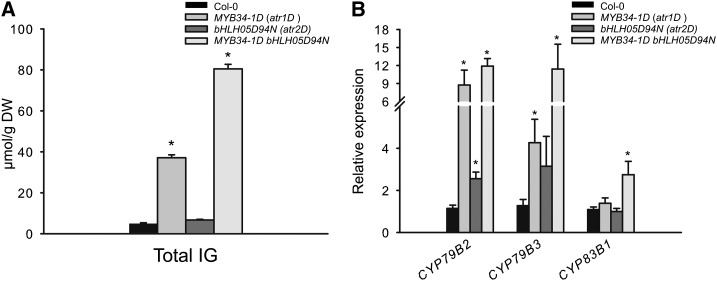
To overcome this problem, we continued to analyze MYB and bHLH gain-of-function alleles of MYB34-1D (atr1D) and bHLH05D94N (atr2D; Bender and Fink, 1998; Smolen et al., 2002) The MYB34-1D line harbors a mutation upstream of the MYB34 open reading frame, which leads to a constitutively high expression level of MYB34 (Bender and Fink, 1998) and to an accumulation of IGs (Celenza et al., 2005; Fig. 5A). The dominant allele bHLH05D94N of bHLH05 is caused by an amino acid exchange in the conserved N-terminal domain, which leads to the up-regulation of some stress-responsive genes (Smolen et al., 2002), but no significant changes in IG levels compared with wild-type plants were recorded (Fig. 5A). Thus, the gain of bHLH05 function alone as well as constitutive overexpression of bHLH05 is not sufficient to activate the production of IGs in plants (Fig. 5A; Supplemental Fig. S5). Analysis of IG in the double MYB34-1D bHLH05D94N mutant compared with single MYB34-1D and bHLH05D94N mutants revealed that IG levels in the double mutant were 2-fold higher than in the single MYB34-1D mutant and up to 20-fold higher than in ecotype Columbia-0 of Arabidopsis (Col-0) and bHLH05D94N (Fig. 5A). In agreement with this finding, the expression level of IG biosynthesis genes was significantly higher than that in the corresponding single MYB34-1D or bHLH05D94N mutants or Col-0 (Fig. 5B). Together, the analysis of IG biosynthesis in gain-of-function mutants and overexpression lines revealed that increased activity of bHLH05 alone is not sufficient to modulate IG production in plants, but increasing the activity of both MYB34 (in MYB34-1D mutants) and bHLH05 (in bHLH05D94N mutants) had a strong additive effect on the accumulation of IG. The levels of AGs in double bHLH-MYB gain-of-function mutants were negatively affected by simultaneous overexpression of MYB34 and bHLH05, which is comparable with the GSL profile of MYB34ox and MYB51ox plants (Gigolashvili et al., 2007a) and agrees with the reciprocal negative regulation of AG and IG biosynthetic branches (Supplemental Fig. S6).
Figure 5.
Combined gain of function of MYB34 and bHLH05 leads to doubling of GSL levels in Arabidopsis plants. A, Total IG content of the dominant alleles of MYB34-1D (atr1D) and bHLH05D94N (atr2D) are shown. For GSL analysis (A) and expression analysis (Col-0 = 1) of CYP79B2, CYP79B3, and CYP83B1 (B), leaves of 6-week-old plants were harvested. Values are means ± se from four independent experiments with four biological replicates each for A and three independent experiments with four biological replicates each for B (n = 16 and 12, respectively). Values marked with asterisks are significantly different (Student’s t test; P < 0.05) from control plants (Col-0). DW, Dry weight.
The Mutation in the MYB Interaction Region/JAZ Interaction Domain of bHLH05 Impairs the Protein-Protein Interaction with JAZ1 and Stimulates Interaction with MYB34
The previous experiment revealed that gain of function of bHLH05D94N causes a doubling in the levels of IGs in MYB34-1D. The additive effect of bHLH05D94N originates from the D94N mutation, which is localized in the N-terminal conserved amino acid region of the protein. This conserved region was predicted to be important for the interaction of subgroup IIIe bHLH proteins with JAZ and MYB (Fernández-Calvo et al., 2011; Schweizer et al., 2013a). In the following experiment, we analyzed the protein-protein interactions of both gain-of-function D94N bHLH05 and native bHLH05 proteins with JAZ1 and MYB34 via the LUMIER assay.
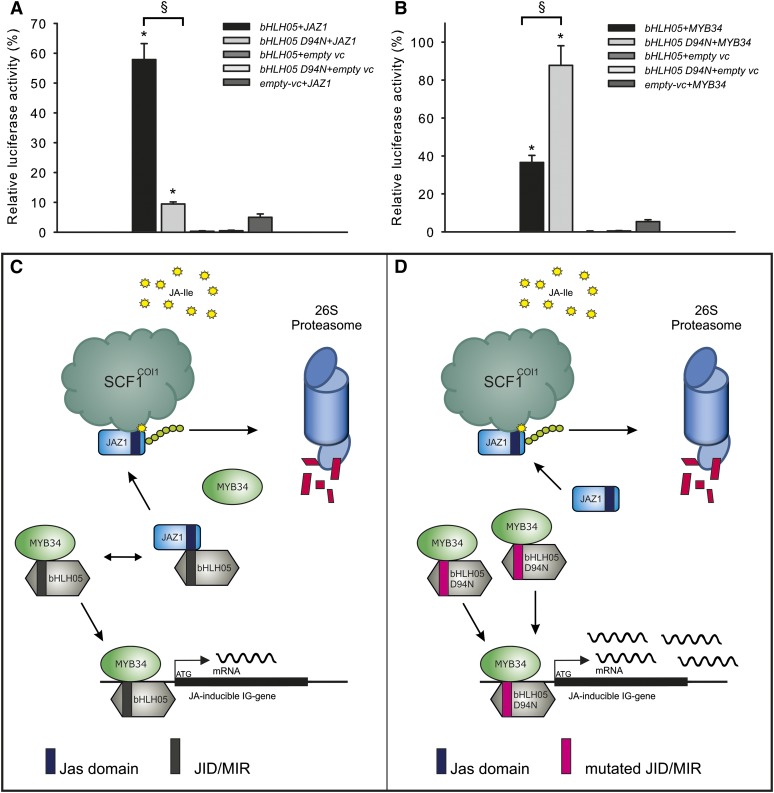
Figure 6A shows that the physical interaction between bHLH05D94N and JAZ1 is strongly attenuated compared with the interaction of native bHLH05 and JAZ1, substantiating the role of D94N, which is predicted to be localized in the MYB interaction region (MIR)/JAZ interaction domain (JID) interaction region, to alter the interaction ability of the bHLH05 protein. In agreement with this finding, bHLH28, which is also unable to interact with JAZ proteins, contains a nonconservative amino acid exchange (Gly to Lys) in the conserved protein region, also predicted to be a JID/MIR interaction region (Fernández-Calvo et al., 2011). Furthermore, together with the impaired interaction of bHLH05D94N with JAZs, an improved interaction of bHLH05D94N with MYB34 was observed (Fig. 6B). This Gly to Lys exchange in the N-terminal JID/MIR interaction region of bHLH28 is most probably also the reason for its increased interaction with MYBs (Fig. 3).
Figure 6.
Mutation D94N in the N-terminal part of bHLH05 prevents interaction of bHLH05 with JAZ1 and stimulates interaction with MYB34. A and B, LUMIER assays for the interaction of native bHLH05 and the mutated bHLH D94N (Asn at position 94) proteins with JAZ1 (A) and MYB34 (B) are shown. Interaction between luciferase-tagged MYB34 and JAZ1 on one side and the protein A-tagged bHLH05 and bHLH05 D94N on the other side are shown. The proteins were coexpressed in human cells (HEK293TN) and coimmunoprecipitated with IgG Dynabeads. Values marked with asterisks are significantly different from both empty vector controls (empty vc) without coding sequence fusion (Student’s t test; P < 0.05). Bars marked with § are significantly different from each other (Student’s t test; P < 0.001). C and D, Model for protein-protein interactions of native bHLH05 protein (C) and mutated bHLH05D94N protein (D) with MYB34 and JAZ1 proteins (without JA addition). C, The interaction of native bHLH05 with JAZ1 and MYB34 is balanced and bHLH05 interacts with both proteins, allowing production of IG only at basal (wild-type) levels. D, The mutation bHLH05D94N impairs the interaction with JAZ1 and triggers the strong interaction of bHLH05D94N with MYB34 followed by enhanced transcription of IG pathway genes and an increased accumulation of IG levels. The JAZ1 protein interacts less with bHLH05D94N and is therefore free for ubiquitinylation by CORONATINE INSENSITIVE1 (COI1) and degradation by the 26S proteasome. The D94N mutation of bHLH05 mimics the presence of JA. Jas domain, The interaction domain of JAZ proteins used during the physical interaction with bHLH proteins; JID/MIR, a JAZ Interaction Domain/MYB Interaction Domain (a part of bHLH proteins, which allows the physical interaction with JAZ and MYB proteins).
Altogether, the data demonstrate the importance of the N-terminal part of bHLH05 protein for the interaction with JAZ and MYB, which can thereby affect the regulatory ability of these proteins. Importantly, similar mutations in the N-terminal MYB-interacting region of the bHLH proteins DELILA (DEL from Antirrhinum majus) and Myc-RP (from Perilla frutescens) positively affect anthocyanin biosynthesis (Pattanaik et al., 2006, 2008).
An Analysis of Single and Multiple bhlh Knock-Out Mutants Reveals a Special Role of bHLH04 and bHLH05 in the Regulation of GSL in the Absence of JA
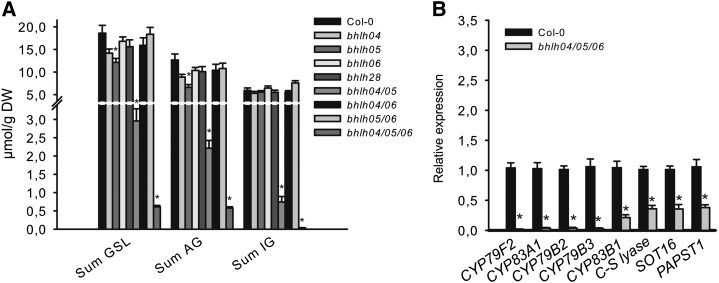
To study the role of bHLH04, bHLH05, bHLH06, and bHLH28 in the regulation of GSL, single and multiple bhlh mutants were generated (for details, see the “Materials and Methods”) and analyzed. None of the T-DNA insertion lines (bhlh04, bhlh05, bhlh06, and bhlh28) or double and triple homozygous loss-of-function mutants (bhlh04/05, bhlh05/06, bhlh04/06, and bhlh04/05/06) showed an obvious phenotype. The GSL levels in leaves of single bhlh04, bhlh05, bhlh06, and bhlh28 or double bhlh04/06 and bhlh05/06 mutants were comparable with those of wild-type plants (Fig. 7A; Supplemental Fig. S7). This contrasted with the significantly reduced GSL levels in double bhlh04/05 and triple bhlh04/05/06 mutants (Fig. 7A; Supplemental Fig. S7), because bhlh04/05 contained only 15% of wild-type GSL levels and bhlh04/05/06 accumulated almost no GSL. These data reveal an essential role for bHLH04, bHLH05, and bHLH06 and a minor role for bHLH28 in the regulation of GSL accumulation.
Figure 7.
GSL accumulation is significantly reduced in rosette leaves of double bhlh04/05 and triple bhlh04/05/06 mutants. A, GSL levels in single and multiple bhlh mutants are shown. For GSL analysis, leaves of 6-week-old plants were harvested and the sum of the three major IGs (I3M, 4MO-I3M, and 1MO-I3M), the sum of the four main AGs (3MSOP, 4MSOB, 5MSOP, and 8MSOO), and the sum of IGs and AGs are shown as Sum GSL. Results are means ± se from three independent experiments with four biological replicates each (n = 12). Values marked with asterisks are significantly different from Col-0 (Student’s t test; P < 0.01). B, Expression of GSL biosynthetic genes in the triple bhlh mutant is shown. Relative expression values of biosynthesis genes for AG (CYP79F2 and CYP83A1), IG (CYP79B2, CYP79B3, CYP83B1, and SOT16) and both AG and IG (C-S lyase and PAPST1) are shown. Relative expression was measured in leaves of 6-week-old bhlh04/05/06 knock-out lines (Col-0 = 1). Data are means ± se from three independent experiments with three biological replicates each (n = 9). Values marked with asterisks are significantly different from control plants (Student’s t test; P < 0.05). DW, Dry weight.
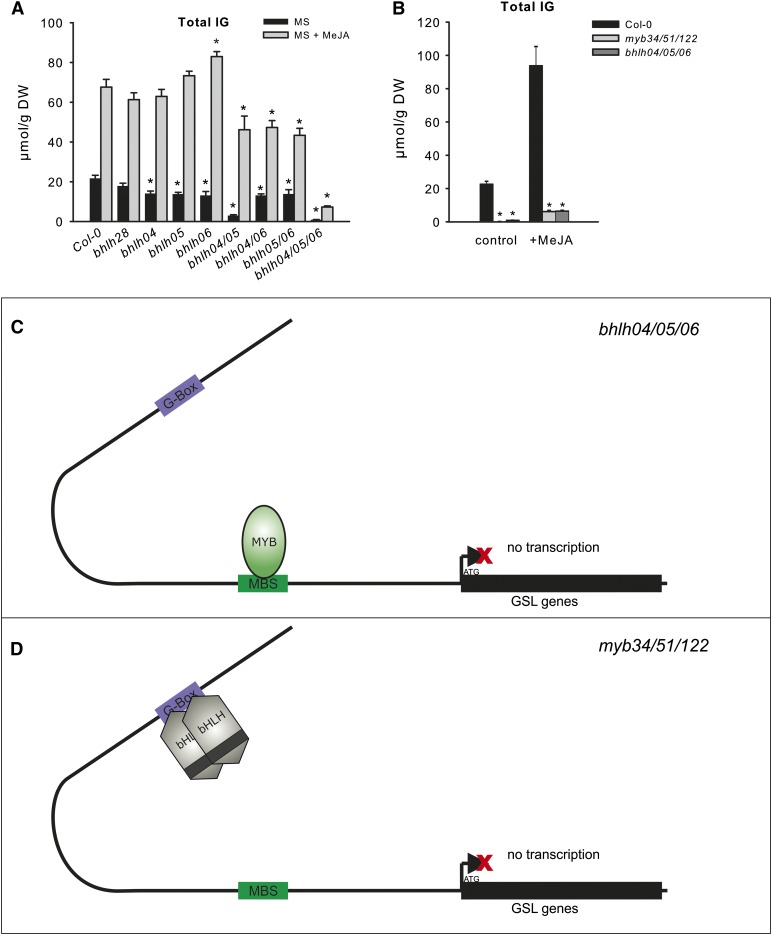
Previous studies have shown an important role of bHLH04, bHLH05, and bHLH06 in multiple JA signaling processes (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011). To understand more about bHLH-mediated IG regulation in response to JA, we treated the single and multiple bhlh mutants with methyl jasmonate (MeJA) and analyzed IG and AG in wild-type and mutant seedlings (Fig. 8; Supplemental Fig. S8). In agreement with the previously reported JA inducibility of bHLHs, the dramatic increase in total IG of Col-0 and single bhlh mutants and only negligible changes in the triple bhlh04/05/06 mutant were detected. Remarkably, the residual levels of IG in the triple bhlh04/05/06 and myb34/51/122 mutants were only marginally affected by JA treatment (Frerigmann and Gigolashvili, 2014; Fig. 8), implying the importance of both bHLH and MYBs in the production of IGs. Furthermore, all analyzed double bhlh mutants (with only single active bHLH04, bHLH05, or bHLH06) were attenuated in JA-induced IG biosynthesis to a similar extent (Fig. 8), underlining the importance of all three bHLH proteins in JA-induced GSL biosynthesis. Furthermore, bHLH06 was able to complement the low IG level of bhlh04/05 under these conditions, implying a specific role of bHLH06 in MeJA signaling.
Figure 8.
Production of IGs in single and multiple bhlh mutants in response to MeJA treatment. Model for the IG production in bhlh04/05/06 and myb34/51/122 mutants. A, The IG content of single and multiple bhlh mutants after MeJA treatment is shown. For GSL analysis, the mutant and wild-type (Col-0) plants were grown on MS plates for 2 weeks followed by transfer to plates supplemented with 50 µm of MeJA. The sum of the three major IGs (I3M, 4MO-I3M, and 1MO-I3M) are shown. Results are means ± se from four two independent experiments with four biological replicates each (n = 8). Values marked with asterisks are significantly different from Col-0 (Student’s t test; P < 0.01). B, Comparison of IG content in bhlh04/05/06 and myb34/51/122 in the presence or absence of MeJA. Mutant and wild-type (Col-0) plants were grown on MS plates free of additives or MS supplemented with 50 µm of MeJA. The sum of the three major IGs (I3M, 4MO-I3M, and 1MO-I3M) is presented. Results are means ± se from three independent experiments with four biological replicates each (n = 12). Values marked with asterisks are significantly different from Col-0 (Student’s t test; P < 0.05). C and D, A model for the biosynthesis of GSL by MYB and bHLH in Arabidopsis in triple bhlh04/05/06 and myb34/51/122 mutants is shown. Biosynthesis of GSL is blocked in the absence of both bHLH (C) and MYB (D) proteins; thus, both types of TFs are necessary to produce GSLs in planta. DW, Dry weight.
The data here reveal the specific importance of bHLH04 and bHLH05 in the regulation of both IGs and AGs in the absence of JA. Under standard growth conditions, bHLH06 only slightly contributes to the production of GSL, because its function is more important in response to JA. Despite the interaction with MYBs, bHLH28 appears to play only a minor role in GSL regulation in both conditions and further analysis of this protein is required to understand its possible role in GSL biosynthesis.
Attenuated Expression of GSL Pathway Genes in the Triple bhlh04/05/06 Mutant
Because IG and AG levels are lower in the triple bhlh04/05/06 mutant, we measured the expression of genes involved in the biosynthesis of IG, AG, or both pathways by real-time quantitative reverse transcription PCR (qRT-PCR). As shown in Figure 7B, the transcript analysis of the triple bhlh04/05/06 mutant revealed the dramatically reduced expression of genes involved in the biosynthesis of IGs (CYP79B2, CYP79B3, and CYP83B1), AGs (CYP79F2 and CYP83A1), as well as transcript levels of genes involved in the biosynthesis of both IGs and AGs (C-S lyase, Sulfotransferase16 [SOT16], and PAPS-Transporter1 [PAPST1]).
Notably, the expression of MYB34 and MYB122, which regulate the production of IG (Celenza et al., 2005; Gigolashvili et al., 2007a; Frerigmann and Gigolashvili, 2014) was hardly altered (Supplemental Fig. S9). However, the steady-state level of MYB51 was approximately 3-fold higher in bhlh04/05/06 compared with Col-0. The low levels of GSL in this triple mutant could be sensed by plants, which can increase the GSL production via a negative feedback loop (Mugford et al., 2009) in the bhlh04/05/06 mutant.
Transactivation Assays with bHLH Proteins and promoter-uidA Constructs of GSL Biosynthesis Genes via Transient Expression with Cultured Arabidopsis Cells
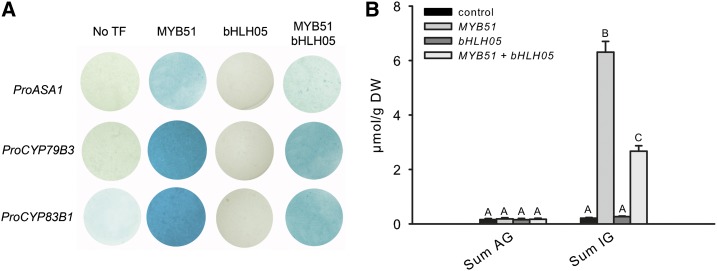
It was previously shown that R2R3 MYBs that regulate IG biosynthesis can activate the transcription of numerous promoter-reporter constructs with GSL biosynthesis genes via transient expression of cultured Arabidopsis cells (Gigolashvili et al., 2007a, 2008). Here, we showed that bHLHs interact with MYBs to control GSL production, and that the triple bhlh04/05/06 mutant contains less than 1% of the GSL present in wild-type plants. This suggests that the GSL pathway might be under transcriptional control by bHLH proteins or by MYB-bHLH complexes. To test the hypothesis that genes involved in the biosynthesis of GSL are controlled by bHLHs or MYB-bHLH complexes, we used a cotransformation assay (Berger et al., 2007; Gigolashvili et al., 2007a, 2008) to identify TFs that bind to promoters of GSL synthesis genes. For this, constructs containing promoters of the IG biosynthetic genes ANTHRANILATE SYNTHASE ALPHA1, CYP79B3, and CYP83B1 fused to the uidA (GUS) reporter gene were used as reporter constructs, and bHLH05 or a mixture of bHLH05 and MYB51 were used as effector constructs. Reporter and effector constructs were coexpressed in cultured Arabidopsis cells and the transactivation potential of these TFs toward promoters of GSL biosynthesis genes was estimated histochemically, using 5-bromo-4-chloro-3- indolyl-β-d-glucuronic acid (X-Gluc) staining (Fig. 9A) or was determined by measurements of GUS activity (Fig. 9B). In the absence of an effector construct, the reporter construct containing the promoters of GSL biosynthetic genes revealed only faint GUS activity (Fig. 9, A and B). The MYBs could induce the transcription of all tested promoters of GSL biosynthetic pathway in trans, indicating a direct role of MYB51 in the binding and activation of GSL biosynthesis genes. However, this was not the case for bHLH proteins, because we did not detect any induced transcription of reporter gene expression after coexpression with bHLHs. Furthermore, coexpression of MYB and bHLH with the GSL promoters did not reveal any increase in GUS activity, but even attenuated the positive transactivation effect observed for MYBs (Fig. 9, A and B). Finally, not only were the expression levels of reporter constructs not positively affected by bHLH, but the accumulation of GSL was also not increased in these Arabidopsis wild-type cells overexpressing bHLH proteins (Fig. 9B). These data support our finding that plants overexpressing bHLH05 (Supplemental Fig. S5) or containing the constitutively active form of this protein (bHLH05D94N; Fig. 5) failed to accumulate more IGs than the wild type. By contrast, the overexpression of MYB51 in cultured cells was followed by both the increased transcription of promoter-GUS constructs and production of GSL in this transient expression assay.
Figure 9.
Transactivation assay of GSL biosynthesis promoters with bHLH TFs. TF coexpression assay to determine the DNA binding and transactivation potential of bHLH05 toward target promoters of the GSL biosynthesis pathway genes ASA1, CYP79B3, and CYP83B1. A, The promoters of ASA1, CYP79B3, and CYP83B1 were fused to the uidA (GUS) reporter gene. Cultured Arabidopsis Col-0 cells were inoculated with the supervirulent A. tumefaciens strain LBA4404.pBBR1MCS.virGN54D containing either only the reporter construct or the reporter construct and in addition, Pro-35S:MYB51, Pro-35S:bHLH05, and the 1:1 mixture of Pro-35S:MYB51 and Pro-35S:bHLH05 effector constructs. The GUS staining indicates transactivation of a promoter by an effector. Histochemical GUS staining of cultured cells was performed with X-Gluc 4 d after transfection. B, Accumulation of GSL in cultured cells transfected with Pro-35S:MYB51, Pro-35S:bHLH05, and the 1:1 mixture of Pro-35S:MYB51 Pro-35S:bHLH05. The sum of the three major IGs (I3M, 4MO-I3M, and 1MO-I3M) and the sum of the four main AGs (3MSOP, 4MSOB, 5MSOP, and 8MSOO) are shown. Results are means ± se from three independent biological replicates (n = 3). Values marked with asterisks are significantly different from Col-0 (Student’s t test; P < 0.01). DW, Dry weight.
DISCUSSION
Protein-protein interactions play a central role in the regulation of scores of cellular processes (Martinez, 2002; Istrail and Davidson, 2005). Despite the identification of numerous TFs involved in plant regulatory networks (Lee et al., 2007; Benhamed et al., 2008; Kaufmann et al., 2009; Morohashi and Grotewold, 2009; Oh et al., 2009), our understanding of the molecular mechanisms of these protein complexes in the regulation of plant gene expression remains poor, including the regulation of GSL biosynthesis. Given the importance of transcriptional regulation in the generation of diverse types of GSL under various environmental conditions, only few regulatory proteins that are crucial for the production of GSL biosynthesis in Arabidopsis have been described. In this study, we identified a new class of TFs, which together with R2R3-MYBs, are crucial for the production of GSL in vivo. Using a yeast two-hybrid assay, we identified bHLH05 as an interacting partner of MYB51 and showed that bHLH05, bHLH04, bHLH06, and bHLH28 interact with R2R3-MYB proteins both in vitro and in vivo. This specific MYB-bHLH interaction plays an essential role in the transcriptional activation of GSL biosynthesis genes and the production of these secondary metabolites in planta.
bHLH Proteins Are Regulators of GSL Biosynthesis in Arabidopsis
The bHLH04, bHLH05, and bHLH06 proteins control JA-dependent responses, such as root growth inhibition, defense against bacterial pathogens, and insect herbivory (Fernández-Calvo et al., 2011). These TFs have almost identical DNA-binding specificities to the canonical G-box (CACGTG), suggesting their importance in the recognition of target genes involved in JA responses (Fernández-Calvo et al., 2011). Furthermore, a recent chromatin immunoprecipitation sequencing (ChIP-seq) analysis revealed that bHLH06 is able to bind the promoter of GSL biosynthesis genes in vivo, all containing canonical G-boxes (Schweizer et al., 2013a). In this study, we identified the GSL biosynthesis pathway as a target of these bHLH proteins and dissected the specific role of bHLH04, bHLH05, and bHLH06 in the regulation of GSL biosynthesis.
Gene expression analysis of the triple bhlh04/05/06 mutant revealed that GSL biosynthetic genes are down-regulated in the bhlh04/05/06 triple mutant (Fig. 7B). However, not only the GSL biosynthetic genes, but also the production of IG and AG is impaired in the bhlh04/05/06 triple mutant (Fig. 7A), pointing to a crucial role of bHLH04, bHLH05, and bHLH06 in the regulation of GSL biosynthesis.
In contrast with the GSL profile of the triple bhlh04/05/06 mutant, the single bhlh mutants and bhlh04/06 and bhlh05/06 double mutants showed similar GSL levels to those in wild-type plants, indicating functional redundancy between bHLH04, bHLH05, and bHLH06. However, unlike bhlh04/06 and bhlh05/06 mutants, the double bhlh04/05 mutant was strongly affected in the production of GSL (Fig. 7), pointing to a special role of bHLH04 and bHLH05 in the production of GSL in the absence of JA. In agreement with the low GSL levels of the double bhlh04/05 mutant, the latter was reported to be more susceptible against generalist herbivores than bhlh04/06 and bhlh05/06 (Fernández-Calvo et al., 2011). Further analysis of GSL levels in multiple bhlh mutants revealed the inability of bHLH06 to fully complement GSL biosynthesis in the bhlh04/05 mutant under standard growth conditions (Fig. 7). This contrasted with GSL biosynthesis levels in seedlings of bhlh04/05 after MeJA treatment, because bhlh04/05 can be fully complemented by bHLH06 (Fig. 8). Notably, IG levels of double bhlh05/06 and bhlh04/06 mutants treated with MeJA treatment were comparable with IG levels of MeJA-treated bhlh04/05, pointing to involvement of all three bHLH proteins in JA-triggered GSL biosynthesis (Fig. 8). Based on this observation, it can be assumed that mainly bHLH06 and secondarily bHLH04 and bHLH05 are important in GSL regulation via JA signaling, which differs from the regulation of constitutive levels of GSL mediated mainly by bHLH04 and bHLH05.
The mRNA microarray analysis of bhlh06/myc2 identified a list of bHLH06-regulated genes, including genes involved in wound/insect responses, flavonoid biosynthesis, and oxidative stress tolerance (Dombrecht et al., 2007). The authors found that the bhlh06/myc2 mutant accumulated more IG in response to MeJA (Fig. 8) and the genes involved in IG biosynthesis were also upregulated. Dombrecht et al. (2007) also suggested that bHLH06 is a negative regulator of GSL accumulation. However, this is apparently not the case, because bHLH06 as well as bHLH04 and bHLH05 are positive regulators of IG biosynthesis, and bHLH04 and bHLH05 compensate for the absence of bHLH06 by inducing the production of IGs in bhlh06 in response to MeJA (Guo et al., 2013b).
Jasmonate, bHLHs, and GSL Regulation
Glucosinolate metabolism in the Brassicaceae has evolved to optimize plant fitness in changing environments (Giamoustaris and Mithen, 1995; Brader et al., 2001; Hiruma et al., 2010). Diverse pathogens trigger the production of GSL by inducing the JA, ethylene, abscisic acid, salicylic acid, or Glc signaling pathways (Kiddle et al., 1994; Doughty et al., 1995; Brader et al., 2001; León et al., 2001; Kliebenstein et al., 2002; Mikkelsen et al., 2003; Farnham et al., 2004; Lorenzo et al., 2004; Dombrecht et al., 2007; Yan and Chen, 2007; Erb et al., 2011; Guo et al., 2013a). This can modulate GSL accumulation via MYBs (Hirai et al., 2007; Sønderby et al., 2007; Gigolashvili et al., 2007a, 2007b; Frerigmann and Gigolashvili, 2014) and other regulatory proteins in GSL biosynthesis. JA is an important regulator of GSL synthesis, which affects the expression of MYB TFs and GSL biosynthesis genes (Doughty et al., 1995; Brader et al., 2001; Mikkelsen et al., 2003; Dombrecht et al., 2007; Frerigmann and Gigolashvili, 2014). Glc signaling, which also positively regulates GSL biosynthesis, acts synergistically with JA, because Glc-induced GSL biosynthesis can be enhanced by the addition of JA (Guo et al., 2013a).
We identified the bHLH proteins using yeast two-hybrid screening, which were known previously from the JA signaling pathway to regulate the JA-inducible genes (Fernández-Calvo et al., 2011; Schweizer et al., 2013a, 2013b). We also showed that bHLH04, bHLH05, and bHLH06 are regulators of GSL biosynthesis in response to MeJA in Arabidopsis. In the absence of MeJA, the bHLH proteins form fewer protein complexes with MYBs and significantly more protein dimers with JAZ proteins (Fernández-Calvo et al., 2011). The bHLH proteins that interact with JAZ are inactive and the production of GSL in the absence of JA is attenuated. In the presence of JA, the JAZ proteins are degraded by the Skp1–Cullin–F-box protein - COI1 complex, triggering the physical interaction of bHLH with MYBs and the formation of active MYB-bHLH complexes, which activate GSL production.
Notably, in the absence of JA, wild-type plants accumulate basal levels of GSL, owing to the formation of less-abundant but still active MYB-bHLH pairs. bHLH04 and bHLH05 especially appear to form active protein complexes with MYBs in standard growth conditions, because the loss of function of both TFs has a dramatic effect on GSL production (Fig. 7). In contrast with bHLH04 and bHLH05, the role of bHLH06 in the production of GSL is particularly important in the presence of JA, because mutation of bhlh06 has no negative effect on GSL production in the absence of JA (Fig. 7), whereas bHLH06 can complement the GSL-deficient bhlh04/05 mutant upon JA treatment (Fig. 8).
Both MYB and bHLH TFs Are Necessary to Regulate GSL Biosynthesis in Arabidopsis
In this study, we demonstrated that the GSL biosynthetic pathway is not only under transcriptional control by MYBs as was previously shown (Hirai et al., 2007; Sønderby et al., 2007; Gigolashvili et al., 2007a, 2007b, 2008; Beekwilder et al., 2008; Malitsky et al., 2008; Sønderby et al., 2010a; Li et al., 2013; Frerigmann and Gigolashvili, 2014), but is also tightly regulated by bHLHs. This is supported by the following observations. First, bHLH04, bHLH05, bHLH06, and bHLH28 interact with known R2R3-MYB regulators of IG and AG biosynthesis (Figs. 1 and 2). Second, the amount of IGs in the triple bhlh04/05/06 mutant is as strongly impaired as in the triple myb34/51/122 mutant (Figs. 7 and 8; Frerigmann and Gigolashvili, 2014). Third, combinatorial gain of function of MYB34 and bHLH05 proteins has an additive effect on IG biosynthesis in Arabidopsis. Thus, the IG levels in the MYB34-1D bHLH05 D94N gain-of-function allele are twice as high as that in MYB34-1D and 20 times greater than that in bHLH05 D94N and Col-0 (Fig. 5). Similarly, MeJA treatment of MYB34-1D caused IG levels to double (Supplemental Fig. S10), supporting the importance of bHLH proteins released from the JAZ complex to activate the production of IGs. Fourth, analysis of IG levels in double myb51 bhlh05 mutants (Fig. 4) revealed synergistic/additive effects of both MYB51 and bHLH05 in the accumulation of IG in seedlings. Fifth, treatment of myb34/51/122 with MeJA, which triggers the release of bHLHs from the JAZ complex, does not lead to an increased production of IGs in mutants that lack MYBs (Fig. 8; Frerigmann and Gigolashvili, 2014). Finally, it is not possible to induce the production of IG in bhlh04/05/06 by solely overexpressing MYB51 (Supplemental Fig. S11), demonstrating the importance of both bHLH and MYB proteins for the production of GSL.
Our findings on the importance of bHLH in GSL regulation agree with ChIP-seq experiments, which showed that the G-boxes of GSL biosynthesis genes are enriched in the chromatin of MeJA-treated bHLH06:FLAG plants (Schweizer et al., 2013a); thus, bHLH06 binds DNA. Similar DNA-binding activities to GSL biosynthesis genes are therefore predicted for bHLH04 and bHLH05. Although regulatory elements of GSL biosynthesis genes can be bound by bHLH06, the role of bHLH proteins in the direct transcriptional control of GSL genes remains controversial. The transactivation assay with promoter-reporter constructs of GSL biosynthesis genes and 35S:bHLH05 revealed no ability of these bHLHs to activate the transcription of GSL genes in trans (Fig. 9). In contrast with the behavior of bHLH proteins, the MYBs strongly induced the promoters of GSL biosynthesis pathway genes in trans. Furthermore, transactivation activity of bHLHs cannot be achieved even in the presence of both bHLHs and R2R3-MYB in the same cell (Fig. 9, A and B), pointing to an alternative role to the transcriptional control of GSL genes for bHLH proteins. Notably, bHLH05 protein not only failed to activate transiently introduced promoter-reporter constructs (Fig. 9A), and to positively affect GSL production in gain-of-function bHLH05D94N (Fig. 5) and 35S:bHLH05 plants (Supplemental Fig. S5), but it additionally inhibited the GSL accumulation in the bHLH05 overexpressor plants and cultured Arabidopsis cells containing both bHLH05 and MYB constructs (Fig. 9; Supplemental Fig. S5). The inhibitory effect of bHLH05 protein observed in these experiments could be explained by its recently reported ability of bHLH to activate the transcription of JAZ proteins (Niu et al., 2011). It could be speculated that the newly transcribed JAZ proteins not only inactivate the transgenic bHLH05, but also sequester endogenous bHLH05 from the functional bHLH05-MYBx complex by replacing it with unfunctional bHLH05-JAZx. Thus, the inability of bHLHs to activate both transient promoter-reporter constructs and endogenous GSL genes cannot be attributed to limitations of the Arabidopsis cell-culture system, because promoters of GSL biosynthetic genes harboring both G-boxes and MYB binding motifs (shown in Supplemental Text S1 for CYP79B2, CYP79B3, and CYP81B1; Higo et al., 1999) were activated by MYB51 in trans. In addition, analysis of GSL levels of cultured Arabidopsis cells revealed that the promoters of GSL biosynthesis genes could be manipulated by overexpressing MYBs, but not bHLH proteins (Fig. 9A).
Combinatorial Gene Regulation by MYB-bHLH Complexes
The physical interaction between bHLH and MYB factors in plants plays an important role in different plant processes and has become a paradigm in plant combinatorial gene regulation (Feller et al., 2011). Several studies that aimed to understand transcriptional gene regulation by the MYB-bHLH complex have addressed this in trichome patterning and the differentiation of epidermal cells (Glover et al., 1998; Bernhardt et al., 2005; Pesch and Hülskamp, 2009; Pesch et al., 2013) in Arabidopsis. Furthermore, the MYB-bHLH protein complexes involved in the regulation of flavonoid genes in Arabidopsis and also in other species such as rice (Oryza sativa), maize (Zea mays), cotton (Gossypium hirsutum), apple (Malus domestica), strawberry (Fragaria spp.), snapdragon (Antirrhinum majus), beefsteakplant (P. frutescens), gerbera (Gerbera hybrida), and clustered gentian (Gentiana triflora; Martin et al., 1991; Grotewold et al., 2000; Hu et al., 2000; Aharoni et al., 2001; Sompornpailin et al., 2002; Elomaa et al., 2003; Humphries et al., 2005; Schwinn et al., 2006; Sweeney et al., 2006; Espley et al., 2007; Furukawa et al., 2007; Nakatsuka et al., 2008; Brueggemann et al., 2010; Lin-Wang et al., 2010) have been rigorously characterized.
In this study, we identified the GSL biosynthesis pathway as a target of the MYB-bHLH complex. We monitored the transcriptional activation of transiently introduced or endogenous GSL biosynthesis genes by MYBs and bHLH, and addressed the special role of bHLH proteins for GSL biosynthesis. The role of bHLHs in the MYB-bHLH complex remains open, because it is not clear why the interaction of bHLH with MYBs is required, or why the simultaneous overexpression of both bHLH and MYB proteins does not induce the transcription of promoter-reporter constructs in transiently transformed Arabidopsis cells. To explain this, an alternative role for bHLHs other than the direct activation of gene transcription can be assumed. Such an indirect activation of gene transcription is also the case for bHLH-mediated gene regulation in flavonoid biosynthesis, which we consider below.
Similar to the R2R3-MYBs that regulate GSL biosynthesis, the C1 protein, which belongs to the R2R3 MYB group and regulates flavonoid biosynthesis in maize, possesses a functional transcription activation domain at its C terminus (Sainz et al., 1997a), which can bind DNA in vitro and activate gene transcription (Sainz et al., 1997b). However, the function of R2R3 MYBs in flavonoid biosynthesis is dependent on bHLH proteins, which is also true for R2R3 MYBs in GSL biosynthesis. It was initially thought that the requirement of C1 to interact with R1 (bHLH protein) is a consequence of a low affinity of C1 for DNA (Sainz et al., 1997b); however, it was shown that even when the DNA-binding ability of this protein increased (via the mutated C1 protein), C1 remains dependent from bHLH (Hernandez et al., 2004). This appears to be the case not only in flavonoid and GSL biosynthesis, because two other proteins belonging to R2R3-MYB TF family known as GLABRA1 and WEREWOLF, which contain transcription activation domains and are able to bind DNA, are also dependent on the bHLH factors GLABRA3/ENHANCER OF GLABRA3 (Lee and Schiefelbein, 2001; Zhang et al., 2003). In summary, the R2R3 MYBs can bind and activate their target genes both transiently and in planta, whereas bHLHs are essential for the regulation of target genes in planta but are dispensable for the activation of transiently expressed promoter-reporter constructs in wild-type Arabidopsis cells.
One hypothesis that can explain the requirement of bHLHs for MYBs is the inhibition hypothesis. It can be suggested that MYBs that regulate GSL biosynthesis can interact with other inhibitor proteins that retain the ability of MYBs to activate GSL biosynthesis genes. Thus, the bHLH-mediated release of MYBs from inhibition will be followed by the MYB-mediated activation of GSL biosynthesis genes. Interestingly, several independent research groups have recently demonstrated that the bHLH subgroup IIId TFs (bHLH03, bHLH13, bHLH14, and bHLH17) are targets of JAZ proteins and therefore inhibitors of JA signaling, which can indeed serve as antagonists of the bHLH subgroup IIIe (bHLH04, bHLH05, and bHLH06) analyzed in this study (Nakata et al., 2013; Sasaki-Sekimoto et al., 2013; Song et al., 2013; Fonseca et al., 2014). Furthermore, these bHLH subgroup IIId TFs function redundantly to negatively regulate JA-mediated plant defense; therefore, it is highly probable that bHLH03, bHLH13, bHLH14, and bHLH17 factors are transcriptional repressors of GSL biosynthesis and antagonize bHLH04, bHLH05, and bHLH06. To verify this hypothesis, it needs to be shown that bHLH03, bHLH13, bHLH14, and bHLH17 interact with MYBs regulating GSL biosynthesis or bind the promoters of GSL biosynthesis genes. However, the existence of inhibitors alone cannot explain the absolute requirement of bHLH proteins for the regulation of GSL biosynthesis by the bHLH-MYB complex. If bHLH04, bHLH05, and bHLH06 were only needed to out-sequester these inhibitor proteins, then the simultaneous overexpression of bHLH05 and MYB51 in vitro would have caused an increase in transcription of GSL promoter-reporter constructs (Fig. 9) by sequestering the bHLH03, bHLH13, bHLH14, and bHLH17 proteins from the unfunctional MYB51-bHLHx complex and replacing it with MYB51-bHLH05.
An alternative hypothesis that can additionally explain the requirement of bHLHs for MYBs and can reconcile all findings presented in this study (including the contradictory results on the dispensability of bHLH in wild-type cultured cells and indispensability in planta) relates to the role of mediator proteins and/or chromatin in the bHLH-mediated regulation of GSL. To assist this regulatory role, bHLH requires an additional interaction partner that can modify chromatin. The protein R-interacting factor1 (RIF1) can modify chromatin in flavonoid biosynthesis (Hernandez et al., 2007). The RIF1 protein is a nuclear, AGENET domain-containing EMSY-like protein, which is recruited by the bHLH protein R to modify chromatin, whereas R2R3-MYBs can proceed with transcriptional gene activation. Thus, it can be speculated that the function of bHLHs in GSL biosynthesis might link the transcriptional activation of genes with chromatin functions by tethering chromatin-modifying proteins to DNA and thereby allowing the modulation of gene expression by histone-modifying (acetylation/methylation) proteins. To facilitate the tethering of RIF-like proteins to DNA, the bHLH proteins need either to be able to bind DNA or to be kept in close proximity to DNA by interacting with MYBs. Notably, this appears to be true for GSL-regulating bHLHs, because ChIP-seq experiments with bHLH06-overexpressing plants revealed the binding of bHLH06 to G-boxes of GSL genes in the presence of R2R3 MYBs (Schweizer et al., 2013a). Whether bHLH proteins can bind the promoters of GSL genes in the absence of MYBs that regulate IGs or AGs needs to be addressed in the future. Interestingly, chromatin immunoprecipitation experiments were unable to detect DNA binding of bHLH protein to one of the pathway gene promoters in the absence of MYB genes (Hernandez et al., 2007). This suggests that R2R3-MYBs may influence the recruitment of bHLH-like factors to DNA, perhaps by conformational changes that expose the bHLH domain for dimerization and DNA binding (Feller et al., 2011).
Emerging evidence has revealed the interaction of the Mediator25 (MED25) protein with bHLH04, bHLH05, and bHLH06 as well as involvement of the Mediator complex in the JA-induced transcriptional regulation (Kidd et al., 2009; Çevik et al., 2012; Chen et al., 2012). Notably, among bHLH proteins interacting with MED25, several important TFs (AP2/ERF1, ORA59, and TDR1/ERF98) known to function in the control of JA-associated gene expression were also identified (Çevik et al., 2012; Chen et al., 2012). Mediator is a conserved protein complex known to promote the transcription of protein-coding genes by RNA polymerase II in eukaryotes. Mediator is highly conserved in a wide range of eukaryotes (Chadick and Asturias, 2005; Malik and Roeder, 2005; Bäckström et al., 2007; Chen et al., 2012). Remarkably, several recent studies demonstrated that besides interacting directly with polymerase II, Mediator has multiple functions and can interact with and coordinate the action of numerous other coactivators and corepressors, including those acting at the level of chromatin (Kidd et al., 2010; Malik and Roeder, 2010; Borggrefe and Yue, 2011; Chen and Roeder, 2011; Conaway and Conaway, 2011; Hentges, 2011; Kidd et al., 2011; Kim and Chen, 2011; Ries and Meisterernst, 2011; Chen et al., 2012). Altogether, we suggest that the Mediator complex could be an integrative point that coordinates GSL biosynthesis and JA signaling through interaction with multiple TFs. Proteins, such as MED25, which is known to act downstream of JA signaling and to interact with bHLH proteins, are probably essential to regulate GSL biosynthesis via the MYB-bHLH complex. Further investigation of individual subunits of the Mediator complex and their interaction partners could provide the potential for discovering regulatory proteins functioning in association with bHLH-MYB complex to regulate GSL biosynthesis. These could be proteins involved in chromatin modification like RIF1, as well as proteins important for function of RNA polymerase II transcription apparatus binding to promoter regions of GSL genes.
We propose a model that we believe occurs in planta (Fig. 10), in which MYBs and bHLHs are recruited to the promoters of GSL genes to activate the transcription of GSL biosynthesis genes; MYB proteins bind to MYB-boxes present in GSL genes, whereas bHLH proteins bind to G-boxes in the same genes. The function of bHLHs is probably to tether the mediator complex and chromatin-modifying factors to DNA, which unwind chromatin and make it accessible to MYBs and the RNA polymerase II transcription apparatus, which can then activate the transcription of GSL biosynthesis genes.
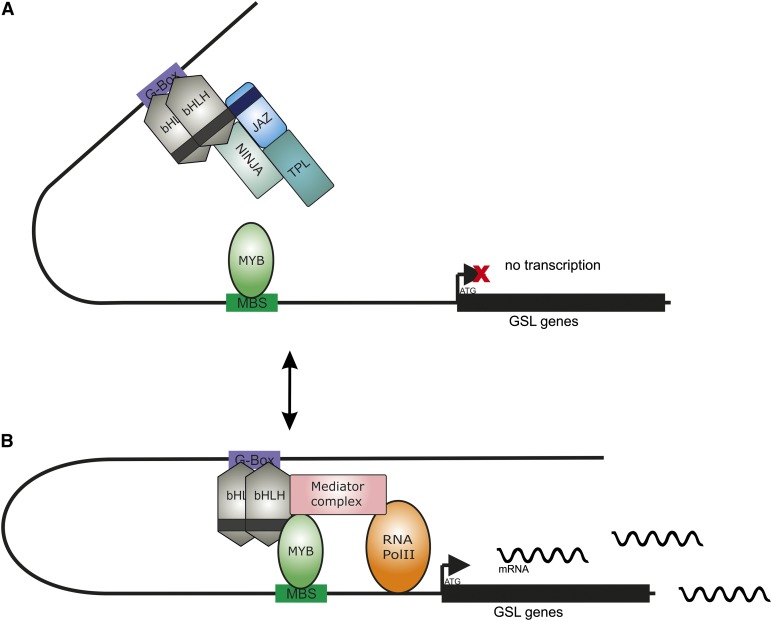
Figure 10.
Working model for regulation of GSL by MYB and bHLH in Arabidopsis. In the absence of JA, JAZ repressors bind to bHLH and inhibit the interaction between the bHLH proteins and R2R3-MYBs, attenuating the potential of the MYB-bHLH complex to activate the promoters of GSL pathway genes. Competitive binding to bHLH proteins by JAZs and MYBs as well as preferential binding of some bHLH proteins (mainly bHLH05 and bHLH04) to MYBs rather than to JAZ allows the basal transcriptional activity of the bHLH-MYB complex and allows the production of the moderate level of GSL present in wild-type plants. In the presence of JA, the bHLH proteins form more protein complexes with MYBs. Once the MYBs and bHLH are simultaneously recruited to the promoter of GSL genes, they activate the transcription of GSL biosynthesis genes. MYBs binds to MYB-boxes present in GSL genes, whereas bHLH proteins bind to G-boxes present in the same genes and the transcriptional of GSL genes is activated. The function of bHLHs is fulfilled by the tethering of the mediator complex, and probably the chromatin-modifying factors to DNA, which unwind chromatin, make it accessible to the MYBs and RNA polymerase II.
MATERIALS AND METHODS
Arabidopsis Loss-of-Function Mutants Used in This Study
The Arabidopsis (Arabidopsis thaliana) loss-of-function mutants used in this study are all in the Col-0 genetic background. For bHLH04, bHLH05, and bHLH28, the following insertion lines were obtained from the Nottingham Arabidopsis Stock Centre: At4g17880/bHLH04 (GK491E10), At5g46760/bHLH05 (GK445B11), and At5g46830/bHLH28 (SALK_119765C). Homozygous lines were identified by PCR for all three mutants and the position of the insertions was confirmed by sequencing. The absence of mRNA transcripts was additionally confirmed by RT-PCR. The bhlh06/jin1.9 mutant (AT1G32640/bHLH06/MYC2) was kindly provided by Dombrecht et al. (2007). The T-DNA insertion mutants for MYB34 and MYB51 were previously described and are known as myb34 (AT5G60890; WiscDsLox424F3; Frerigmann and Gigolashvili, 2014) and myb51/hig1 (AT1G18570; GK228B12; Gigolashvili et al., 2007a). For the sake of simplicity, the lines will be herein named as bhlh04, bhlh05, bhlh06, bhlh28, myb34, and myb51. The multiple mutants bhlh04/05, bhlh04/06, bhlh05/06, bhlh04/05/06, bhlh04/myb34, bhlh04/myb51, bhlh05/myb34, bhlh05/myb51, bhlh06/myb34, and bhlh06/myb51 were generated by crossing the corresponding parental homozygous lines and genotyping F2 segregating progenies to select homozygous mutations (primers for genotyping are listed in Supplemental Table S1). The homozygosity of mutants containing bhlh06 was confirmed by analyzing the population for long roots on Murashige and Skoog (MS) plates supplemented with 50 µm of MeJA, compared with the respective control lines. The isolation of triple and quadruple mutants with bhlh28 was not achieved due to the close linkage of the bHLH05 (At5g46760) locus to bHLH28 (At5g46830).
Arabidopsis Gain-of-Function Alleles and Overexpression Lines Used in This Study
For the generation of MYB51 and bHLH05 overexpression lines, the respective full-length coding sequences were amplified with gene-specific primers (Supplemental Table S2) and cloned into the Gateway pENTR/D-TOPO vector. The final destination clones were generated by LR (Invitrogen) recombination with pGWB2 (Pro::35S; kindly provided by Nakagawa et al., 2007) and/or pBatTL-K-GFP (Jörgens et al., 2010) followed by Agrobacterium tumefaciens-mediated transformation into wild-type (Col-0) plants. Successful transformed lines were selected using the respective marker genes and were analyzed for elevated transcript levels by qRT-PCR (PCR primers listed in Supplemental Table S3).
The gain-of-function alleles MYB34-1D (atr1D, AT5G60890; Bender and Fink, 1998) and bHLH05-D94N (atr2D, AT5G46760; Smolen et al., 2002) and the double gain-of-function allele MYB34-1D bHLH05-D94N (atr1D atr2D; Smolen et al., 2002) were kindly provided by the respective authors. The MYB51-1D (HIG1-1D) activation-tagged line and 35S:MYB51 overexpression plants were available from a previous study (Gigolashvili et al., 2007a)
Protein-Protein Interaction Analysis Using BiFC
To test the protein-protein interaction in planta, BiFC was used (Walter et al., 2004). The coding sequences of MYB51 and bHLH05 were transferred from the entry clones into the gateway pCL112 YFP N-terminal and pCL113 YFP C-terminal vectors, respectively (kindly provided by Sean Chapman, SCRI, and recently described by Pesch et al., 2013). The nuclear-localized factor bHLH133, which was cloned into the pCL113 YFP C-terminal vector, was used as a negative control for the interaction with MYB51. All plasmids of interest were transformed into A. tumefaciens and used for transient expression in tobacco via leaf infiltration. YFP fluorescence in tobacco was monitored 3.5 d after infiltration via a Leica DMRE fluorescence microscope (excitation at 514 nm and emission at 520-560 nm).
Protein-Protein Interaction Analysis Using LUMIER
The LUMIER assay is based on the techniques of Barrios-Rodiles et al. (2005) for mammalian proteins and Pesch et al. (2013) for plant proteins. The LUMIER assay was performed as described by Pesch et al. (2013), with slight modifications. To fuse luciferase from Renilla reniformis or protein A from Staphylococcus aureus to MYB and bHLH proteins, the two destination vectors pcDNA3-Rluc-GW and pTREX-dest30-ntPrA were recombined by LR reaction with the respective entry clones. The entry clone containing the gain-of-function protein D94N bHLH05 was generated by amplifying the full-length coding sequence from the bHLH05-D94N mutant (atr2D, AT5G46760; Smolen et al., 2002) followed by cloning into the Gateway pENTR/D-TOPO vector. The entry clone for the native bHLH05 was generated as described above (Arabidopsis gain-of-function alleles and overexpression lines used in this study).Generated constructs were transiently coexpressed in HEK293TN cells (BioCat/SBI LV900A-1) as hybrid proteins. About 0.4 μg of each plasmid DNA was cotransfected into 1 × 105 HEK293TN cells in 48-well plates using 1 μL of Lipofectamine 2000 (Invitrogen). After 48 h, the medium was removed and cells were washed with phosphate-buffered saline (PBS). The cells were collected by centrifugation (600g, 15 min) and then lysed on ice in 180 μL of ice-cold lysis buffer (20 mm Tris, pH 7.5, 250 mm NaCl, 1% Triton X-100, 10 mm EDTA, 10 mm dithiothreitol, protease inhibitor cocktail [catalog no. 11697498001, Roche] and phosphatase inhibitor cocktail [catalog no. 04693116001, Roche]) for 1 h. Subsequently, lysates were centrifuged at 21,380g for 15 min at 4°C, and the 50 µL of supernatant was mixed with 3 μL of PBS-prewashed sheep antirabbit IgG-coated magnetic beads (2 mg/mL final concentration, Dynabeads M280; Invitrogen) and incubated for 1 h on ice. The beads were than washed five times with cold PBS using a magnetic plate washer (Hydro Flex; Tecan) and luminescence was measured with 200 µL of luciferase buffer (1.1 m NaCl, 2.2 mm EDTA, 200 mm KxPO4, 0.44 mg/mL bovine serum albumin, and 2.5 µm coelenterazine) in a microtiter plate reader (Tecan). For the luciferase input control, 10 µL of the lysate was diluted to 50 µL with PBS and measured accordingly. Luciferase activity was determined for the input and the pull-down. Lysis buffer, PBS, and untransformed cells served as a negative control. Pull-down efficiency was calculated by the following: [relative luminescence unit pull-down/(relative luminescence unit input × 5)] × 100.
RNA Extraction and Expression Analysis by qRT-PCR
Total RNA was extracted from rosette leaves or seedlings of different mutants using TRIzol buffer (Life Technologies) followed by RNase-free DNAse (Roth) treatment to remove genomic DNA contamination. The expression of GSL biosynthesis genes was analyzed by real-time qRT-PCR analysis using the fluorescent intercalating dye SYBR-Green in a GeneAmp 5700 Sequence Detection System (Applied Biosystems). The Arabidopsis ACTIN2 gene was used as an endogenous control. A two-step RT-PCR analysis was performed. First, total RNAs (10 µg per reaction) were reverse transcribed into cDNAs, using the First-Strand cDNA Synthesis SSII Kit (Life Technologies) according to the manufacturer’s instructions. Subsequently, the cDNAs were used as a template in real-time PCR experiments with the SYBR-Green Master Kit System (Life Technologies) and gene-specific primers (for primer sequences, see Supplemental Table S3) according to the manufacturer’s instructions. The relative quantification of expression levels was performed using the comparative Δ cycle threshold method, and the calculated relative expression values were normalized to ACTIN2 and compared with the expression level in wild-type plants (Col-0 = 1). If not specified in the figure legend, three technical replicates and three biological replicates from independently grown plants were analyzed.
Generation and Analysis of probHLH05-uidA Plants
For in planta expression analysis of bHLH05, two different ProbHLH05 constructs were generated. The first construct included the first two exons, intron, and −1325 bp of the promoter region. The second construct comprised only the promoter region from −1325 to −4 bp and no sequences from the coding part of the gene. Both pieces of DNA were cloned into an entry vector and subsequently transferred by LR into the pGWB3 vector containing a uidA (GUS) fusion. All stably transformed plants containing only a −1325 to −4 bp part of promoter region exhibited similar tissue-specific expression patterns although the intensity of blue staining slightly differed from plant to plant. The promoter fusion construct including −1325 to −4 bp, the translation initiation codon, and the first two exons did not show any GUS activity in stably transformed plants and were therefore eliminated from further analysis. Histochemical detection of GUS activity was performed using X-Gluc as a substrate. Incubation in 80% (v/v) ethanol was performed to remove chlorophyll.
HPLC Analysis of desulpho-GSL
The isolation and analysis of GSL content was performed by using the desulpho-GSL method (Thies, 1979) on an ultra-performance liquid chromatography device (Eschborn; Waters) as previously described (Gigolashvili et al., 2012).
Promoter Transactivation Assay with bHLH05 and MYB51 and Promoters of GSL Biosynthesis Genes in Cultured Arabidopsis Cells
An Arabidopsis suspension-culture cell line was maintained in 50 mL of Arabidopsis (AT) medium. The AT medium contained 4.3 g/L MS basal salts (Duchefa), 1 mg/L 2,4-dichlorophenoxyacetic acid, 4 mL of vitamin B5 mixture (Sigma-Aldrich), and 30 g/L Suc (pH 5.8). Cells were gently agitated at 160 rounds per minute in the dark at 22°C. Promoters of the GSL biosynthetic genes ASA1, CYP79B3, and CYP83B1 were generated as reported in Gigolashvili et al. (2007a). The overexpression constructs for bHLH05 and MYB51 generated as described above (Arabidopsis gain-of-function alleles and overexpression lines used in this study) together with GSL promoter-reporter constructs were transferred into the supervirulent A. tumefaciens strain LBA4404.pBBR1MCS.virGN54D.
To assess the transactivation potential of bHLH05 and MYB51 proteins against promoters or GSL genes, the effector constructs with TFs and the promoter-reporter uidA constructs driven by the promoters of GSL biosynthesis genes were used. Thus, the effector constructs in the supervirulent A. tumefaciens strain, the antisilencing A. tumefaciens strain 19K (Voinnet et al., 1999), and each of the reporter constructs were taken from fresh yeast extract broth plates, grown overnight, resuspended in 1 mL of AT medium and used for cotransfection. To estimate the transactivation potential, three clones of A. tumefaciens containing effector, reporter, and antisilencing constructs (19K) were mixed in a 1:1:1 ratio and 75 µL of this suspension was added to 3 mL of cultured Arabidopsis cells. When the combinatorial effect of two effectors (bHLH05 and MYB51) was desired, two effector constructs, one promoter-reporter construct, and 19K strains were mixed in a 1:1:1:1 ratio and used for the contransfection of cells. After 4 to 5 d of coculturing (in the dark, 22°C, 160 rpm), cells were treated with 100 μL X-Gluc solution for 1 h to overnight at 37°C or cells were centrifuged, the proteins isolated and GUS activity determined using 4-methylumbelliferyl-β-d-glucuronide as a substrate. The amount of 4-methylumbelliferone formed was recorded fluorometrically (excitation, 340 nm; emission, 465 nm). For this, 200 μL of substrate buffer (protein extraction buffer with 1 mm 4-methylumbelliferyl-β-d-glucuronide) and 25 μL of protein extract were mixed, incubated at 37°C in the dark, and measured every 10 to 30 min on a multiwell plate reader. The slope of the increase in 4-methylumbelliferone was determined in the linear range and divided by the amount of protein.
Plant Growth Conditions and MeJA Treatment
Seeds of Arabidopsis Col-0 and of mutant lines were stratified for 2 to 7 d in the dark at 4°C to break seed dormancy. Plants were grown in growth cabinets with a 8-h/16-h light/dark cycle and 21°C/18°C day/night temperature, 40% humidity, and a mean photon flux density of 150 μmol m−2 s−1. A minimum of 100 mg of rosette material was harvested from 4- to 5-week-old plants, immediately frozen in liquid nitrogen, and kept at −80°C until DNA and RNA extraction or GSL analysis.
For MeJA treatment, wild-type and mutant plants were grown vertically on sterile MS plates for 2 weeks and then transferred onto MS plates supplemented with 50 µm of MeJA or without any supplementation. Seedlings were grown for an additional 2 d or 1 week on these media until they were harvested and used for transcript or GSL analysis.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Biosynthesis of aliphatic and indolic glucosinolates in Arabidopsis.
Supplemental Figure S2. BiFC reveals an interaction of indolic MYB51, MYB34 and MYB122 but also of MYB28 and MYB29 with bHLH05 and bHLH04.
Supplemental Figure S3. Expression level of bHLH subgroup IIIe is comparable in roots and shoots.
Supplemental Figure S4. Levels of individual IG and AG in seedlings of various double myb bhlh mutants.
Supplemental Figure S5. GSL accumulation in rosette leaves of Arabidopsis is not positively affected by the overexpression of 35S:bHLH05.
Supplemental Figure S6. Levels of individual IG and AG in single and double gain-of-function alleles of MYB34 and bHLH05.
Supplemental Figure S7. Levels of individual IGs and AGs in single and multiple bhlh mutants.
Supplemental Figure S8. Levels of individual AG and IGs in the bhlh04/05/06 mutant treated with MeJA.
Supplemental Figure S9. Expression of MYB34, MYB51 and MYB122 genes is not reduced in bhlh04/05/06 mutant.
Supplemental Figure S10. Induction of indolic glucosinolates in MYB34-1D by MeJA treatment.
Supplemental Figure S11. Accumulation of indolic GSLs in rosette leaves of triple bhlh04/05/06 is not positively affected by the overexpression of MYB51.
Supplemental Table S1. Primer sequences for qPCR analysis.
Supplemental Table S2. Primers used for the screening of T-DNA insertion lines.
Supplemental Table S3. Primer sequences for cloning.
Supplemental Data Text S1. Putative Cis Elements for MYB and bHLH detected by PLACE in CYP79B2, CYP79B3 and CYP83B1.
Acknowledgments
We thank Dr. Stanislav Kopriva and Dr. John Chandler for critical reading of the article and Dr. Ulf-Ingo Flügge for continuous support.
Glossary
- GSL
glucosinolate
- TF
transcription factor
- cDNA
complementary DNA
- BiFC
bimolecular fluorescence complementation
- IG
indolic glucosinolate
- LUMIER
luminescence-based mammalian interactome
- AG
aliphatic glucosinolate
- T-DNA
transfer DNA
- Col-0
ecotype Columbia-0 of Arabidopsis
- MeJA
methyl jasmonate
- qRT
quantitative reverse transcription
- X-Gluc
5-bromo-4-chloro-3- indolyl-β-d-glucuronic acid
- ChIP-seq
chromatin immunoprecipitation sequencing
- MS
Murashige and Skoog
- YFP
yellow fluorescent protein
- PBS
phosphate-buffered saline
- AT
Arabidopsis medium
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (project nos. GI 824/1–1 and EXC 1028).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abel S, Bürstenbinder K, Müller J. (2013) The emerging function of IQD proteins as scaffolds in cellular signaling and trafficking. Plant Signal Behav 8: e24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, De Vos CH, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O’Connell AP. (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28: 319–332 [DOI] [PubMed] [Google Scholar]
- Bäckström S, Elfving N, Nilsson R, Wingsle G, Björklund S. (2007) Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol Cell 26: 717–729 [DOI] [PubMed] [Google Scholar]
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, et al. (2005) High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307: 1621–1625 [DOI] [PubMed] [Google Scholar]
- Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L. (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39: 366–380 [DOI] [PubMed] [Google Scholar]
- Beekwilder J, van Leeuwen W, van Dam NM, Bertossi M, Grandi V, Mizzi L, Soloviev M, Szabados L, Molthoff JW, Schipper B, et al. (2008) The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS ONE 3: e2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J, Fink GR. (1998) A Myb homologue, ATR1, activates tryptophan gene expression in Arabidopsis. Proc Natl Acad Sci USA 95: 5655–5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamed M, Martin-Magniette ML, Taconnat L, Bitton F, Servet C, De Clercq R, De Meyer B, Buysschaert C, Rombauts S, Villarroel R, et al. (2008) Genome-scale Arabidopsis promoter array identifies targets of the histone acetyltransferase GCN5. Plant J 56: 493–504 [DOI] [PubMed] [Google Scholar]
- Bennett RN, Wenke T, Freudenberg B, Mellon FA, Ludwig-Müller J. (2005) The tu8 mutation of Arabidopsis thaliana encoding a heterochromatin protein 1 homolog causes defects in the induction of secondary metabolite biosynthesis. Plant Biol (Stuttg) 7: 348–357 [DOI] [PubMed] [Google Scholar]
- Berger B, Stracke R, Yatusevich R, Weisshaar B, Flügge UI, Gigolashvili T. (2007) A simplified method for the analysis of transcription factor-promoter interactions that allows high-throughput data generation. Plant J 50: 911–916 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132: 291–298 [DOI] [PubMed] [Google Scholar]
- Bodnaryk RP. (1992) Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry 31: 2671–2677 [Google Scholar]
- Bodnaryk RP. (1994) Potent effect of jasmonates on indole glucosinolates in oilseed rape and mustard. Phytochemistry 35: 301–305 [Google Scholar]
- Borggrefe T, Yue X. (2011) Interactions between subunits of the Mediator complex with gene-specific transcription factors. Semin Cell Dev Biol 22: 759–768 [DOI] [PubMed] [Google Scholar]
- Brader G, Tas E, Palva ET. (2001) Jasmonate-dependent induction of indole glucosinolates in Arabidopsis by culture filtrates of the nonspecific pathogen Erwinia carotovora. Plant Physiol 126: 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun P. (2004) Transcription factors as tools for metabolic engineering in plants. Curr Opin Plant Biol 7: 202–209 [DOI] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62: 471–481 [DOI] [PubMed] [Google Scholar]
- Brueggemann J, Weisshaar B, Sagasser M. (2010) A WD40-repeat gene from Malus x domestica is a functional homologue of Arabidopsis thaliana TRANSPARENT TESTA GLABRA1. Plant Cell Rep 29: 285–294 [DOI] [PubMed] [Google Scholar]
- Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EG, Hall RD, Bovy AG, Luo J, et al. (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26: 1301–1308 [DOI] [PubMed] [Google Scholar]
- Celenza JL, Quiel JA, Smolen GA, Merrikh H, Silvestro AR, Normanly J, Bender J. (2005) The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol 137: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çevik V, Kidd BN, Zhang P, Hill C, Kiddle S, Denby KJ, Holub EB, Cahill DM, Manners JM, Schenk PM, et al. (2012) MEDIATOR25 acts as an integrative hub for the regulation of jasmonate-responsive gene expression in Arabidopsis. Plant Physiol 160: 541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadick JZ, Asturias FJ. (2005) Structure of eukaryotic Mediator complexes. Trends Biochem Sci 30: 264–271 [DOI] [PubMed] [Google Scholar]
- Chen R, Jiang H, Li L, Zhai Q, Qi L, Zhou W, Liu X, Li H, Zheng W, Sun J, Li C. (2012) The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell 24: 2898–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Roeder RG. (2011) Mediator-dependent nuclear receptor function. Semin Cell Dev Biol 22: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Sun L, Qi T, Zhang B, Peng W, Liu Y, Xie D. (2011) The bHLH transcription factor MYC3 interacts with the Jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol Plant 4: 279–288 [DOI] [PubMed] [Google Scholar]
- Chini A, Boter M, Solano R. (2009) Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS J 276: 4682–4692 [DOI] [PubMed] [Google Scholar]
- Clarke DB. (2010) Glucosinolates, structures and analysis in food. Anal Methods 2: 310–325 [Google Scholar]
- Conaway RC, Conaway JW. (2011) Origins and activity of the Mediator complex. Semin Cell Dev Biol 22: 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Strack D. (2003) Phytochemistry meets genome analysis, and beyond. Phytochemistry 62: 815–816 [DOI] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al. (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19: 2225–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty KJ, Kiddle GA, Pye BJ, Wallsgrove RM, Pickett JA. (1995) Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry 38: 347–350 [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15: 573–581 [DOI] [PubMed] [Google Scholar]
- Elomaa P, Uimari A, Mehto M, Albert VA, Laitinen RA, Teeri TH. (2003) Activation of anthocyanin biosynthesis in Gerbera hybrida (Asteraceae) suggests conserved protein-protein and protein-promoter interactions between the anciently diverged monocots and eudicots. Plant Physiol 133: 1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Köllner TG, Degenhardt J, Zwahlen C, Hibbard BE, Turlings TCJ. (2011) The role of abscisic acid and water stress in root herbivore-induced leaf resistance. New Phytol 189: 308–320 [DOI] [PubMed] [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49: 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham M, Wilson P, Stephenson K, Fahey J. (2004) Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed 123: 60–65 [Google Scholar]
- Feller A, Hernandez JM, Grotewold E. (2006) An ACT-like domain participates in the dimerization of several plant basic-helix-loop-helix transcription factors. J Biol Chem 281: 28964–28974 [DOI] [PubMed] [Google Scholar]
- Feller A, Machemer K, Braun EL, Grotewold E. (2011) Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J 66: 94–116 [DOI] [PubMed] [Google Scholar]
- Fernández-Calvo P, Chini A, Fernández-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, et al. (2011) The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Fernández-Calvo P, Fernández GM, Díez-Díaz M, Gimenez-Ibanez S, López-Vidriero I, Godoy M, Fernández-Barbero G, Van Leene J, De Jaeger G, et al. (2014) bHLH003, bHLH013 and bHLH017 are new targets of JAZ repressors negatively regulating JA responses. PLoS ONE 9: e86182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H, Gigolashvili T. (2014) MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol Plant 7: 814–828 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Maekawa M, Oki T, Suda I, Iida S, Shimada H, Takamure I, Kadowaki K. (2007) The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J 49: 91–102 [DOI] [PubMed] [Google Scholar]
- Giamoustaris A, Mithen R. (1995) The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann Appl Biol 126: 347–363 [Google Scholar]
- Gigolashvili T, Berger B, Flügge UI. (2009) Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem Rev 8: 3–13 [Google Scholar]
- Gigolashvili T, Berger B, Mock HP, Müller C, Weisshaar B, Flügge UI. (2007a) The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 50: 886–901 [DOI] [PubMed] [Google Scholar]
- Gigolashvili T, Engqvist M, Yatusevich R, Müller C, Flügge UI. (2008) HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol 177: 627–642 [DOI] [PubMed] [Google Scholar]
- Gigolashvili T, Geier M, Ashykhmina N, Frerigmann H, Wulfert S, Krueger S, Mugford SG, Kopriva S, Haferkamp I, Flügge UI. (2012) The Arabidopsis thylakoid ADP/ATP carrier TAAC has an additional role in supplying plastidic phosphoadenosine 5′-phosphosulfate to the cytosol. Plant Cell 24: 4187–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigolashvili T, Yatusevich R, Berger B, Müller C, Flügge UI. (2007b) The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 51: 247–261 [DOI] [PubMed] [Google Scholar]
- Glover BJ, Perez-Rodriguez M, Martin C. (1998) Development of several epidermal cell types can be specified by the same MYB-related plant transcription factor. Development 125: 3497–3508 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53: 814–827 [DOI] [PubMed] [Google Scholar]
- Grotewold E. (2008) Transcription factors for predictive plant metabolic engineering: Are we there yet? Curr Opin Biotechnol 19: 138–144 [DOI] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. (2000) Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc Natl Acad Sci USA 97: 13579–13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb CD, Zipp BJ, Ludwig-Müller J, Masuno MN, Molinski TF, Abel S. (2004) Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J 40: 893–908 [DOI] [PubMed] [Google Scholar]
- Guo J, Chen YZ, Li MS, Shi L, Yan XF. (2013b) Does MYC2 really play a negative role in jasmonic acid-induced indolic glucosinolate biosynthesis in Arabidopsis thaliana? Russ J Plant Physiol 60: 100–107 [Google Scholar]
- Guo R, Shen W, Qian H, Zhang M, Liu L, Wang Q. (2013a) Jasmonic acid and glucose synergistically modulate the accumulation of glucosinolates in Arabidopsis thaliana. J Exp Bot 64: 5707–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Zou C, Lehti-Shiu MD, Shinozaki K, Shiu S-H. (2008) Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol 148: 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges KE. (2011) Mediator complex proteins are required for diverse developmental processes. Semin Cell Dev Biol 22: 769–775 [DOI] [PubMed] [Google Scholar]
- Hernandez JM, Feller A, Morohashi K, Frame K, Grotewold E. (2007) The basic helix loop helix domain of maize R links transcriptional regulation and histone modifications by recruitment of an EMSY-related factor. Proc Natl Acad Sci USA 104: 17222–17227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JM, Heine GF, Irani NG, Feller A, Kim M-G, Matulnik T, Chandler VL, Grotewold E. (2004) Different mechanisms participate in the R-dependent activity of the R2R3 MYB transcription factor C1. J Biol Chem 279: 48205–48213 [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, Araki R, Sakurai N, Suzuki H, Aoki K, et al. (2007) Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc Natl Acad Sci USA 104: 6478–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Onozawa-Komori M, Takahashi F, Asakura M, Bednarek P, Okuno T, Schulze-Lefert P, Takano Y. (2010) Entry mode-dependent function of an indole glucosinolate pathway in Arabidopsis for nonhost resistance against anthracnose pathogens. Plant Cell 22: 2429–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Reddy VS, Wessler SR. (2000) The rice R gene family: two distinct subfamilies containing several miniature inverted-repeat transposable elements. Plant Mol Biol 42: 667–678 [DOI] [PubMed] [Google Scholar]
- Humphries JA, Walker AR, Timmis JN, Orford SJ. (2005) Two WD-repeat genes from cotton are functional homologues of the Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 (TTG1) gene. Plant Mol Biol 57: 67–81 [DOI] [PubMed] [Google Scholar]
- Istrail S, Davidson EH. (2005) Logic functions of the genomic cis-regulatory code. Proc Natl Acad Sci USA 102: 4954–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörgens CI, Grünewald N, Hülskamp M, Uhrig JF. (2010) A role for ABIL3 in plant cell morphogenesis. Plant J 62: 925–935 [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Muiño JM, Jauregui R, Airoldi CA, Smaczniak C, Krajewski P, Angenent GC. (2009) Target genes of the MADS transcription factor SEPALLATA3: integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol 7: e1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DA, Hoffman CS. (2002) Gap repair transformation in fission yeast to exchange plasmid-selectable markers. Biotechniques 33: 978–982, 980, 982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd BN, Aitken EA, Schenk PM, Manners JM, Kazan K. (2010) Plant mediator: mediating the jasmonate response. Plant Signal Behav 5: 718–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd BN, Cahill DM, Manners JM, Schenk PM, Kazan K. (2011) Diverse roles of the Mediator complex in plants. Semin Cell Dev Biol 22: 741–748 [DOI] [PubMed] [Google Scholar]
- Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, Kazan K. (2009) The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell 21: 2237–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiddle GA, Doughty KJ, Wallsgrove RM. (1994) Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J Exp Bot 45: 1343–1346 [Google Scholar]
- Kim JH, Durrett TP, Last RL, Jander G. (2004) Characterization of the Arabidopsis TU8 glucosinolate mutation, an allele of TERMINAL FLOWER2. Plant Mol Biol 54: 671–682 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Chen X. (2011) The plant Mediator and its role in noncoding RNA production. Front Biol (Beijing) 6: 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Figuth A, Mitchell-Olds T. (2002) Genetic architecture of plastic methyl jasmonate responses in Arabidopsis thaliana. Genetics 161: 1685–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. (2001) Developmentally distinct MYB genes encode functionally equivalent proteins in Arabidopsis. Development 128: 1539–1546 [DOI] [PubMed] [Google Scholar]
- León J, Rojo E, Sánchez-Serrano JJ. (2001) Wound signalling in plants. J Exp Bot 52: 1–9 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M. (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57: 405–430 [DOI] [PubMed] [Google Scholar]
- Levy M, Wang Q, Kaspi R, Parrella MP, Abel S. (2005) Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J 43: 79–96 [DOI] [PubMed] [Google Scholar]
- Li S. (2014) Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal Behav 9: e27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sawada Y, Hirai A, Sato M, Kuwahara A, Yan X, Hirai MY. (2013) Novel insights into the function of Arabidopsis R2R3-MYB transcription factors regulating aliphatic glucosinolate biosynthesis. Plant Cell Physiol 54: 1335–1344 [DOI] [PubMed] [Google Scholar]
- Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC. (2010) An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Roeder RG. (2005) Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem Sci 30: 256–263 [DOI] [PubMed] [Google Scholar]
- Malik S, Roeder RG. (2010) The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet 11: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malitsky S, Blum E, Less H, Venger I, Elbaz M, Morin S, Eshed Y, Aharoni A. (2008) The transcript and metabolite networks affected by the two clades of Arabidopsis glucosinolate biosynthesis regulators. Plant Physiol 148: 2021–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Prescott A, Mackay S, Bartlett J, Vrijlandt E. (1991) Control of anthocyanin biosynthesis in flowers of Antirrhinum majus. Plant J 1: 37–49 [DOI] [PubMed] [Google Scholar]
- Martinez E. (2002) Multi-protein complexes in eukaryotic gene transcription. Plant Mol Biol 50: 925–947 [DOI] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. (2005) Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA. (2000) Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275: 33712–33717 [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Petersen BL, Glawischnig E, Jensen AB, Andreasson E, Halkier BA. (2003) Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol 131: 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanta TK. (2013) Plant metabolomics: missing link in next generation functional genomics era. J Appl Biol Biotechnol 1: 001–010 [Google Scholar]
- Morohashi K, Grotewold E. (2009) A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet 5: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford SG, Yoshimoto N, Reichelt M, Wirtz M, Hill L, Mugford ST, Nakazato Y, Noji M, Takahashi H, Kramell R, et al. (2009) Disruption of adenosine-5′-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 21: 910–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Nakata M, Mitsuda N, Herde M, Koo AJ, Moreno JE, Suzuki K, Howe GA, Ohme-Takagi M. (2013) A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 25: 1641–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Haruta KS, Pitaksutheepong C, Abe Y, Kakizaki Y, Yamamoto K, Shimada N, Yamamura S, Nishihara M. (2008) Identification and characterization of R2R3-MYB and bHLH transcription factors regulating anthocyanin biosynthesis in gentian flowers. Plant Cell Physiol 49: 1818–1829 [DOI] [PubMed] [Google Scholar]
- Niu Y, Figueroa P, Browse J. (2011) Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J Exp Bot 62: 2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G. (2009) Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanaik S, Xie CH, Kong Q, Shen KA, Yuan L. (2006) Directed evolution of plant basic helix–loop–helix transcription factors for the improvement of transactivational properties. Biochim Biophys Acta 1759: 308–318 [DOI] [PubMed] [Google Scholar]
- Pattanaik S, Xie CH, Yuan L. (2008) The interaction domains of the plant Myc-like bHLH transcription factors can regulate the transactivation strength. Planta 227: 707–715 [DOI] [PubMed] [Google Scholar]
- Pesch M, Hülskamp M. (2009) One, two, three...models for trichome patterning in Arabidopsis? Curr Opin Plant Biol 12: 587–592 [DOI] [PubMed] [Google Scholar]
- Pesch M, Schultheiß I, Digiuni S, Uhrig JF, Hülskamp M. (2013) Mutual control of intracellular localisation of the patterning proteins AtMYC1, GL1 and TRY/CPC in Arabidopsis. Development 140: 3456–3467 [DOI] [PubMed] [Google Scholar]
- Petersen BL, Chen S, Hansen CH, Olsen CE, Halkier BA. (2002) Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 214: 562–571 [DOI] [PubMed] [Google Scholar]
- Pilpel Y, Sudarsanam P, Church GM. (2001) Identifying regulatory networks by combinatorial analysis of promoter elements. Nat Genet 29: 153–159 [DOI] [PubMed] [Google Scholar]
- Pontoppidan B (2001) Studies of the glucosinolate-myrosinase system in relation to insect herbivory on oilseed rape (Brassica napus) and in Arabidopsis thaliana PhD thesis. University of Agricultural Sciences, Uppsala, Sweden
- Ries D, Meisterernst M. (2011) Control of gene transcription by Mediator in chromatin. Semin Cell Dev Biol 22: 735–740 [DOI] [PubMed] [Google Scholar]
- Sainz MB, Goff SA, Chandler VL. (1997a) Extensive mutagenesis of a transcriptional activation domain identifies single hydrophobic and acidic amino acids important for activation in vivo. Mol Cell Biol 17: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz MB, Grotewold E, Chandler VL. (1997b) Evidence for direct activation of an anthocyanin promoter by the maize C1 protein and comparison of DNA binding by related Myb domain proteins. Plant Cell 9: 611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Jikumaru Y, Obayashi T, Saito H, Masuda S, Kamiya Y, Ohta H, Shirasu K. (2013) Basic helix-loop-helix transcription factors JASMONATE-ASSOCIATED MYC2-LIKE1 (JAM1), JAM2, and JAM3 are negative regulators of jasmonate responses in Arabidopsis. Plant Physiol 163: 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster J, Knill T, Reichelt M, Gershenzon J, Binder S. (2006) Branched-chain aminotransferase4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell 18: 2664–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W. (2003) Metabolome diversity: too few genes, too many metabolites? Phytochemistry 62: 837–849 [DOI] [PubMed] [Google Scholar]
- Schweizer F, Bodenhausen N, Lassueur S, Masclaux FG, Reymond P. (2013b) Differential contribution of transcription factors to Arabidopsis thaliana defense against Spodoptera littoralis. Front Plant Sci 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F, Fernández-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P. (2013a) Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinn K, Venail J, Shang Y, Mackay S, Alm V, Butelli E, Oyama R, Bailey P, Davies K, Martin C. (2006) A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. Plant Cell 18: 831–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Shih MC, Li WH. (2005) Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol 139: 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J, et al. (2006) DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47: 10–24 [DOI] [PubMed] [Google Scholar]
- Smolen GA, Pawlowski L, Wilensky SE, Bender J. (2002) Dominant alleles of the basic helix-loop-helix transcription factor ATR2 activate stress-responsive genes in Arabidopsis. Genetics 161: 1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompornpailin K, Makita Y, Yamazaki M, Saito K. (2002) A WD-repeat-containing putative regulatory protein in anthocyanin biosynthesis in Perilla frutescens. Plant Mol Biol 50: 485–495 [DOI] [PubMed] [Google Scholar]
- Sønderby IE, Burow M, Rowe HC, Kliebenstein DJ, Halkier BA. (2010a) A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol 153: 348–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby IE, Geu-Flores F, Halkier BA. (2010b) Biosynthesis of glucosinolates—gene discovery and beyond. Trends Plant Sci 15: 283–290 [DOI] [PubMed] [Google Scholar]
- Sønderby IE, Hansen BG, Bjarnholt N, Ticconi C, Halkier BA, Kliebenstein DJ. (2007) A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE 2: e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Fan M, Zhang X, Gao H, Huang H, Wu D, Guo H, Xie D. (2013) The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Sweeney MT, Thomson MJ, Pfeil BE, McCouch S. (2006) Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 18: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W. (1979) Detection and utilization of a glucosinolate sulfohydrolase in the edible snail, Helix pomatia. Naturwissenschaften 66: 364–365 [Google Scholar]
- Verhage A, Vlaardingerbroek I, Raaymakers C, Van Dam NM, Dicke M, Van Wees SC, Pieterse CM. (2011) Rewiring of the jasmonate signaling pathway in Arabidopsis during insect herbivory. Front Plant Sci 2: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Pinto YM, Baulcombe DC. (1999) Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci USA 96: 14147–14152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wink M. (2010). Biochemistry of Plant Secondary Metabolism, Ed 2 John Wiley & Sons Ltd, Chichester, UK [Google Scholar]
- Wu S, Chappell J. (2008) Metabolic engineering of natural products in plants; tools of the trade and challenges for the future. Curr Opin Biotechnol 19: 145–152 [DOI] [PubMed] [Google Scholar]
- Xu W, Grain D, Bobet S, Le Gourrierec J, Thévenin J, Kelemen Z, Lepiniec L, Dubos C. (2014) Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol 202: 132–144 [DOI] [PubMed] [Google Scholar]
- Yan X, Chen S. (2007) Regulation of plant glucosinolate metabolism. Planta 226: 1343–1352 [DOI] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869 [DOI] [PubMed] [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF. (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40: 22–34 [DOI] [PubMed] [Google Scholar]